Introduction
Oral cancer is responsible for 200,000–350,000
cancer-associated fatalities per year worldwide and is thus ranked
sixth with regard to the cause of mortality due to tumors (1,2).
Beside the time of diagnosis and the consequent size
of the tumor (3), the presence of
lymph node metastasis in the neck is the most important prognostic
indicator (4,5). Oral SCC is disseminated preferentially
by the lymphatic system and mainly the cervical lymph nodes at
levels I and II are affected (6–8). The high
incidence of occult cervical lymph node metastases of ~25% in N0
cases in the clinic underscores the clinical significance and the
resulting therapeutic difficulties (9,10).
The commonly used staging procedures often cannot
predict the absence of CM. Clinical and radiological examination
have approximate false-negative and false-positive rates of 30% in
the determination of CM (11). The
most precise method and the gold standard for the correct N-staging
is the histopathological examination of the surgical specimen
following elective neck dissection (END) (12).
The management of the clinically and radiologically
negative neck, particularly in patients with early oral SCC,
remains a matter of debate, although the majority of centers favor
END for staging of the neck and the removal of occult disease
(11).
In the modern surgical treatment of melanoma or
breast cancer, the presence of regional lymph node metastases is
evaluated by the identification and examination of the sentinel
lymph node (SLN). Radiolabeled colloid solution is injected around
the primary tumor, which drains to the next lymph nodes and
predominantly to the SLN, which may contain metastatic deposits of
the primary tumor. The combination of pre-operative
lymphoscintigraphy and the intraoperative detection of the SLN with
a γ-probe allows the radioactive tracer in the lymph nodes to be
precisely located during the surgery (11,13).
In the past decade, the SLN-technique has been
increasingly used for other malignancies, including head and neck
carcinomas. Technical developments and a gain in experience have
led to a wider use of SNB, even in the complex lymphatic system of
the head and neck region (14).
Multiple small patient series have been published evaluating the
application of SLN biopsy for head and neck cancers, with a
sensitivity of at least 75% for the identification of CM (Table I) (11,15,16). But
the majority of these studies included higher stage SCC and did not
focus on a specific region and a clinical N0 neck.
 | Table I.Epidemiological and clinical data. |
Table I.
Epidemiological and clinical data.
| Patient | Gender | Age, years | Risk factors | No. of SLNs detected
during surgery | No. of CMs | Follow-up time,
months | Relapse |
|---|
| 1 | Male | 21 | No | 2 | 0 | 33 | No |
| 2 | Male | 28 | Yes | 2 | 0 | 39 | No |
| 3 | Male | 32 | No | 4 | 1 | 18 | After 10
months |
| 4 | Female | 33 | Yes | 2 | 0 | 22 | No |
| 5 | Female | 59 | Yes | 2 | 1 | 28 | No |
| 6 | Male | 62 | Yes | 3 | 2 | 40 | No |
| 7 | Female | 63 | No | 2 | 0 | 38 | No |
| 8 | Male | 69 | Yes | 2 | 0 | 40 | No |
| 9 | Female | 75 | Yes | 3 | 0 | 10 | No |
| 10 | Female | 82 | Yes | 2 | 0 | 34 | No |
The aim of the present study was to analyze and
evaluate the applicability of the SLN concept for T1/T2 SCC of the
tongue with a clinical N0 situation.
Patients and methods
Patients
Between 2010 and 2012, 10 patients with SCC of the
tongue were selected from the Department of Oral and Maxillofacial
of the University Medical Center (Johannes Gutenberg-University of
Mainz, Mainz, Germany) to take part in the study. The criteria for
inclusion were: SCC of the tongue, a tumor size <T3 and a
clinical N0 situation. All tumors were classified and staged
according to the 2003 tumor-node-metastasis (TNM) staging system of
the Union for International Cancer Control, and special attention
was paid to the CM (17).
The study protocol was approved by the internal
institutional review board and informed consent was obtained from
all the patients involved in the study. Computed tomography and
ultrasonography of the head and neck region were performed on all
patients prior to the treatment.
Treatment
All patients received peritumorous injections of
technetium-99m-labeled colloidal human serum albumin (0.2 ml; 50
MBq) in an attempt to completely surround the tumor in its deep and
lateral aspects. Injection was performed 1 day prior to surgery.
