Introduction
Bladder transitional cell carcinoma (BTCC) is one of
the most common types of malignant tumor of the urinary system. The
treatment of BTCC is challenging, due to its high occurrence of
metastasis (1). Survival in
muscle-invasive BTCC depends mainly on the pathological stage of
the disease (2), and lymph node
metastasis is currently considered to be the most highly-dependent
variable associated with survival (3).
Evidence from previous studies suggests that
chemokines and their receptors participate in tumor metastasis
(4). CC-chemokine receptor 7 (CCR7)
is naturally a homeostatic chemokine receptor expressed by various
subtypes of immune cells that migrate to and within lymphoid organs
(5). Previous studies have
demonstrated that the overexpression of CCR7 in tumor cells is
significantly associated with lymph node metastasis in gastric,
non-small cell lung, breast and bladder cancer (6,7). Spiral
computed tomography (CT) is commonly used to evaluate lymph
metastasis in bladder cancer. However, the sensitivity and accuracy
of CT is limited (8). A previous
study by Li et al (9) reported
that the use of CT combined with vascular endothelial growth factor
C may improve the accuracy of the diagnosis of lymph node
metastasis in bladder carcinoma.
Therefore, in the present study, the potential role
of CCR7 as an independent predictor for lymph node metastasis in
BTCC was investigated. Furthermore, the sensitivity, specificity
and accuracy of CCR7 and CT for the diagnosis of lymph node
metastasis were evaluated separately and jointly. The results
indicated that CCR7 is a predictive biomarker associated with CT
for the diagnosis of lymph node metastasis in BTCC.
Materials and methods
Patients and treatment
The present study included 115 patients with BTCC
who underwent laparoscopic radical cystectomy and pelvic
lymphadenectomy in the Department of Urology of Xiangya Hospital,
Central South University (Changsha, China) between January 2010 and
December 2013. The study was approved by the ethics committee at
Xiangya Hospital, Central South University. Written informed
consent was obtained from all the participants in the study. The
patients included in the study had undergone radical surgery of the
bladder, surrounding fat tissue, remote end of the ureter and
pelvic lymphadenectomy. The cranial, lateral and caudal borders of
the pelvic lymphadenectomy were the level of the inferior
mesenteric artery, genitofemoral nerve and pelvic floor,
respectively.
The BTCC tissue specimens obtained following the
above surgical procedure were fixed in 10% formaldehyde solution
for postoperative histopathological examination. Normal urothelium
tissue specimens were obtained from ten patients with benign
prostatic hypertrophy via transurethral resection of the bladder.
The histological cell type of the BTCC specimens was determined
according to the 2004 World Health Organization (10) classification by an experienced
pathologist who did not possess any prior knowledge of the
patient's disease data. A total of 14, 42 and 59 specimens were
classified as papillary urothelial neoplasm of low malignant
potential, low-grade urothelial carcinoma and high-grade urothelial
carcinoma, respectively. Tumor staging was reviewed based on the
2009 Union for International Cancer Control-tumor node metastasis
staging system (11). Accordingly, 7,
26, 58 and 24 tumors were staged T1, T2, T3 and T4,
respectively.
Immunohistochemical staining
Immunohistochemical staining of CCR7 was performed
using a ready-to-use SP kit (Zhongshan Jinqiao Biotechnology Co.,
Ltd., Beijing, China), according to the manufacturer's protocol.
Briefly, the 4-µm paraffin-embedded tissue sections were subjected
to deparaffinization and rehydration prior to be immersed in 0.01 M
citric buffer (pH 6.0). The slides were then incubated with 3%
hydrogen peroxide for 10 min, followed by incubation in normal goat
serum for 10 min at room temperature. Anti-CCR7 rabbit monoclonal
immunoglobulin (Ig)G antibody (catalogue no. ab32527; Abcam,
Cambridge, MA, USA) was used at a dilution of 1:200 as primary
antibody. Next, the sections were incubated with goat anti-rabbit
polyclonal antibody for 10 min, and subsequently exposed to
streptavidin peroxidase (Zhongshan Jianqaio Biotechnology) for 10
min. The slides were visualized with 3,3′-diaminobenzidine
(Zhongshan Jianqaio Biotechnology), counterstained with hematoxylin
(Auragene, Changsha, China), prior to be examined by microscopy
(Olympus BH2; Olympus Corporation, Tokyo, Japan). For the negative
controls, the primary antibody was substituted for
phosphate-buffered saline.
