Introduction
Dermatofibrosarcoma protuberans (DFSP) is a
superficial, low-grade, locally aggressive, spindle, fibroblastic,
neoplastic lesion. As a relatively uncommon neoplasm and locally
aggressive cutaneous tumor, it is characterized by high rates of
local recurrence, but a low risk of metastasis (1–4). DFSP
typically presents with a purple or pink asymptomatic plaque or
nodule, with a history of slow but persistent growth (1). DFSPs usually affect young to middle-aged
adult patients (1–4). The tumor was first described in 1924 by
Darier and Ferrand as a ‘progressive and recurring dermatofibroma’,
which is a nodular cutaneous tumor characterized by a prominent
storiform pattern (5). In addition to
the classical form characterized by a storiform pattern of tumor
cells, the pigmented (Bednar's tumor), plaque-like and myxoid
types, and DFSP with sarcomatous areas can be observed (1–4). Classical
DFSP and Bednar's tumors are readily diagnosed, however, the myxoid
type represents a challenge diagnostically. Immunoreactivity for
cluster of differentiation (CD)34 in a spindle cell tumor is the
main immunohistochemical marker for the diagnosis of DFSP (1–4,6,7). Although
DFSP has been regarded as a low-grade sarcoma, certain cases have
sarcomatous transformation, which is characterized by hypercellular
spindle cell fascicles with increased atypia, mitosis tumor
necrosis and usually, the loss of CD34 immunoreactivity (1,3,4).
The objective of the present study was to analyze
the correlation between clinical and pathological factors,
including age, gender, tumor size, anatomical location, mitotic
counts, surgical margin, recurrence and high-grade sarcomatous
transformation, in a large series of patients with DFSP from a
single center.
Materials and methods
Patients and criteria
A retrospective study was performed on DFSP cases
diagnosed by surgical specimens from wide excisional biopsies
obtained from the Department of Pathology, Faculty of Medicine
Ramathibodi Hospital (Mahidol University, Bangkok, Thailand) over a
period of 20 years, between 1994 and 2013. The histopathological
diagnosis of DFSP was reviewed. The criteria for the diagnosis of
DFSP were: i) The presence of spindle cells irregularly organized
in linked fascicles with a storiform arrangement, and
histopathological features compatible with DFSP; and ii) positive
CD34 and vimentin immunohistochemical staining in spindle tumor
cells. Information obtained from the medical records, including
patients age at first diagnosis, gender, smoking history, tumor
size, surgical margin of tumor, anatomical location, first
pathological diagnosis, mitotic counts, modality of treatment,
surgical margin, recurrence and treatment outcomes, were collected
and analyzed. Patients were grouped based on the sarcomatous
transformation or recurrence of the tumor.
Statistical analysis
A comparison between the clinicopathological
features was evaluated using the chi-square test. Univariate
log-rank analysis was performed. Two-tailed Fisher's exact test was
used to evaluate statistical significance between the groups.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using SPSS
software, version 13.0 (SPSS Inc, Chicago, IL, USA).
Study approval
This study was approved by the Committee on Human
Rights related to research involving human subjects of Faculty of
Medicine Ramathibodi Hospital, Mahidol University (ID
11-54-33).
Results
Patient and tumor characteristics, and
treatment modalities
In total, 68 cases consisting of 32 male and 36
female patients, with an age range of 3–86 years old, and a mean
and median age of 40 and 39 years, respectively, met the inclusion
criteria. All patients presented with primary disease without
evidence of metastasis. The characteristics of the DFSP patients
are summarized in Table I. The
anatomical locations of the lesions were as follows: Head and neck,
19 cases; upper extremities, 9 cases; lower extremities, 18 cases;
body, 20 cases; and genitalia, 2 cases. The tumor sizes ranged from
0.2–10 cm (mean, 3.2±2.3 cm; median, 2.5 cm). The most common first
pathological diagnosis was DFSP (53 patients; 77.9%), while a
spindle cell tumor was the first diagnosis in 4 patients (5.9%).
Therefore, the overall sensitivity for diagnosis was 83.8%. The
false-negative initial diagnoses included dermatofibroma (5 cases),
neurofibroma (4 cases), fibromatosis and schwannoma (1 case each).