The pre-operative lymphoscintigraphy was performed 30 min after the
injection. Static images were accomplished in lateral and
antero-posterior projections, and the radioactive lymph nodes were
marked on the skin and controlled by B-mode sonography.
Beside the resection of the tongue tumor, all
patients received an END at levels I–III. Using a hand-held γ-probe
(Gamma Finder® II, World Of Medicine USA, Inc., Orlando, Florida,
USA), the SLN was identified in vivo and dissected
separately. Next, the remaining neck was re-evaluated for the
absence of radioactivity. All lymph nodes with radioactivity were
dissected and considered as SLNs. Afterwards, the proposed END was
performed. The SLNs and all neck specimens from the subsequent END
were sent for histopathological examination.
Results
The cohort consisted of 5 (50%) female and 5 (50%)
male patients, with an average age of 52 years and a range of 21–82
years (female: Mean, 62 years; range, 33–82 years; male: Mean, 42
years; range, 21–69 years). The majority of the patients (70%)
showed a risk profile regarding smoking and alcohol consumption
(Table I).
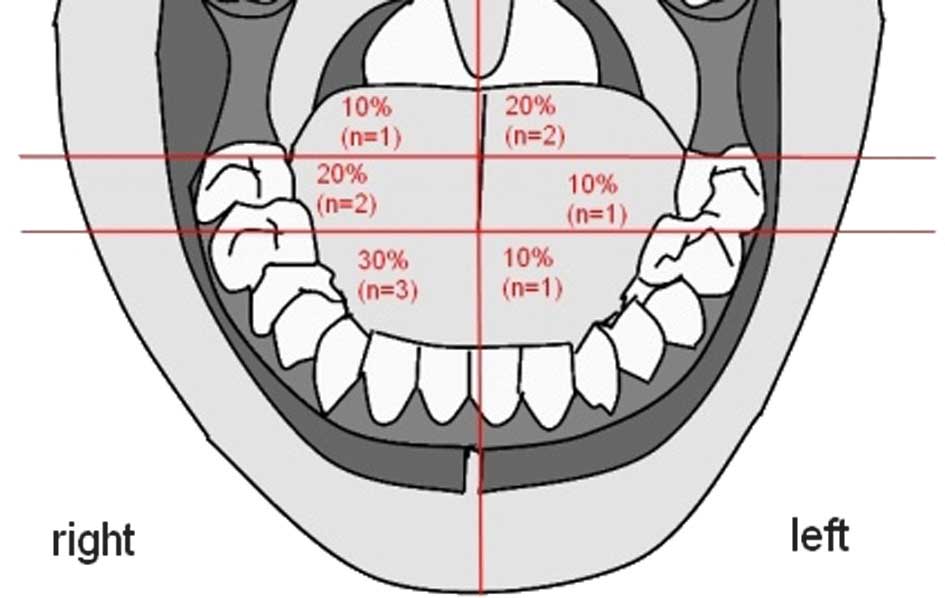
SCC was evenly spread in the tongue without a
preference for a side, however, 70% was located in the front and
middle section of the tongue (Fig.
1).
The pathological TNM stage of the patients is shown
in Table II; 80% of the patients
presented with a T1 tumor and 20% with a T2 tumor. No distant
metastases were detected following primary staging. The majority of
the patients (90%) presented with a tumor of grade G1-G2.
 | Table II.pTNM classification following
surgical therapy. |
Table II.
pTNM classification following
surgical therapy.
| pTNM stage | Patients, n
(%) |
|---|
| T-Stage |
|
|
pT1 | 8 (80) |
|
pT2 | 2 (20) |
| N-stage |
|
|
pN0 | 7 (70) |
|
pN1 | 2 (20) |
|
pN2 | 1 (10) |
| M-stage |
|
|
cM0 | 10 (100) |
| Grade |
|
| G1 | 4 (40) |
| G2 | 5 (50) |
| G3 | 1 (10) |
In all patients, SLN could be detected
intraoperatively. On average, 2.4 SLNs per patient were found.