Under a low-power microscopy field, each slide was
evaluated in ten randomly selected areas that contained tumor
cells, ensuring that ≥100 tumor cells/field were examined. The
evaluation was conducted by two independent investigators, who were
unaware of the clinicopathological data. Two scoring systems were
used, including staining intensity and percentage of positive
cells. The staining intensity was scored on a semiquantitative
4-points scale as follows: i) A score of 0 was assigned to the
negative control; ii) a score of 1 indicated weak cytoplasmic and
nuclear staining, which was slightly darker than the negative
control; iii) a score of 2 was indicative of moderate staining,
defined as an intensity exhibiting a score of 1–3; and iv) 3
corresponded to strong staining, which was equivalent to or darker
than the positive control. The percentage of stained cells was also
scored on a semiquantitative 4-points scale as follows: i) 0
indicated <10% positive cells; ii) 1 was assigned if the
percentage of positive cells in the sample was 10–25%; iii) 2
corresponded to 25–50% positive cells present in the sample; and
iv) 3 was assigned to those samples displaying >50% positive
cells. The staining intensity and percentage of stained cells
scores were then combined as follows: i) Those specimens presenting
a total score of 0–1 were assigned a value of I; ii) II was
assigned to those specimens with a total score of 2; iii) III
indicated a total score of 3–4; and iv) IV was assigned to samples
exhibiting a total score of 5–6.
CT evaluation
All patients included in the study had undergone CT
scanning of the abdomen and pelvis ≥5 days prior to cystectomy and
pelvic lymphadenectomy, which had been conducted on SOMATOM
Sensation 64 eco (Siemens AG, Munich, Germany), with a slice
thickness and layer of 5 mm. As contrast agent, 80 ml Ultravist®
(Bayer AG, Leverkusen, Germany) was used. The CT images were
reevaluated by urologists and uroradiologists who were unaware of
the final pathological results. A pelvic lymph node >10 mm in
size was considered as positive lymph node metastasis.