There were 62 cases of DFSP without sarcomatous transformation and
6 cases of sarcomatous transformation in DFSP. A total of 62
patients underwent a wide excision only, 4 patients underwent a
wide excision with radiotherapy (4500–7000 cGy), 1 patient
underwent a wide excision with radiotherapy followed by
chemotherapy (Adriamycin for undifferentiated sarcoma arising in
DFSP) and 1 patient underwent a wide excision with imatinib
targeted therapy (800 mg daily for 4 months). Of the 6 cases with
sarcomatous transformation, 4 underwent a wide excision only. The
study also noted 1 patient who developed distant pulmonary
metastasis.
 | Table I.Clinicopathological characteristics of
DFSP patients. |
Table I.
Clinicopathological characteristics of
DFSP patients.
| Characteristics | Cases, n | % |
|---|
| Gender |
|
|
| Male | 32 | 47.1 |
|
Female | 36 | 52.9 |
| Smoking (n=48) |
|
|
| Yes | 5 | 10.4 |
| No | 43 | 89.6 |
| Anatomical
location |
|
|
| Head and
neck | 19 | 27.9 |
| Upper
extremities | 9 | 13.2 |
| Lower
extremities | 18 | 26.5 |
| Body | 20 | 29.4 |
|
Genitalia | 2 | 2.9 |
| First pathological
diagnosis |
|
|
| DFSP | 53 | 77.9 |
|
Dermatofibroma | 5 | 7.4 |
|
Neurofibroma | 4 | 5.9 |
| Spindle
cell tumor | 4 | 5.9 |
|
Schwannoma | 1 | 1.5 |
|
Fibromatosis | 1 | 1.5 |
| Recurrence |
|
|
| Yes | 26 | 38.2 |
| No | 42 | 61.8 |