Fig. 2 shows the distribution of SLNs
and CMs at the neck level. A total of 2 SLNs were found in 7
patients, 3 SLNs in 2 patients and 1 SLN in 1 patient were
detected. In 7 cases, the SLNs and the residual neck dissection
were negative for cervical lymph node metastasis.
In total, 30% (n=3) of the patients exhibited lymph
node metastases, which were detected by the SLN biopsy, without
further CM in the other neck nodes. One patient exhibited skip
metastasis; the patient presented with a CM in a SLN at level IV,
which had bypassed the common upper neck level I–III.
Additional CMs were developed in 1 patient after 10
months in the contralateral neck, with lung metastasis after 18
months.
The median follow-up time for the patients was 32
months (range, 8–39 months). During the follow-up, none of the
other 9 patients experienced local or cervical recurrence.
If the case with the contralateral CM recurrence
after 10 months is defined as a false-negative result, then the
sensitivity and specificity of the SLN biopsy in the patient group
were 75% (3/4 patients with CM were detected) and 100% (6/6 patient
without CN were detected), respectively, and the false negative
rate was 25%.
Discussion
The demographic data of the present SCC patients,
with a mean age of 52 years and the high presence of risk factors,
are comparable with the international literature (15–20). SCC
was evenly spread in the tongue and was identical to the
distribution pattern in the literature (21).
The management of patients with early oral SCC with
a clinically negative neck remains controversial. The majority of
clinics prefer the END instead of a wait-and-see strategy due to
the high rate of occult metastases. However, 70–80% of this patient
group are ultimately pN0 and are theoretically overtreated with a
selective neck dissection (SND) (22,23).
Although an SND is less invasive than a modified radical
dissection, measurable morbidity does exist, including shoulder
dysfunction, contour changes, pain and lower lip paresis (24–27).
Although the SND has proven reliability and worldwide acceptance,
it is an extended surgery compared with the SLN biopsy, meaning a
longer surgical time, higher costs and greater morbidity.
Functional outcome and post-operative complications following an
SLN biopsy are also significantly better than after an SND
(28,29).
The concept of an SLN biopsy provides the
possibility of accurate pathological cervical node staging, whilst
minimizing the invasiveness of the procedure and its associated
morbidity. In addition, pre-operative lymphoscintigraphy and
intraoperative detection with a hand-held γ-probe have the
additional advantage of identifying aberrant drainage pathways
(22,23). In the present study, a contralateral
SLN could be detected in 1 patient and a CM was found at level IV,
which had bypassed the common upper neck level I–III (skip
metastasis).
The SLN biopsy has the benefit of concentrating only
on the relevant nodes for pathological examination. This selection
allows a more in-depth evaluation of the small number of sentinel
nodes, using step serial sections and immunohistochemistry
(22,30,31).
However, if there are multiple SLNS at different levels, the number
of SLNs that should be removed for the examination remains unknown.
The majority of studies recommend the removal of at least 2–3 SLNs
to reduce the possibility of false-negative results (32–34). In
the present study, an average of 2.4 SLNs were detected per
patient.
There are a number of studies focusing on the use of
SLN in SCC (Table III) (11,15,16,26,33,36,38–44).
But only few studies do have a homogenous clientele with only small
tumors and a clinical N0 neck in which the SLN is of importance. In
addition the majority of these studies did not focus on a specific
region (oral cavity vs. oropharynx). The sensitivity of the SLN
biopsy for head and neck cancer varies in the literature between 75
and 100%.
 | Table III.Published sensitivity rates for SLN
biopsy. |
Table III.
Published sensitivity rates for SLN
biopsy.