Statistical analysis
Statistical analyses were performed using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). The
association between the variables was assessed by χ2
test, Fisher's exact probability test, Mann-Whitney U test or
stepwise logistic regression analysis. Logistic regression analysis
was employed for univariate analysis, while multiple logistic
regressions were used for multivariate analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
CCR7 levels in patients with BTCC and
normal controls
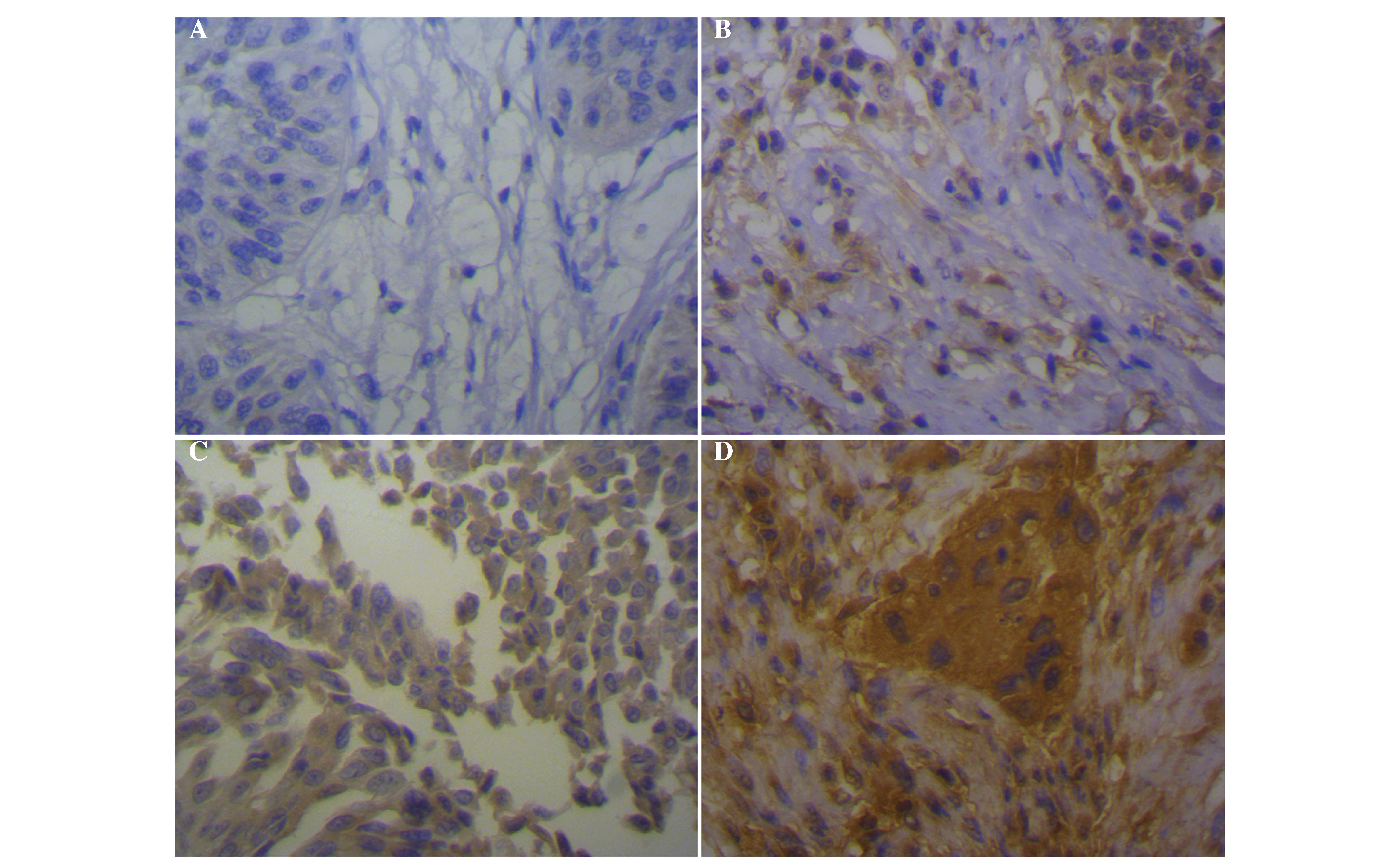
Expression of CCR7 was detected in the cytoplasm and
membrane of tumor cells, but low or no staining for CCR7 was
observed in the normal transitional epithelium (Fig. 1). The frequency of detectable CCR7 in
the BTCC samples was markedly higher than in the normal bladder
tissues (P<0.01; Table I).
 | Table I.Expression levels (score I–IV) of
CC-chemokine receptor 7 in patients with bladder transitional cell
carcinoma and normal controls. |
Table I.
Expression levels (score I–IV) of
CC-chemokine receptor 7 in patients with bladder transitional cell
carcinoma and normal controls.
|
| CC-chemokine receptor
7 (no.) |
|
|---|
|
|
|
|
|---|
| Tissues (no. of
patients) | I | II | III | IV | P-value |
|---|
| Bladder transitional
cell carcinoma (115) | 16 | 29 | 48 | 22 | <0.01 |
| Normal (10) | 8 | 2 | 0 | 0 | – |
Association of CCR7 with lymph node
metastasis in BTCC
In the positive lymph node metastasis group, 0, 5,
14 and 23 samples displayed expression of CCR7 of score I, II, III
and IV, respectively. By contrast, in the negative lymph node
metastasis group, the number of cases displaying expression levels
of CCR7 of score I, II, III and IV were 15, 36, 22 and 0,
respectively. These results indicated that the overexpression of
CCR7 was significantly associated with pelvic lymph node metastasis
(P<0.05; Table II).
 | Table II.Association between the expression
levels (score I–IV) of CC-chemokine receptor 7 and lymph node
metastasis in bladder transitional cell carcinoma. |
Table II.
