Introduction
Lung cancer, which is the most frequently diagnosed
cancer worldwide, is the leading cause of cancer-associated
mortality in males and is also common in females worldwide
(1,2).
Therefore, improvements in the diagnosis and treatment of lung
cancer are urgently required. Overall, ~80% of lung cancer lesions
are diagnosed as non-small cell lung cancer (NSCLC) (3). Currently, complete surgical resection
with adjuvant platinum-based chemotherapy is the standard treatment
for patients with resected stage II or IIIa NSCLC (4,5). However,
various toxicities occur in post-operative chemotherapy and the
overall survival time varies in patients, even in those at the same
stage of NSCLC and with the same histological type of cancer
(6). Previously, studies have
reported that genetic biomarkers, such as excision repair
cross-complementation group 1 (ERCC1), breast cancer 1 (BRCA1),
ribonucleotide reductase M1 (RRM1) and class III β-tubulin (TUBB3),
are closely associated with the clinical outcome of patients
administered with chemotherapy (7,8).
One of the major determinants of cisplatin
resistance is the nucleotide excision repair capacity. In
particular, ERCC1 acts as a key component in DNA repair, as well as
BRCA1 (9–12). RRM1 is a component of DNA and the
essential enzyme producing the deoxynucleoside involved in DNA
synthesis and repair (13,14). Tubulin dimers consisting of TUBB3
compose microtubule polymers that interfere with the reaction to
paclitaxel by slowing or blocking the transition from metaphase to
anaphase in the mitotic cell cycle (15,16).
Overall, these capable biomarkers have been
demonstrated as prognostic and predictive markers in certain
studies, but not in others (17–20).
Accordingly, the present prospective, randomized,
non-interventional study was performed to verify the predictive
value of the protein expression of ERCC1, BRCA1, RRM1 and TUBB3 in
patients with NSCLC that received adjuvant cisplatin-based
chemotherapy at the Sun Yat-Sen University Cancer Center
(Guangzhou, Guangdong, China).
Materials and methods
Patients and treatment
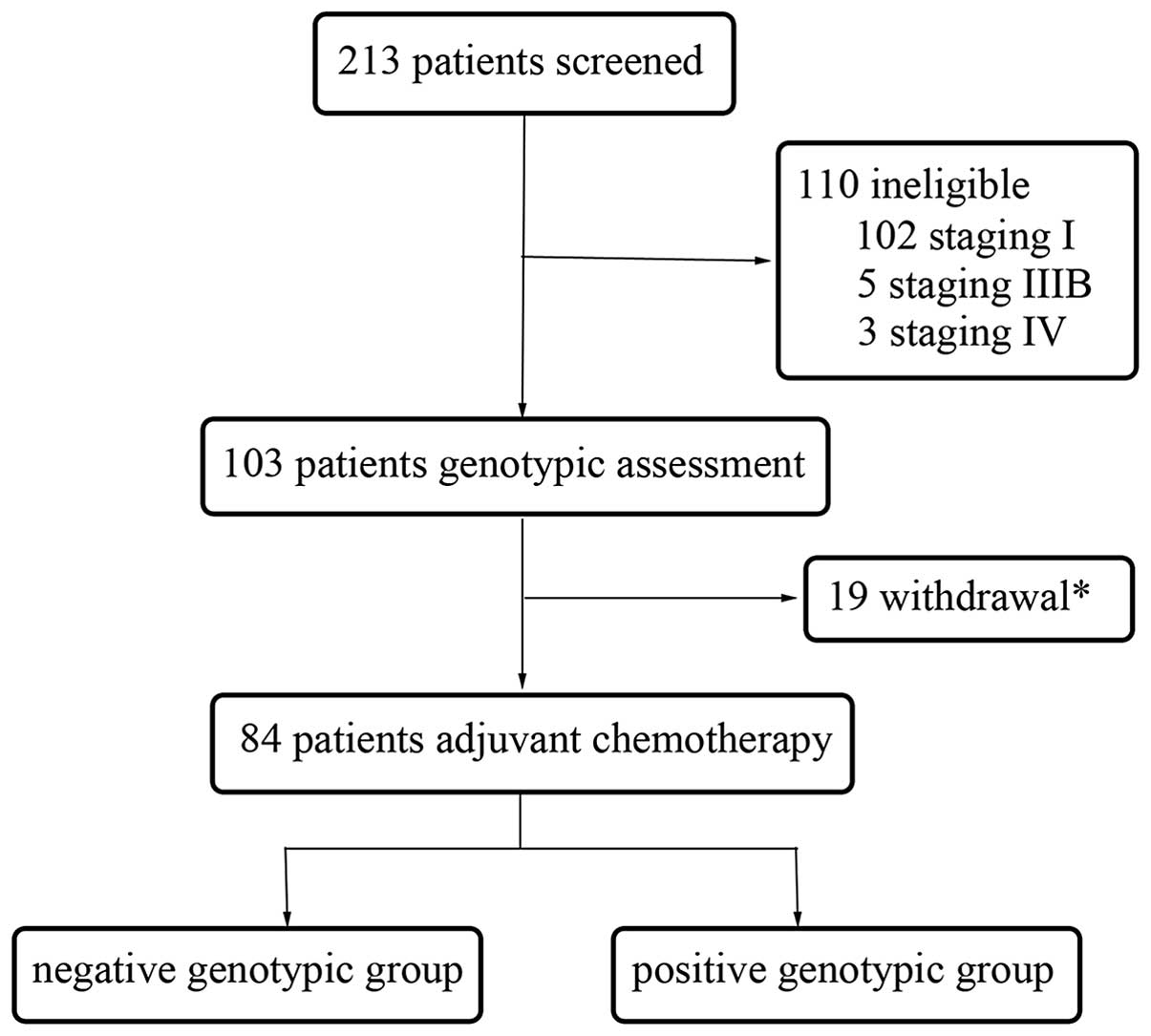
A total of 213 NSCLC patients were recruited in the
present prospective, randomized, non-interventional study at the
Sun Yat-Sen University Cancer Center between February 2009 and June
2013. All patients provided written informed consent and the
present study was approved by the Ethics Committee of Sun Yat-Sen
University Cancer Center. The baseline assessment prior to surgery
involved obtaining the medical history of patients and performing
physical, hematological and biochemical examinations, chest X-rays,
electrocardiograms, pulmonary function tests, computed tomography
of the thorax and abdomen, and magnetic resonance imaging of the
brain. Bone scans were required only if bone metastasis was
suspected. All patients received lobectomy or pneumonectomy. The
expression of ERCC1, BRCA1, RRM1 and TUBB3 was tested following the
surgical procedure. The pathological stage was identified
post-operatively according to the 7th edition of the Union for
International Cancer Control Tumor, Node, Metastasis (TNM)
Classification for lung cancer (21).