| Number of
surgeries |
|
|
| 1 | 50 | 73.5 |
| 2 | 12 | 17.6 |
| 3 | 3 | 4.4 |
| ≥4 | 3 | 4.4 |
| Sarcomatous
transformation |
|
|
| Yes | 6 | 8.8 |
| No | 62 | 91.2 |
| Modality of
treatment |
|
|
| Wide
excision only | 62 | 91.1 |
| Wide
excision with radiotherapy | 4 | 5.9 |
| Wide
excision with radiotherapy and chemotherapy | 1 | 1.5 |
| Wide
excision with imatinib targeted therapy | 1 | 1.5 |
| Surgical margin
(n=45) |
|
|
| Free (≥1
cm) | 11 | 24.4 |
| Not free
(<1 cm) | 34 | 75.6 |
Sarcomatous transformation
Of the 68 patients with DFSP, 6 developed
sarcomatous transformation. The characteristics of sarcomatous
transformation in the DFSP patients are summarized in Table II. Case 1 underwent surgery 19 times,
with 18 surgeries for recurrence at the head and neck region since
the time of first diagnosis at the age of 41 years. The surgical
resected margin was not disease-free and the tumor invaded the
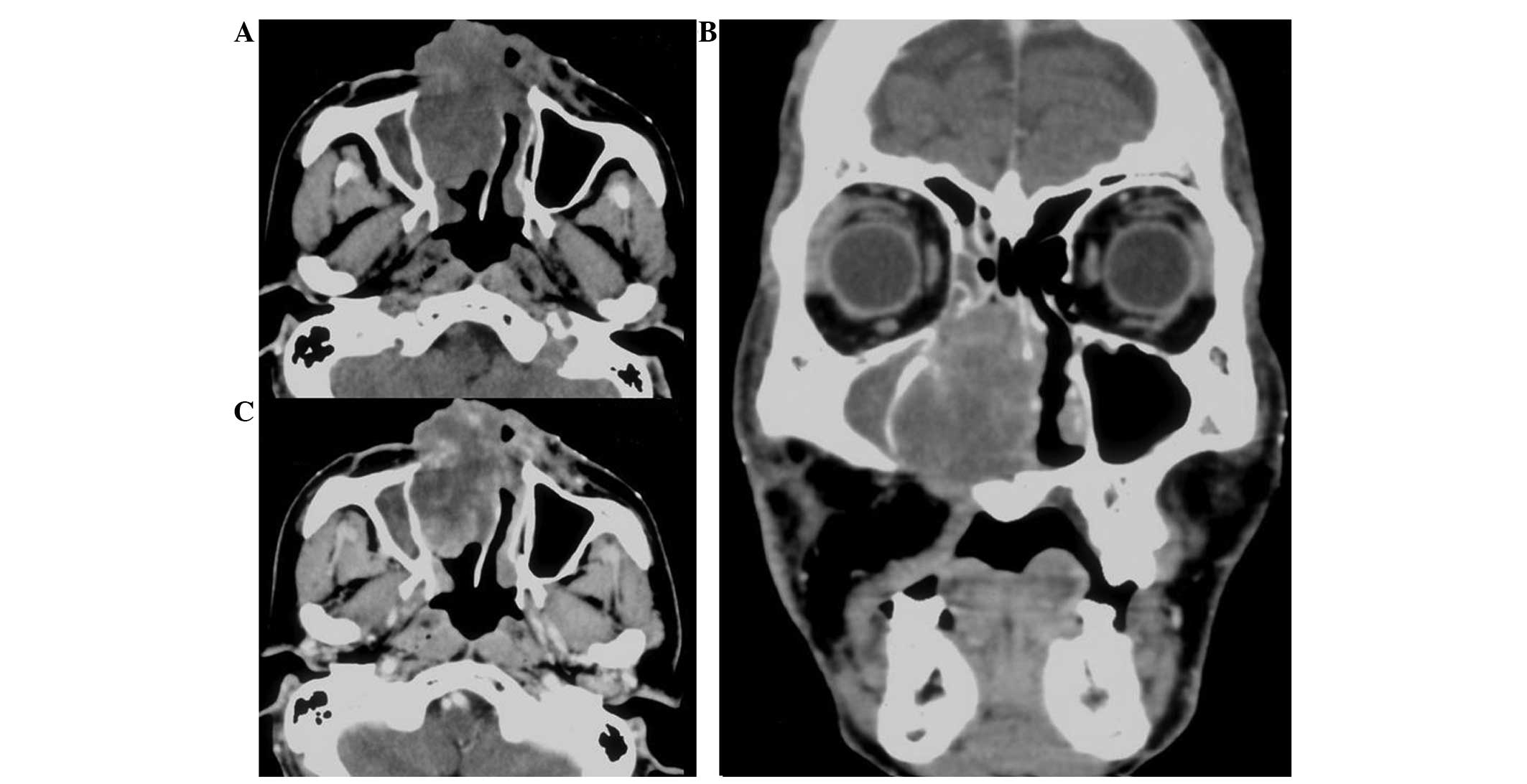
periosteum, pericranium and bony structures (Fig. 1). The tumor showed a classical area of
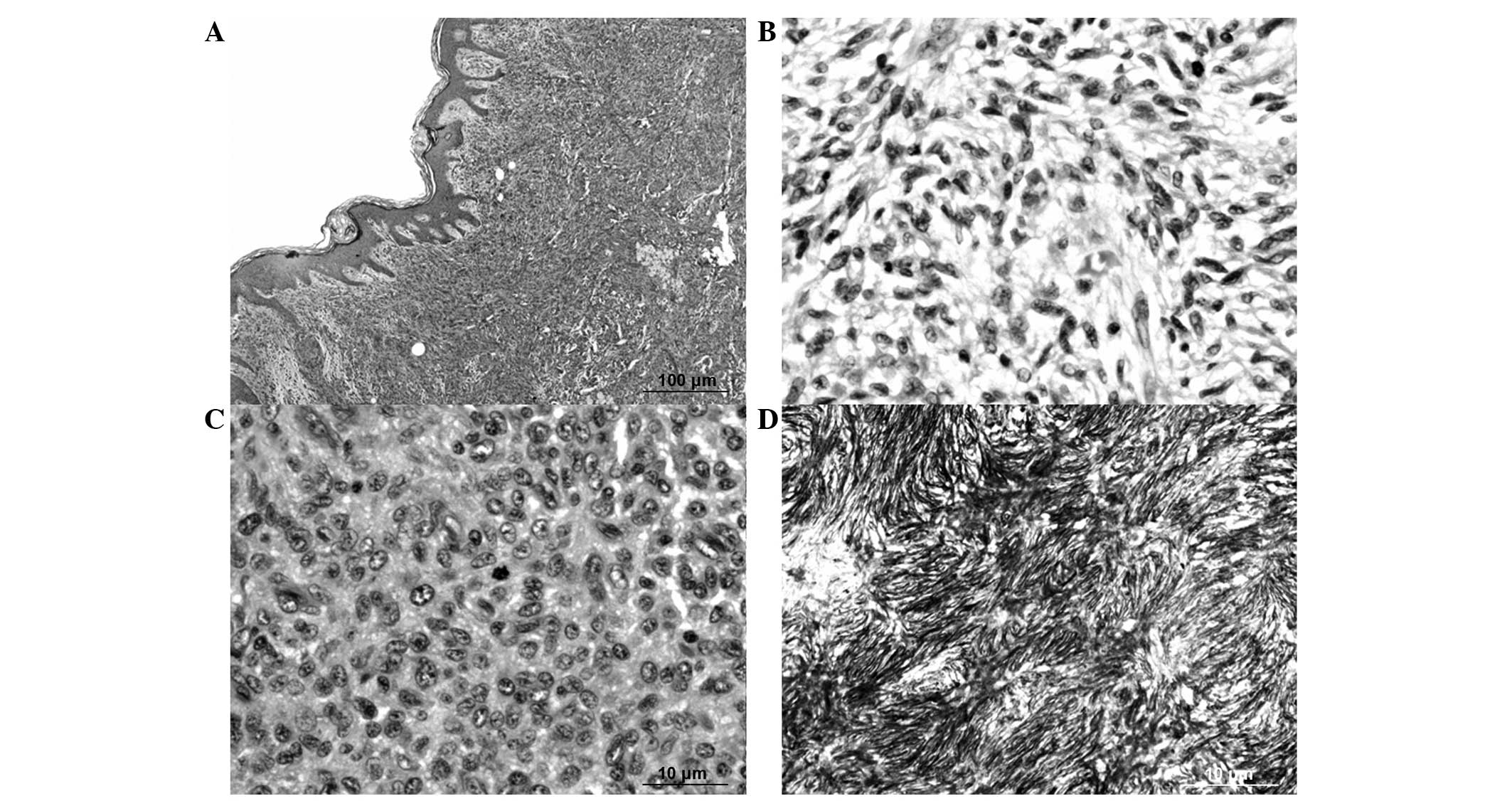
spindle tumor cells arranged in a storiform pattern, with focal
active mitoses [20/10 high-power fields (HPF)] (Fig. 2). The patient developed secondary
transformation of malignant fibrous histiocytoma (MFH) after
receiving 18 surgical resections. The patient subsequently