Association between the expression
levels (score I–IV) of CC-chemokine receptor 7 and lymph node
metastasis in bladder transitional cell carcinoma.
|
| CC-chemokine receptor
7 (no.) |
|
|---|
|
|
|
|
|---|
| Lymph node metastasis
(no. of patients) | I | II | III | IV | P-value |
|---|
| Positive (42) | 0 | 5 | 14 | 23 | <0.05 |
| Negative (73) | 15 | 36 | 22 | 0 | – |
Association of BTCC lymph node
metastasis with clinicopathological features
BTCC pelvic lymph node metastasis was closely
associated with certain clinicopathological features of the
patients, including tumor size, pathological grade, clinical stage,
recurrence and expression levels of CCR7. Among these,
overexpression of CCR7 was identified as an independent indicator
for lymph node metastasis in multivariate analysis, based on the
results of stepwise logistic regression analysis (Table III).
 | Table III.Univariate and multivariate analyses
for bladder transitional cell carcinoma lymph node metastasis. |
Table III.
Univariate and multivariate analyses
for bladder transitional cell carcinoma lymph node metastasis.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | Comparison | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Age (years) | <60 or ≥60 | – | >0.05 | – | – |
| Tumor size (cm) | <3 or ≥3 | – | >0.05 | – | – |
| Pathological grade
(WHO 2004) | PUNLMP-LGPUC or
HGPUC | 0.409
(0.061–0.813) | <0.05 | – | >0.05 |
| Clinical stage
(UICC-TNM) | T1-T2 or T3-T4 | 0.233
(0.042–0.671) | <0.01 | – | >0.05 |
| Detection (time) | First or
recurrence | – | >0.05 | – | – |
| CCR7 expression
levels (score) | I–II or III–IV | 3.860
(1.710–7.590) | <0.01 | 2.930
(1.650–6.710) | <0.05 |
Separate and joint evaluation of CCR7
and CT scan for the detection of BTCC lymph node metastasis
Based on the expression levels of CCR7, the samples
were classified into two groups, according to their score levels.
Thus, CCR7 scores of I and II were included in the low expression
or negative group, whereas CCR7 scores of III and IV corresponded
to the high expression or positive group. Among the 115 cases with
BTCC analyzed, 37 displayed CCR7 scores of III–IV and lymph node
metastasis; 22 displayed CCR7 scores of III–IV but no lymph node
metastasis; 51 exhibited CCR7 scores of I–II but no lymph node
metastasis; and 5 presented CCR7 scores of I–II in addition to
lymph node metastasis. The sensitivity, specificity and accuracy of
CCR7 in the evaluation of lymph node metastasis were 88.1, 69.9 and
76.5%, respectively.
CT examination revealed 37 patients with lymph node
metastasis, including 29 in stage T3 and 8 in stage T4. The
positive rate was 32.2% (37/115). Positive CT and presence of lymph
node metastasis were observed in 22 patients, while 15 patients
presented positive CT but negative lymph node metastasis, 20
displayed negative CT but positive lymph node metastasis, and 58
exhibited negative CT and absence of lymph node metastasis. The
sensitivity, specificity and accuracy of CT in the evaluation of
lymph node metastasis were 52.4, 79.5 and 69.6%, respectively.
When CCR7 was combined with CT scan in the detection
of lymph node metastasis, the sensitivity, specificity and accuracy
were 92.3% (39/42 cases), 83.6% (61/73 cases) and 87.0% (100/115
cases), respectively (Table IV).
 | Table IV.Sensitivity, specificity and accuracy
of CCR7 alone, CT alone and CCR7 combined with CT for the diagnosis
of bladder transitional cell carcinoma lymph node metastasis. |
Table IV.