Subsequent to surgery, the patients were randomly
assigned to receive 75 mg/m2 cisplatin plus 75
mg/m2 docetaxel or 500 mg/m2 pemetrexed
chemotherapy every three weeks for four cycles. Prior to the
administration of the first cycle of chemotherapy within four weeks
of the surgical procedure, the patients were assessed
post-operatively, including a physical examination and a two-view
chest X-ray. Prior to each chemotherapy cycle, the patients
underwent a physical examination with routine biochemistry
examination and blood counts. Patients were excluded if they
possessed concurrent uncontrolled illness, or demonstrated an
Eastern Cooperative Oncology Group (ECOG) performance status >1.
Other exclusion criteria were significant weight loss (≥5%),
inadequate liver or renal function and an age >80 years old.
Immunohistochemical (IHC) and
pathological assessments
A standard protocol was used for the immunostaining
of the samples that were detected as NSCLC by hematoxylin and eosin
staining. Briefly, formalin-fixed, paraffin-embedded specimens were
sliced into 4 µm sections and baked for 1 h at 65°C. The specimens
were exposed to 10 mM citrate buffer (pH 6.0) for 10 min. Tumor
sections were incubated for 60 min with the mouse monoclonal
anti-human ERCC1 antibody (clone, 4F9; #UM500008; OriGene
Technologies, Inc., Rockville, MD, USA; dilution, 1:150), rabbit
polyclonal anti-BRCA1 antibody (#AR345-5R; BioGenex Laboratories,
Inc., San Ramon, CA, USA; dilution, 1:50), rabbit monoclonal
anti-RRM1 antibody (clone, EP242; #AC-0217RUO; Epitomics, Abcam,
Cambridge, UK; dilution, 1:100) and mouse monoclonal anti-TUBB3
antibody (clone SDL3D10; #MU177-UC; BioGenex Laboratories, Inc.;
dilution, 1:50). The tissue sections were incubated with polyclonal
goat anti-rabbit (#ab150077; Abcam; dilution, 1:200) and goat
anti-mouse (#ab150115; Abcam; dilution, 1:200) IgG biotinylated
secondary antibodies for 30 min at 37°C. The sections were then
incubated with a streptavidin-horseradish peroxidase complex
(Sigma-Aldrich, St. Louis, MO, USA) for 5 min at room temperature.
Finally, the sections were developed with diaminobenzidine and
counterstained with hematoxylin. Negative controls were also run
simultaneously.
Each section of the tissue specimens was evaluated
independently under a light microscope (CX21; Olympus Corporation,
Tokyo, Japan) by two pathologists, and eight random fields were
used to assess the expression levels of ERCC1, BRCA1, RRM1 and
TUBB3, and also to calculate an average score. In addition, the two
pathologists were blinded to the clinical status of the patients.
For each patient specimen, these biomarkers were assessed by
intensity, which was scored as follows: 0, no staining; 1, weak
staining; 2, moderate staining; and 3, strong staining.
Statistical analysis
The primary endpoint was DFS time, which was defined
as the time from the date of surgery to the date of tumor
recurrence or distant metastasis. The date was limited to the time
of the last tumor assessment if disease recurrence or distant
metastasis did not occur. The secondary endpoint was the overall
survival (OS) time, which was defined as the time from the date of
the surgical procedure to the date of mortality from any cause.