| First author
(ref.) | Year | No. of patients
with oral SCC/no. of patients | Cancer | T-Stage | No. of detected CM
by SLN/no. of all CM | Sensitivity, % |
|---|
| Hyde et al
(38) | 2003 | 19/19 | Oral cancer | 1–4 | 3/4 | 75 |
| Gallegos et
al (33) | 2005 | 48/48 | Oral cancer | 1–2 | 13/17 | 77 |
| Frerich et
al (39) | 2007 | 28/40 | Oropharyngeal
cancer | 1–2 | 8/10 | 80 |
| Chone et al
(11) | 2008 | 24/35 | Oropharyngeal
cancer | 1–3 | 9/11 | 82 |
| Werner et al
(30) | 2004 | 11/90 | Oropharyngeal
cancer | 1–3 | 20/23 | 87 |
| Civantos et
al (23) | 2010 | 140/140 | Oral cancer | 1–2 | 34/40 | 90 |
| Tschopp et
al (40) | 2005 | 25/31 | Oropharyngeal
cancer | 1–3 | 14/15 | 93 |
| Shoaib et al
(41) | 2001 | 37/37 | Oral cancer | 1–4 | 16/17 | 94 |
| Ionna et al
(42) | 2002 | 40/40 | Oral cancer | 1–2 | 4/4 | 100 |
| Höft et al
(43) | 2004 | 22/50 | Oropharyngeal
cancer | 1–4 | 12/12 | 100 |
| Bilde et al
(44) | 2008 | 51/51 | Oral cancer | 1–2 | 11/11 | 100 |
| Current study | 2015 | 10/10 | Oral cancer | 1–2 | 4/5 | 80 |
The sensitivity of the SLN biopsy for head and neck
cancer varies in the literature between 75 and 100%. This has to be
compared with the rate of regional recurrence after SND, which is
recorded as between 6–30% in the literature (35–37). In
the present patient group, the sensitivity of the SLN biopsy was
75% when defining the contralateral CM recurrence after 10 months
in 1 patient as a false-negative result.
Although further studies are necessary to confirm
the results, patients with cN0 and early-stage oral SCC may benefit
from an SLN biopsy by avoiding the morbidity of a neck
dissection.
References
|
1
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kowalski LP and Carvalho AL: Influence of
time delay and clinical upstaging in the prognosis of head and neck
cancer. Oral Oncol. 37:94–98. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Capote A, Escorial V, Muñoz-Guerra MF,
Rodriguez-Campo FJ, Gamallo C and Naval L: Elective neck dissection
in early-stage oral squamous cell carcinoma-does it influence
recurrence and survival? Head Neck. 29:3–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hiratsuka H, Miyakawa A, Nakamori K, Kido
Y, Sunakawa H and Kohama G: Multivariate analysis of occult lymph
node metastasis as a prognostic indicator for patients with
squamous cell carcinoma of the oral cavity. Cancer. 80:351–356.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kowalski LP, Bagietto R, Lara JR, Santos
RL, Tagawa EK and Santos IR: Factors influencing contralateral
lymph node metastasis from oral carcinoma. Head Neck. 21:104–110.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pimenta Amaral TM, Da Silva Freire AR,
Carvalho AL, Pinto CA and Kowalski LP: Predictive factors of occult
metastasis and prognosis of clinical stages I and II squamous cell
carcinoma of the tongue and floor of the mouth. Oral Oncol.
40:780–786. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Woolgar JA: The topography of cervical
lymph node metastases revisited: the histological findings in 526
sides of neck dissection from 439 previously untreated patients.
Int J Oral Maxillofac Surg. 36:219–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mishra P and Sharma AK: A 3-year study of
supraomohyoid neck dissection and modified radical neck dissection
type I in oral cancer: with special reference to involvement of
level IV node metastasis. Eur Arch Otorhinolaryngol. 267:933–938.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuen AP, Ho CM, Chow TL, Tang LC, Cheung
WY, Ng RW, Wei WI, Kong CK, Book KS, Yuen WC, et al: Prospective
randomized study of selective neck dissection versus observation
for N0 neck of early tongue carcinoma. Head Neck. 31:765–772. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chone CT, Magalhes RS, Etchehebere E,
Camargo E, Altemani A and Crespo AN: Predictive value of sentinel
node biopsy in head and neck cancer. Acta Otolaryngol. 128:920–924.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Woolgar JA, Beirne JC, Vaughan ED,
Lewis-Jones HG, Scott J and Brown JS: Correlation of
histopathologic findings with clinical and radiologic assessments
of cervical lymph-node metastases in oral cancer. Int J Oral
Maxillofac Surg. 24:30–37. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stoeckli SJ, Alkureishi LW and Ross GL:
Sentinel node biopsy for early oral and oropharyngeal squamous cell
carcinoma. Eur Arch Otorhinolaryngol. 266:787–793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuriakose MA and Trivedi NP: Sentinel node
biopsy in head and neck squamous cell carcinoma. Curr Opin
Otolaryngol Head Neck Surg. 17:100–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bertz J, Dahm S, Haberland J, Kraywinkel
K, Kurth BM and Wolf U: Spread of cancers in Germany. Development
of prevalence between 1990 and 2010. A publication of the Centre
for Cancer Registry Data at the RKI (Westkreuz-Druckerei, Berlin).