Sensitivity, specificity and accuracy
of CCR7 alone, CT alone and CCR7 combined with CT for the diagnosis
of bladder transitional cell carcinoma lymph node metastasis.
|
| Lymph node metastasis
(no. of patients) |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Predictor | Positive | Negative | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|
| CCR7 |
|
| 88.1 | 69.9 | 76.5 |
|
Positive | 37 | 22 |
|
|
|
|
Negative | 5 | 51 |
|
|
|
| CT |
|
| 52.4 | 79.5 | 69.6 |
|
Positive | 22 | 15 |
|
|
|
|
Negative | 20 | 58 |
|
|
|
| CCR7 plus CT |
|
| 92.3 | 83.6 | 87.0 |
|
Positive | 39 | 12 |
|
|
|
|
Negative | 3 | 61 |
|
|
|
Discussion
Despite remarkable advances in the treatment of
bladder cancer, the morbidity and mortality rates for this disease
remain high. In USA, the estimated incidence and number of
mortalities associated with bladder cancer in 2012 were 73,510 and
14,880 cases, respectively (12).
Thus, the identification of the risks behind disease recurrence and
mortality in bladder cancer is critical for adequate surveillance
and selection of adjuvant therapies (13,14).
Numerous studies have previously investigated potential prognostic
factors for patients with bladder cancer, in order to guide
therapeutic approaches and improve survival outcomes (15). Lymph node metastasis was previously
identified as an important prognostic factor in patients with BTCC,
since the survival rate of patients with lymph node metastasis of
bladder cancer is usually low (16).
Chemokines are small secreted proteins that may
contribute to the regulation of leukocyte adherence to endothelial
cells, leukocyte migration through transendothelial membranes and
tissue invasion (17). Previous
studies revealed that the binding of chemokines to their specific
receptors led to the activation of numerous signaling pathways
involved in the growth and metastasis of tumor cells (18). CCR7, which was formerly considered to
be an orphan receptor, has recently been identified as a chemokine
receptor for chemokine (C-C motif) ligand 12, which is expressed in
several human cell lines and vascular endothelial cells (19). The expression of CCR7 on tumor cells
has mainly been reported for breast, non-small cell lung, prostate,
head and neck, stomach and colorectal cancer, in addition to
melanoma, chronic lymphocytic and T cell leukemia and non-Hodgkin's
lymphoma (20). Yates et al
(21) demonstrated that the
expression of CCR7 in bladder carcinoma cells regulated processes
such as proliferation, chemotactic motility and formation of
signaling complexes in these cells and the expression levels of
CCR7 were observed to be upregulated in bladder tumor tissues and
exfoliated urothelial cells present in the urine of patients with
bladder carcinoma, which was associated with prognosis
According to previous studies, the overexpression of
CCR7 appears to be implicated in lymph node metastasis in patients
with esophageal, pancreatic, gastric and breast cancer, and
demonstrated that the overexpression of CCR7 was an independent
factor of lymph node metastasis in these patients (22–24). In
the present study, CCR7 was observed to be highly expressed in BTCC
tissues, compared with normal bladder tissues. In addition, the
frequency of detectable CCR7 expression was observed to be
significantly higher in patients with pelvic lymph node metastasis,
which is consistent with previous data reported in the literature
(21). Furthermore, the logistic
regression analysis conducted in the present study identified the
overexpression of CCR7 as an independent factor influencing pelvic
lymph node metastasis in BTCC.
CT is commonly used to evaluate bladder cancer and
provide information on lymph node metastasis. However the
sensitivity and accuracy of diagnosing lymph node metastasis
remains poor (8). In previous
studies, Ficarra et al (25)
diagnosed pathological lymph node involvement in 45 patients, but
CT only identified 19 of these patients. CT appears to be limited
to the detection of metastatic lymph nodes of ≥0.5 cm in size. In
the present study, postoperative pathological examination results
were used as standards, and the sensitivity, specificity and
accuracy of CT for the evaluation of lymph node metastasis were
calculated to be 52.4, 79.5 and 69.6%, respectively, which is
somewhat in agreement with previous studies. These results confirm
the limitations of CT for detecting lymph node metastasis in
bladder carcinoma. Thus, a more sensitive method to detect lymph
node metastasis at early stage is required.