The Kaplan-Meier method was used to estimate the DFS
and OS times using SPSS version 17 software (SPSS, Inc., Chicago,
IL, USA). Pearson's χ2 test and Fisher's exact test were
also applied to study the association between these biomarkers and
patient characteristics that consisted of gender, age, histology,
smoking status, alcohol intake and tumor stage. The DFS time was
analyzed by subgroups using the Cox proportional hazards model,
with variables consisting of age, gender, histology, smoking
status, alcohol intake and tumor stage. All P-values were two-sided
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
During the follow-up period, 34 patients experienced
recurrence, of which 22 patients had succumbed to cancer by the
cut-off date for analysis (June 1, 2014). The median follow-up
period was 28.0 months (range, 4.9–49.5 months). Table I lists the characteristics of the
patients and the pathological findings. For all 84 patients in the
present study, the median age at disease diagnosis was 58 years,
ranging between 37 and 76 years old, with 68% of the patients being
men. The most common histological type was adenocarcinoma (53
patients; 63%), followed by squamous cell carcinoma (22 patients;
26%) and other types (9 patients; 11%), including large-cell
carcinoma, bronchoalveolar carcinoma and mixed types. The number of
patients with stage II and III tumors was 36 (43%) and 48 (57%),
respectively.
 | Table I.Patient characteristics according to
the expression of ERCC1, BRCA1, RRM1 and TUBB3. |
Table I.
Patient characteristics according to
the expression of ERCC1, BRCA1, RRM1 and TUBB3.
|
|
| ERCC1 expression | BRCA1 expression | RRM1 expression | TUBB3 expression |
|---|
|
|
|
|
|
|
|
|---|
| Characteristics | Total, n (%) | − | + | P-value | − | + | P-value | − | + | P-value | − | + | P-value |
|---|
| NSCLC patients | 84
(100) | 38 | 46 |
| 73 | 11 |
| 11 | 73 |
| 8 | 76 |
|
| Gender |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Female | 27 (32) | 12 | 15 | 0.92 | 23 | 4 | 1.00 | 4 | 23 | 1.00 | 3 | 24 | 1.00 |
|
Male | 57 (68) | 26 | 31 |
| 50 | 7 |
| 7 | 50 |
| 5 | 52 |
|
| Age |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
≤60 | 66 (79) | 33 | 33 | 0.09 | 56 | 10 | 0.50 | 8 | 58 | 0.91 | 6 | 60 | 1.00 |
|
>60 | 18 (21) | 5 | 13 |
| 17 | 1 |
| 3 | 15 |
| 2 | 16 |
|
| Smoking status |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Smoker | 52 (62) | 22 | 30 | 0.49 | 44 | 8 | 0.65 | 7 | 45 | 1.00 | 5 | 47 | 1.00 |
|
Non-smoker | 32 (38) | 16 | 16 |