2010.Robert Koch Institute, Berlin, 2010.
|
|
16
|
Mashberg A, Boffetta P, Winkelman R and
Garfinkel L: Tobacco smoking, alcohol drinking and cancer of the
oral cavity and oropharynx among U.S. veterans. Cancer.
72:1369–1375. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blot WJ, McLaughlin JK, Winn DM, et al:
Smoking and drinking in relation to oral and pharyngeal cancer.
Cancer Res. 48:3282–3287. 1988.PubMed/NCBI
|
|
18
|
Hashibe M, Brennan P, Benhamou S, et al:
Alcohol drinking in never users of tobacco, cigarette smoking in
never drinkers and the risk of head and neck cancer: pooled
analysis in the international head and neck cancer epidemiology
consortium. J Natl Cancer Inst. 99:777–789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maier H, Tisch M, Conradt C and
Pötschke-Langer M: Alcohol drinking and cancer of the upper
aerodigestive tract in women. Dtsch Med Wochenschr. 124:851–854.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Petti S: Lifestyle risk factors for oral
cancer. Oral Oncol. 45:340–350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cooper JS, Porter K, Mallin K, et al:
National Cancer Database report on cancer of the head and neck:
10-year update. Head Neck. 31:748–758. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alkureishi LW, Ross GL, Shoaib T, et al:
Sentinel node biopsy in head and neck squamous cell cancer: 5-year
follow-up of a European multicenter trial. Ann Surg Oncol.
17:2459–2464. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Civantos FJ, Zitsch RP, Schuller DE,
Agrawal A, Smith RB, Nason R, Petruzelli G, Gourin CG, Wong RJ,
Ferris RL, et al: Sentinel lymph node biopsy accurately stages the
regional lymph nodes for T1-T2 oral squamous cell carcinomas:
Results of a prospective multi-institutional trial. J Clin Oncol.
28:1395–1400. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chepeha DB, Taylor RJ, Chepeha JC, Teknos
TN, Bradford CR, Sharma PK, Terrell JE and Wolf GT: Functional
assessment using constant's shoulder scale after modified radical
and selective neck dissection. Head Neck. 24:432–436. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nibu K, Ebihara Y, Ebihara M, Kawabata K,
Onitsuka T, Fujii T and Saikawa M: Quality of life after neck
dissection: A multicenter longitudinal study by the japanese
clinical study group on standardization of treatment for lymph node
metastasis of head and neck cancer. Int J Clin Oncol. 15:33–38.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Terrell JE, Ronis DL, Fowler KE, Bradford
CR, Chepeha DB, Prince ME, Teknos TN, Wolf GT and Duffy SA:
Clinical predictors of quality of life in patients with head and
neck cancer. Arch Otolaryngol Head Neck Surg. 130:401–408. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferlito A, Rinaldo A, Silver CE, Gourin
CG, Shah JP, Clayman GL, Kowalski LP, Shaha AR, Robbins KT, Suárez
C, et al: Elective and therapeutic selective neck dissection. Oral
Oncol. 42:14–25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murer K, Huber GF, Haile SR and Stoeckli
SJ: Comparison of morbidity between sentinel node biopsy and
elective neck dissection for treatment of the n0 neck in patients
with oral squamous cell carcinoma. Head Neck. 33:1260–1264. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schiefke F, Akdemir M, Weber A, Akdemir D,
Singer S and Frerich B: Function, postoperative morbidity and
quality of life after cervical sentinel node biopsy and after
selective neck dissection. Head Neck. 31:503–512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Werner JA, Dünne AA, Ramaswamy A, Dalchow
C, Behr T, Moll R, Folz BJ and Davis RK: The sentinel node concept
in head and neck cancer: Solution for the controversies in the N0