In the present study, the diagnosis of lymph node
metastasis using CCR7 identified 42 cases that were positive for
lymph node metastasis, 37 of which were confirmed to be correct. In
56 cases that appeared to be absent of lymph node metastasis based
on their expression levels of CCR7, 5 presented lymph nodes
metastasis, according to pathological diagnosis. The sensitivity,
specificity and accuracy of CCR7 in the evaluation of lymph node
metastasis were observed to be 88.1, 69.9 and 76.5%, respectively.
Thus, the sensitivity of CCR7 appeared to be higher than that of CT
(88.1 vs. 52.4%, respectively), but its specificity was lower (69.9
vs. 79.5%). Therefore, the expression levels of CCR7 were combined
with the results of CT scan, and the combined sensitivity increased
to 92.3% (39/42 cases), whereas the combined specificity and
accuracy were determined to be 83.6% (61/73 cases) and 87.0%
(100/115 cases, respectively). Thus, a combination of CT and CCR7
detection may improve the sensitivity of the diagnosis of lymph
node metastasis in BTCC.
In conclusion, the results of the present study
suggest the potential clinical application of CCR7 as a predictive
biomarker for diagnosing lymph node metastasis in BTCC.
Furthermore, the combined evaluation of CCR7 and CT appears to be a
more reliable marker for lymph node metastasis in BTCC, compared to
the diagnosis by CT or CCR7 alone. These results require further
confirmation by large sample and multi-center prospective
studies.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China) (grant no.
30700832) and the Fundamental Research Funds for the Central
Universities of Central South University (Changsha, China) (grant
no. 72150050586).
Glossary
Abbreviations
Abbreviations:
|
CCR7
|
CC-chemokine receptor 7
|
|
CT
|
computed tomography
|
|
BTCC
|
bladder transitional cell
carcinoma
|
|
PUNLMP
|
papillary urothelial neoplasm of low
malignant potential
|
|
LGPUC
|
low-grade urothelial carcinoma
|
|
HGPUC
|
high-grade urothelial carcinoma
|
References
|
1
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stein JP, Lieskovsky G, Cote R, Groshen S,
Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M,
et al: Radical cystectomy in the treatment of bladder cancer:
Long-term results in 1,054 patients. J Clin Oncol. 19:666–675.
2001.PubMed/NCBI
|
|
3
|
Herr HW: Superiority of ratio based lymph
node staging for bladder cancer. J Urol. 169:943–945. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sarvaiya PJ, Guo D, Ulasov I, Gabikian P
and Lesniak MS: Chemokines in tumor progression and metastasis.