| 29 | 3 |
| 4 | 28 |
| 3 | 29 |
|
| Alcohol Intake |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 6 (7) | 2 | 4 | 0.86 | 6 | 0 |
1.00a | 0 | 6 |
1.00a | 0 | 6 |
1.00a |
| No | 78 (93) | 36 | 42 |
| 67 | 11 |
| 11 | 67 |
| 8 | 70 |
|
| Histology |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adenocarcinoma | 53 (63) | 23 | 30 | 0.67 | 45 | 8 | 0.76 | 8 | 45 | 0.76 | 2 | 51 | 0.03 |
|
Squamous cell carcinoma | 22 (26) | 12 | 10 |
| 20 | 2 |
| 2 | 20 |
| 3 | 19 |
|
|
Others | 9
(11) | 4 | 5 |
| 8 | 1 |
| 1 | 8 |
| 3 | 6 |
|
| Stage |
|
|
|
|
|
|
|
|
|
|
|
|
|
| II | 36 (43) | 16 | 20 | 0.90 | 34 | 2 | 0.15 | 6 | 30 | 0.60 | 4 | 32 | 1.00 |
|
IIIa | 48 (57) | 22 | 26 |
| 39 | 9 |
| 6 | 42 |
| 4 | 41 |
|
IHC assessment
All patients were divided into two groups,
consisting of the negative group, with a staining score of 0, and
the positive group, with a staining score of 1–3, according to the
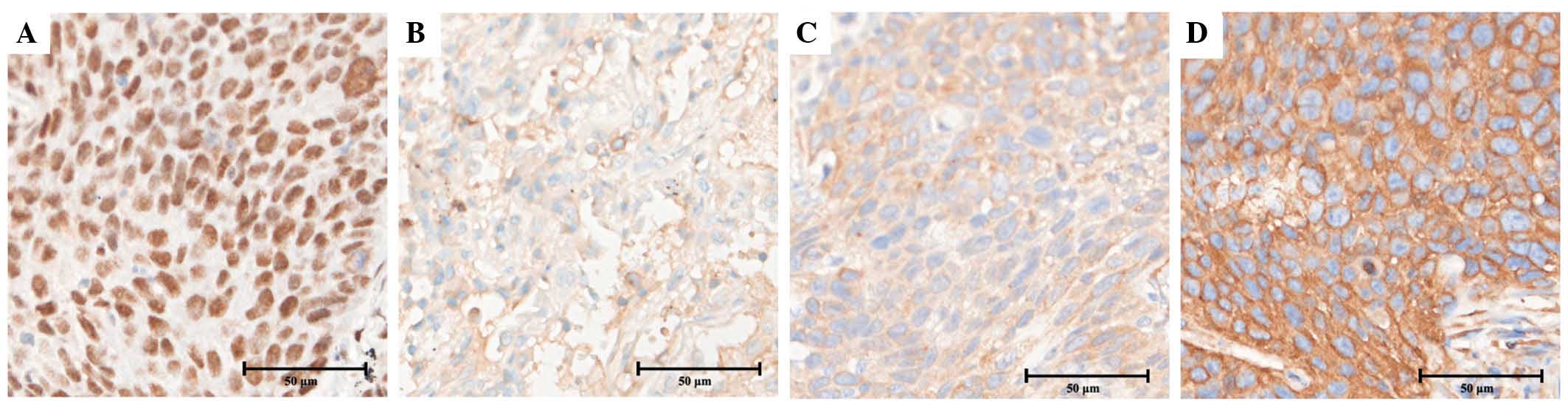
expression of ERCC1, BRCA1, RRM1 and TUBB3, respectively (Fig. 1). Fig. 2
shows the appearance of specimens stained for the expression of
ERCC1, BRCA1, RRM1 and TUBB3 in squamous cell carcinoma. The
nuclear expression of ERCC1 was identified in 46 out of 84 tissues
(54.8%), cytoplasmic BRCA1 expression was identified in 11 out of
84 tissues (13.1%), cytoplasmic RRM1 expression was identified in
73 out of 84 tissues (86.9%) and cytoplasmic TUBB3 expression was
identified in 76 out of 84 tissues (90.5%). There was a notable
positive association between pathological histology and TUBB3
expression (P=0.03), but not expression of ERCC1, BRCA1 or RRM1.
However, there was no significant association between the
expression of these biomarkers and the clinicopathological
variables, which consisted of age, gender, smoking status, alcohol
intake and TNM stage.
Survival analysis
The DFS time was significantly longer in the
ERCC1-negative group compared with the ERCC1-positive group
(median, 33.6 vs. 19.0 months; P=0.032; Fig. 3A. A similar result was demonstrated in
the BRCA1-positive and BRCA1-negative groups (median, 24.4 vs. 23.9
months; P=0.019; Fig. 3B), but not in
the RRM1-positive and RRM1-negative groups (median, 23 vs. 24
months; P=0.205; Fig. 3C) or
TUBB3-positive and TUBB3-negative groups (median, 33.4 vs. 23.3
months; P=0.382; Fig. 3D). In
addition, the combined expression of ERCC1 and BRCA1 was assessed
to determine the DFS time. A markedly increased benefit from
chemotherapy was identified in the absence of ERCC1 and BRCA1