neck? Head Neck. 26:603–611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ambrosch P and Brinck U: Detection of
nodal micrometastases in head and neck cancer by serial sectioning
and immunostaining. Oncology (Williston Park). 10:1221–1226;
discussion 1226, 1229. 1996.PubMed/NCBI
|
|
32
|
Antonio JK, Santini S, Politi D, Sulfaro
S, Spaziante R, Alberti A, Pin M and Barzan L: Sentinel lymph node
biopsy in squamous cell carcinoma of the head and neck: 10 years of
experience. Acta Otorhinolaryngol Ital. 32:18–25. 2012.PubMed/NCBI
|
|
33
|
Gallegos-Hernández JF, Hernández-Hernández
DM, Flores-Díaz R, Sierra-Santiesteban I, Pichardo-Romero P,
Arias-Ceballos H, Minauro-Muñoz G and Alvarado-Cabrero I: The
number of sentinel nodes identified as prognostic factor in oral
epidermoid cancer. Oral Oncol. 41:947–952. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Atula T, Shoaib T, Ross GL, Gray HW and
Soutar DS: How many sentinel nodes should be harvested in oral
squamous cell carcinoma? Eur Arch Otorhinolaryngol. 265(Suppl 1):
S19–S23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fasunla AJ, Greene BH, Timmesfeld N,
Wiegand S, Werner JA and Sesterhenn AM: A meta-analysis of the
randomized controlled trials on elective neck dissection versus
therapeutic neck dissection in oral cavity cancers with clinically
node-negative neck. Oral Oncol. 47:320–324. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu TR, Chen FJ, Yang AK, Zhang GP, Song
M, Liu WW, Chen WC, Chen YF, Ouyang D and Li QL: Elective neck
dissection in clinical stage I squamous cell carcinoma of the
tongue: Does it improve regional control or survival time? Oral
Oncol. 47:136–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ebrahimi A, Ashford BG and Clark JR:
Improved survival with elective neck dissection in thick
early-stage oral squamous cell carcinoma. Head Neck. 34:709–716.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hyde NC, Prvulovich E, Newman L,
Waddington WA, Visvikis D and Ell P: A new approach to
pre-treatment assessment of the N0 neck in oral squamous cell
carcinoma: The role of sentinel node biopsy and positron emission
tomography. Oral Oncol. 39:350–360. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Frerich B, Förster M, Schiefke F,
Wittekind C, Hemprich A and Sabri O: Sentinel lymph node biopsy in
squamous cell carcinomas of the lips and the oral cavity-a single
center experience. J Surg Oncol. 95:97–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tschopp L, Nuyens M, Stauffer E, Krause T
and Zbären P: The value of frozen section analysis of the sentinel
lymph node in clinically N0 squamous cell carcinoma of the oral
cavity and oropharynx. Otolaryngol Head Neck Surg. 132:99–102.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shoaib T, Soutar DS, MacDonald DG,
Camilleri IG, Dunaway DJ, Gray HW, McCurrach GM, Bessent RG,
MacLeod TI and Robertson AG: The accuracy of head and neck
carcinoma sentinel lymph node biopsy in the clinically N0 neck.
Cancer. 91:2077–2083. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ionna F, Chiesa F, Longo F, Manola M,
Villano S, Calabrese L, Lastoria S and Mozzillo N: Prognostic value
of sentinel node in oral cancer. Tumori. 88(Suppl 1): S18–S19.
2002.PubMed/NCBI
|
|
43
|
Höft S, Maune S, Muhle C, Brenner W, Czech
N, Kampen WU, Jänig U, Laudien M, Gottschlich S and Ambrosch P:
Sentinel lymph-node biopsy in head and neck cancer. Br J Cancer.
91:124–128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bilde A, von Buchwald C, Therkildsen MH,
Mortensen J, Kirkegaard J, Charabi B and Specht L: Need for
intensive histopathologic analysis to determine lymph node
metastases when using sentinel node biopsy in oral cancer.
Laryngoscope. 118:408–414. 2008. View Article : Google Scholar : PubMed/NCBI
|