Oncotarget. 4:2171–2185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Comerford I, Harata-Lee Y, Bunting MD,
Gregor C, Kara EE and McColl SR: A myriad of functions and complex
regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive
immune system. Cytokine Growth Factor Rev. 24:269–283. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cassier PA, Treilleux I, Bachelot T,
Ray-Coquard I, Bendriss-Vermare N, Ménétrier-Caux C, Trédan O,
Goddard-Léon S, Pin JJ, Mignotte H, et al: Prognostic value of the
expression of C-Chemokine receptor 6 and 7 and their ligands in
non-metastatic breast cancer. BMC Cancer. 11:2132011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cunningham HD, Shannon LA, Calloway PA,
Fassold BC, Dunwiddie I, Vielhauer G, Zhang M and Vines CM:
Expression of the C-C chemokine receptor 7 mediates metastasis of
breast cancer to the lymph nodes in mice. Transl Oncol. 3:354–361.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baltaci S, Resorlu B, Yagci C, Turkolmez
K, Gogus C and Beduk Y: Computerized tomography for detecting
perivesical infiltration and lymph node metastasis in invasive
bladder carcinoma. Urol Int. 81:399–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Qi F, Miao J, Zu X, He W, Wang L and
Qi L: Vascular endothelial growth factor-C associated with computed
tomography used in the diagnosis of lymph node metastasis of
bladder carcinoma. Arch Med Res. 41:606–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Montironi R and Lopez-Beltran A: The 2004
WHO classification of bladder tumors: A summary and commentary. Int
J Surg Pathol. 13:143–153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng L, Montironi R, Davidson DD and
Lopez-Beltran A: Staging and reporting of urothelial carcinoma of
the urinary bladder. Mod Pathol. 22:S70–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim H, Kim M, Kwak C, Kim HH and Ku JH:
Prognostic significance of lymphovascular invasion in radical
cystectomy on patients with bladder cancer: A systematic review and
meta-analysis. PLoS One. 9:e892592014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zaravinos A, Volanis D, Lambrou GI,
Delakas D and Spandidos DA: Role of the angiogenic components,
VEGFA, FGF2, OPN and RHOC, in urothelial cell carcinoma of the
urinary bladder. Oncol Rep. 28:1159–1166. 2012.PubMed/NCBI
|
|
15
|
Kamat AM, Vlahou A, Taylor JA, Hudson ML,
Pesch B, Ingersoll MA, Todenhöfer T, van Rhijn B, Kassouf W, Barton
Grossman H, et al: Considerations on the use of urine markers in
the management of patients with high-grade non-muscle-invasive
bladder cancer. Urol Oncol. 32:1069–1077. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Türkölmez K, Tokgöz H, Reşorlu B, Köse K
and Bedük Y: Muscle-invasive bladder cancer: Predictive factors and
prognostic difference between primary and progressive tumors.
Urology. 70:477–481. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kakinuma T and Hwang ST: Chemokines,
chemokine receptors, and cancer metastasis. J Leukoc Biol.
79:639–651. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Homey B, Müller A and Zlotnik A:
Chemokines: Agents for the immunotherapy of cancer? Nat Rev
Immunol. 2:175–184. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hao M, Zheng J, Hou K and Wang J, Chen X,
Lu X, Bo J, Xu C, Shen K and Wang J: Role of chemokine receptor
CXCR7 in bladder cancer progression. Biochem Pharmacol. 84:204–214.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zlotnik A, Burkhardt AM and Homey B:
Homeostatic chemokine receptors and organ-specific metastasis. Nat
Rev Immunol. 11:597–606. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yates TJ, Knapp J, Gosalbez M, Lokeshwar
SD, Gomez CS, Benitez A, Ekwenna OO, Young EE, Manoharan M and
Lokeshwar VB: C-X-C chemokine receptor 7: A functionally associated
molecular marker for bladder cancer. Cancer. 119:61–71. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Irino T, Takeuchi H, Matsuda S, Saikawa Y,
Kawakubo H, Wada N, Takahashi T, Nakamura R, Fukuda K, Omori T and
Kitagawa Y: CC-Chemokine receptor CCR7: A key molecule for lymph
node metastasis in esophageal squamous cell carcinoma. BMC Cancer.
14:2912014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee HJ, Song IC, Yun HJ, Jo DY and Kim S:
CXC chemokines and chemokine receptors in gastric cancer: From
basic findings towards therapeutic targeting. World J
Gastroenterol. 20:1681–1693. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo J, Lou W, Ji Y and Zhang S: Effect of
CCR7, CXCR4 and VEGF-C on the lymph node metastasis of human
pancreatic ductal adenocarcinoma. Oncol Lett. 5:1572–1578.
2013.PubMed/NCBI
|
|
25
|
Ficarra V, Dalpiaz O, Alrabi N, Novara G,
Galfano A and Artibani W: Correlation between clinical and
pathological staging in a series of radical cystectomies for
bladder carcinoma. BJU Int. 95:786–790. 2005. View Article : Google Scholar : PubMed/NCBI
|