expression compared with the presence of either ERCC1 or BRCA1
expression (median, 32.2 vs. 14.8 months; P=0.005; Fig. 3E). However, the OS time was not
significantly different between any of the four biomarkers (data
not shown) and the combination of ERCC1 and BRCA1 expression
(median, 32.2 vs. 23.4 months; P=0.117; Fig. 3F).
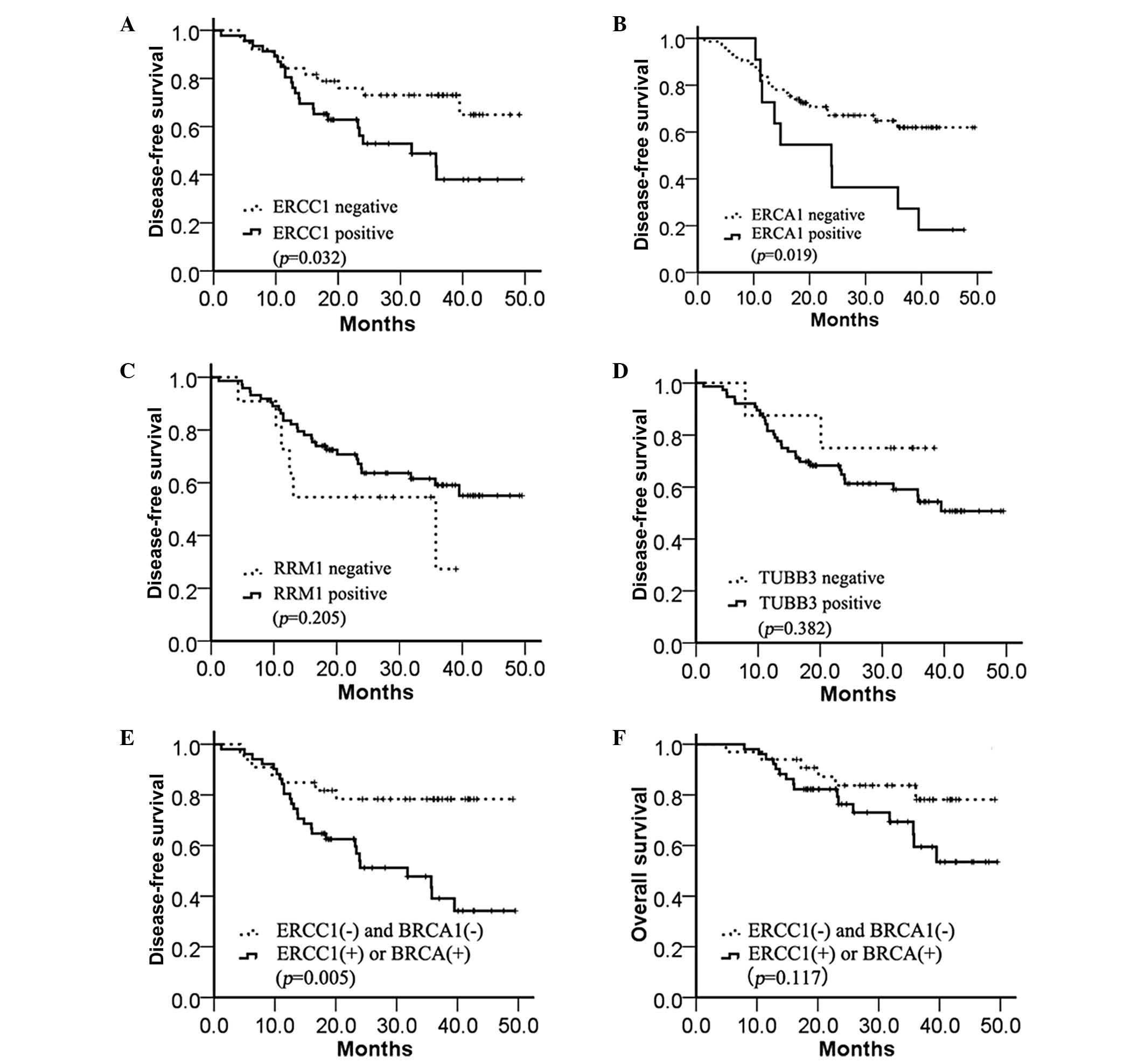 | Figure 3.Kaplan-Meier estimates of the
probability of survival with adjuvant cisplatin-based chemotherapy.
(A) Disease-free survival according to ERCC1 expression (median,
33.6 vs. 19.0 months; P=0.032). (B) Disease-free survival according
to BRCA1 expression (median, 24.4 vs. 23.9 months; P=0.019). (C)
Disease-free survival according to RRM1 expression (median, 23.0
vs. 24.0 months; P=0.205). (D) Disease-free survival according to
TUBB3 expression (median, 33.4 vs. 23.3 months; P=0.382). (E)
Disease-free survival according to ERCC1 and BRCA1 expression
(median, 32.2 vs. 14.8 months; P=0.005). (F) Overall survival
according to ERCC1 and BRCA1 expression (median, 32.2 vs. 23.4
months; P=0.117). ERCC1, excision repair cross-complementation
group 1; BRCA1, breast cancer 1; RRM1, ribonucleotide reductase M1;
TUBB3, class III β-tubulin. |
From the output of Cox regression, a series of
factors were assessed, consisting of age, gender, smoking status,
alcohol intake, histology, pathological staging, ERCC1, BRCA1, RRM1
and TUBB3 expression, and the combination of ERCC1 and BRCA1
expression. These factors were assessed using the univariate Cox
regression analysis, as reported in Table II, to assess the impact of the
factors on the DFS time of patients with NSCLC. The variables found
to impact DFS time in the univariate analysis were ERCC1 [hazard
ratio (HR), 2.166; 95% confidence interval (CI), 1.049–4.474;
P=0.037], BRCA1 (HR, 2.419; 95% CI 1.127–5.193; P=0.023), tumor
stage (HR, 2.352; 95% CI 1.097–5.044; P=0.028) and the combination
of ERCC1 and BRCA1 expression (HR, 3.102; 95% CI 1.343–7.163;
P=0.008). The variables, with the exception of ERCC1 and BRCA1,
which were excluded to avoid confounding bias, were also used in
the multivariate analysis model. In the multivariate analysis
model, the results indicated that the predominant predictors of DFS
time were the combination of ERCC1 and BRCA1 expression (HR, 2.968;
95% CI, 1.203–7.322; P=0.018) and tumor stage (HR, 2.381; 95% CI,
1.069–5.304; P=0.034).
 | Table II.Univariate analysis and multivariate
analysis for presictors of disease-free survival. |
Table II.
Univariate analysis and multivariate
analysis for presictors of disease-free survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | CI | P-value | HR | CI | P-value |
|---|
| Gender |
|
|
|
|
|
|
| Male
vs. female | 0.949 | 0.462–1.949 | 0.887 | 0.868 | 0.354–2.133 | 0.758 |
| Age |
|
|
|
|
|
|
| >60
vs. ≤ 60 years | 1.934 | 0.922–4.057 | 0.081 | 1.683 | 0.700–4.047 | 0.245 |
| Pathology |
|
|
|
|
|
|
| Others
vs. squamous cell carcinoma vs. adenocarcinoma | 1.209 | 0.751–1.949 | 0.425 | 1.643 | 0.949–2.845 | 0.076 |
| Smoking status |
|
|
|
|
|
|
| Smoker
vs. non-smoker | 0.794 | 0.387–1.630 | 0.530 | 0.708 | 0.285–1.758 | 0.457 |
| Drink |
|
|
|
|
|
|
| Yes vs.
no | 0.971 | 0.245–4.303 | 0.971 | 1.737 | 0.341–8.844 | 0.506 |
| Stage |
|
|
|
|
|
|
| Stage
IIIa vs. stage II | 2.352 | 1.097–5.044 | 0.028 | 2.381 | 1.069–5.304 | 0.034 |
| ERCC1
expression |
|
|
|
|
|
|
| Present
vs. absent | 2.166 | 1.049–4.474 | 0.037 |
|
|
|
| BRCA1
expression |
|
|
|
|
|
|
| Present
vs. absent | 2.419 | 1.127–5.193 | 0.023 |
|
|
|
| RRM1
expression |
|
|
|
|
|
|
| Present
vs. absent | 0.568 | 0.234–1.379 | 0.212 | 0.399 | 0.156–1.022 | 0.055 |
| TUBB3
expression |
|
|
|
|
|
|
| Present
vs. absent | 1.874 | 0.448–7.842 | 0.390 | 1.677 | 0.345–8.154 | 0.521 |
| ERCC1+BRCA1
expression |
|
|
|
|
|
|
| ERCC1
or BRCA1 present vs. | 3.102 | 1.343–7.163 | 0.008 | 2.968 | 1.203–7.322 | 0.018 |
| ERCC1
and BRCA1 absent |
|
|
|
|
|
|
Discussion
Despite the development of novel treatments for
patients with resected NSCLC, cisplatin agents have remained as the
standard first-line treatment. However, the positive response and
overall survival rates differ in patients with NSCLC, as well as
the toxicities (4,22,23).
Therefore, the detection of genes, proteins and RNA may facilitate
the selection of individuals or groups that may benefit most from
adjuvant chemotherapy and reduce adverse events. The results of the
present study revealed that the group of post-operative NSCLC
patients administered with adjuvant cisplatin-based chemotherapy
without ERCC1 expression demonstrated a significantly longer DFS
time compared with the group that expressed ERCC1. The results from
the assessment of BRCA1 expression were similar, with the lack of
ERCC1 and BRCA1 expression prolonging the median DFS time by 14.6
and 0.5 months, respectively. The combination of ERCC1 and BRCA1
expression may be a more valuable prognostic predictor in patients
with NSCLC administered with adjuvant cisplatin-based
chemotherapy.
ERCC1 is the limiting factor in the nucleotide
excision repair pathway that recognizes and removes
platinum-induced nucleotide adducts (24,25). In
addition, ERCC1 may participate in the repair of DNA double-strand
breaks (26). Overexpression of ERCC1
was involved in platinum resistance by prohibiting the activation
of the EGFR pathway (27). These
findings are consistent with the clinical evidence that ERCC1 in
NSCLC inhibits platinum efficacy. The International Adjuvant Lung
Cancer Trial (IALT) designed a prospective study to demonstrate
that patients with completely resected NSCLC may demonstrate
improved survival with the administration of adjuvant
cisplatin-based chemotherapy in 2004 (28). Another study designed by IALT revealed
that patients with ERCC1-negative tumors appeared to benefit from
adjuvant cisplatin-based chemotherapy. However, patients with
ERCC1-positive tumors did not benefit from the administered
chemotherapy (7). Certain studies
have also reported improved OS, DFS or progression-free survival
times in patients with a low expression of ERCC1 compared with
patients with high ERCC1 expression (20,28–30). The
present results supported ERCC1 as a predictive biomarker for
cisplatin-based chemotherapy. However, certain trials have failed
to acquire similar results (31–33). This
difference may be due to different antineoplastic protocols,
different components of data and the presence of other genotypes
that disturb the chemotherapeutic efficacy, which require
additional investigation.
BRCA1 increases cisplatin sensitivity through
inhibition of the c-Jun N-terminal kinase pathway in vitro
(34,35). A retrospective study by Taron et
al revealed that the absence of BRCA1 expression resulted in
high sensitivity to cisplatin compared with the cells that
expressed BRCA1 (12). The present
study also revealed that the tissues without BRCA1 expression
demonstrated a longer DFS rate compared with patients that
expressed BRCA1. However, the positive group had just 11 subjects.
Therefore, a larger, multi-centric trial is required. As ERCC1 and
BRCA1 are each associated with nucleotide excision repair, the
present study hypothesized that the benefit from chemotherapy may
be clearer when the expression of ERCC1 and BRCA1 were combined to
assess survival. RRM1 is involved in DNA repair systems, similar to
ERCC1 and BRCA1, as one of the targets of gemcitabine (36). Rosell et al demonstrated RRM1
to be a crucial predictive biomarker of survival in
gemcitabine-treated patients with advanced NSCLC (37). TUBB3 has been investigated to
determine the role of this gene in resistance to paclitaxel and
docetaxel. TUBB3 is also an independent prognostic marker in
patients with resected NSCLC that have not received chemotherapy
(38–40). In the present study, the expression of
RRM1 andTUBB3 was not associated with the DFS time of patients with
NSCLC that received adjuvant cisplatin-based chemotherapy. The
difference in the OS time was not significant in any of the four
biomarkers. There are certain points that should be noted. Firstly,
it is necessary to prolong the follow-up period to reflect the
population outcome. Secondly, the sample size in the present study
was too small to detect a difference in OS time.
In conclusion, the present study demonstrates that
the expression of ERCC1, as detected by immunohistochemistry, acts
as a predictive biomarker for cisplatin-based post-operative
chemotherapy in patients with NSCLC. The expression of BRCA1 and
ERCC1 may be an indicator for the lack of benefit of
cisplatin-based adjuvant chemotherapy, but an enlargement of the
sample size is required. Patients lacking ERCC1 and BRCA1
expression are likely to experience an increased benefit from
adjuvant cisplatin-based chemotherapy.
Acknowledgements
This study was supported by the Science and
Technology Project of Guangdong Province, China (grant no.,
2010B031600315) and the National Natural Science Foundation of
China (grant no., 81372568).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lozano R, Naghavi M, Foreman K, et al:
Global and regional mortality from 235 causes of death for 20 age
groups in 1990 and 2010: A systematic analysis for the Global
Burden of Disease Study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Devesa SS, Bray F, Vizcaino AP and Parkin
DM: International lung cancer trends by histologic type:
Male:female differences diminishing and adenocarcinoma rates
rising. Int J Cancer. 117:294–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
NSCLC Meta-analyses Collaborative Group.
Arriagada R, Auperin A, Burdett S, et al: Adjuvant chemotherapy,
with or without postoperative radiotherapy, in operable
non-small-cell lung cancer: Two meta-analyses of individual patient
data. Lancet. 375:1267–1277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pignon JP, Tribodet H, Scagliotti GV, et
al: LACE Collaborative Group: Lung adjuvant cisplatin evaluation: A
pooled analysis by the LACE Collaborative Group. J Clin Oncol.
26:3552–3559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olaussen KA, Dunant A, Fouret P, et al:
IALT Bio Investigators: DNA repair by ERCC1 in non-small-cell lung
cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med.
355:983–991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vilmar A and Sørensen JB: Excision repair
cross-complementation group 1 (ERCC1) in platinum-based treatment
of non-small cell lung cancer with special emphasis on carboplatin:
A review of current literature. Lung Cancer. 64:131–139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reed E: Platinum-DNA adduct, nucleotide
excision repair and platinum based anti-cancer chemotherapy. Cancer
Treat Rev. 24:331–344. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simon GR, Ismail-Khan R and Bepler G:
Nuclear excision repair-based personalized therapy for non-small
cell lung cancer: From hypothesis to reality. Int J Biochem Cell
Biol. 39:1318–1328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Quinn JE, James CR, Stewart GE, et al:
BRCA1 mRNA expression levels predict for overall survival in
ovarian cancer after chemotherapy. Clin Cancer Res. 13:7413–7420.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taron M, Rosell R, Felip E, et al: BRCA1
mRNA expression levels as an indicator of chemoresistance in lung
cancer. Hum Mol Genet. 13:2443–2449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mann GJ, Musgrove EA, Fox RM and Thelander
L: Ribonucleotide reductase M1 subunit in cellular proliferation,
quiescence, and differentiation. Cancer Res. 48:5151–5156.
1988.PubMed/NCBI
|
|
14
|
Engström Y, Eriksson S, Jildevik I, Skog
S, Thelander L and Tribukait B: Cell cycle-dependent expression of
mammalian ribonucleotide reductase. Differential regulation of the
two subunits. J Biol Chem. 260:9114–9116. 1985.PubMed/NCBI
|
|
15
|
Jordan MA, Toso RJ, Thrower D and Wilson
L: Mechanism of mitotic block and inhibition of cell proliferation
by taxol at low concentrations. Proc Natl Acad Sci USA.
90:9552–9556. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sève P, Isaac S, Trédan O, et al:
Expression of class III β-tubulin is predictive of patient outcome
in patients with non-small cell lung cancer receiving
vinorelbine-based chemotherapy. Clin Cancer Res. 11:5481–5486.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bepler G, Williams C, Schell MJ, et al:
Randomized international phase III trial of ERCC1 and RRM1
expression-based chemotherapy versus gemcitabine/carboplatin in
advanced non-small-cell lung cancer. J Clin Oncol. 31:2404–2412.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tiseo M, Bordi P, Bortesi B, Boni C, et
al: Bio-FAST trial group: ERCC1/BRCA1 expression and gene
polymorphisms as prognostic and predictive factors in advanced
NSCLC treated with or without cisplatin. Br J Cancer.
108:1695–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Booton R, Ward T, Ashcroft L, Morris J,
Heighway J and Thatcher N: ERCC1 mRNA expression is not associated
with response and survival after platinum-based chemotherapy
regimens in advanced non-small cell lung cancer. J Thorac Oncol.
2:902–906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cobo M, Isla D, Massuti B, et al:
Customizing cisplatin based on quantitative excision repair
cross-complementing 1 mRNA expression: A phase III trial in
non-small-cell lung cancer. J Clin Oncol. 25:2747–2754. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sobin LH, Gospodarowicz MK and Wittekind
C: Lung and Pleural Tumors. TNM Classification of Malignant Tumors
(7th). (Hoboken, NJ). Wiley-Blackwell. 136–148. 2010.
|
|
22
|
Arriagada R, Bergman B, Dunant A, Le
Chevalier T, Pignon JP and Vansteenkiste J: International Adjuvant
Lung Cancer Trial Collaborative Group: Cisplatin-based adjuvant
chemotherapy in patients with completely resected non-small-cell
lung cancer. N Engl J Med. 350:351–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Douillard JY, Rosell R, De Lena M, et al:
Adjuvant vinorelbine plus cisplatin versus observation in patients
with completely resected stage IB-IIIA non-small-cell lung cancer
(Adjuvant Navelbine International Trialist Association [ANITA]): A
randomised controlled trial. Lancet Oncol. 7:719–727. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mu D, Hsu DS and Sancar A: Reaction
mechanism of human DNA repair excision nuclease. J Biol Chem.
271:8285–8294. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zamble DB, Mu D, Reardon JT, Sancar A and
Lippard SJ: Repair of cisplatin - DNA adducts by the mammalian
excision nuclease. Biochemistry. 35:10004–10013. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Silva IU, McHugh PJ, Clingen PH and
Hartley JA: Defining the roles of nucleotide excision repair and
recombination in the repair of DNA interstrand cross-links in
mammalian cells. Mol Cell Biol. 20:7980–7990. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lian S, Su H, Zhao BX, Liu WY, Zheng LW
and Miao JY: Synthesis and discovery of pyrazole-5-carbohydrazide
N-glycosides as inducer of autophagy in A549 lung cancer cells.
Bioorg Med Chem. 17:7085–7092. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holm B, Mellemgaard A, Skov T and Skov BG:
Different impact of excision repair cross-complementation group 1
on survival in male and female patients with inoperable
non-small-cell lung cancer treated with carboplatin and
gemcitabine. J Clin Oncol. 27:4254–4259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee SH, Noh KB, Lee JS, et al: Thymidylate
synthase and ERCC1 as predictive markers in patients with pulmonary
adenocarcinoma treated with pemetrexed and cisplatin. Lung Cancer.
81:102–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lord RV, Brabender J, Gandara D, et al:
Low ERCC1 expression correlates with prolonged survival after
cisplatin plus gemcitabine chemotherapy in non-small cell lung
cancer. Clin Cancer Res. 8:2286–2291. 2002.PubMed/NCBI
|
|
31
|
Zheng Z, Chen T, Li X, Haura E, Sharma A
and Bepler G: DNA synthesis and repair genes RRM1 and ERCC1 in lung
cancer. N Engl J Med. 356:800–808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Friboulet L, Olaussen KA, Pignon JP, et
al: ERCC1 isoform expression and DNA repair in non-small-cell lung
cancer. N Engl J Med. 368:1101–1110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee KH, Min HS, Han SW, et al: ERCC1
expression by immunohistochemistry and EGFR mutations in resected
non-small cell lung cancer. Lung Cancer. 60:401–407. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Harkin DP, Bean JM, Miklos D, et al:
Induction of GADD45 and JNK/SAPK-dependent apoptosis following
inducible expression of BRCA1. Cell. 97:575–586. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Potapova O, Haghighi A, Bost F, et al: The
Jun kinase/stress-activated protein kinase pathway functions to
regulate DNA repair and inhibition of the pathway sensitizes tumor
cells to cisplatin. J Biol Chem. 272:14041–14044. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pitterle DM, Kim YC, Jolicoeur EM, Cao Y,
O'Briant KC and Bepler G: Lung cancer and the human gene for
ribonucleotide reductase subunit M1 (RRM1). Mamm Genome.
10:916–922. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rosell R, Danenberg KD, Alberola V, et al:
Spanish Lung Cancer Group: Ribonucleotide reductase messenger RNA
expression and survival in gemcitabine/cisplatin-treated advanced
non-small cell lung cancer patients. Clin Cancer Res. 10:1318–1325.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Koh Y, Jang B, Han SW, et al: Expression
of class III beta-tubulin correlates with unfavorable survival
outcome in patients with resected non-small cell lung cancer. J
Thorac Oncol. 5:320–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Burkhart CA, Kavallaris M and Band Horwitz
S: The role of β-tubulin isotypes in resistance to antimitotic
drugs. Biochim Biophys Acta. 1471:O1–O9. 2001.PubMed/NCBI
|
|
40
|
Gan PP, Pasquier E and Kavallaris M: Class
III β-tubulin mediates sensitivity to chemotherapeutic drugs in non
small cell lung cancer. Cancer Res. 67:9356–9363. 2007. View Article : Google Scholar : PubMed/NCBI
|