developed pulmonary metastasis and finally succumbed to the disease
20 years after the initial diagnosis of DFSP and 17 months after
the diagnosis of MFH. No autopsy was performed.
 | Table II.Summary of patients with sarcomatous
transformation in DFSP. |
Table II.
Summary of patients with sarcomatous
transformation in DFSP.
| Case no. | Gender | Age, years | Tumor size, cm | Margin, cm | Location | Mitosis,/10HPF | First pathological
diagnosis | Type of sarcomatous
transformation | No. of surgeries |
|---|
| 1 | Male | 41 |
7.5 | 0 | Head and neck | 20 | Neurofibroma | MFH | 19 |
| 2 | Male | 46 |
1.5 | 1 | Lower extremity | 11 | Neurofibroma | Fibrosarcoma | 2 |
| 3 | Male | 37 |
7.5 | <1 | Lower extremity | 10 | Dermatofibroma | Undifferentiated
sarcoma | 5 |
| 4 | Female | 86 |
3.2 |
0.1 | Lower extremity | 5 | DFSP | MFH | 2 |
| 5 | Female | 33 | 7 |
0.1 | Upper extremity | 5 | DFSP | Fibrosarcoma | 4 |
| 6 | Male | 39 |
6.5 | 2 | Body | 10 | DFSP | Fibrosarcoma | 2 |
Case 2 experienced tumor recurrence at the lower
extremity, although the surgical margin used was 1 cm. Mitotic
activity was 11/10 HPF. Case 3 experienced undifferentiated
sarcomatous transformation of the tumor at the lower extremity. The
mitotic rate was 10/10 HPF. The patient underwent a below-knee
amputation and received 6500 cGy radiotherapy followed by adjuvant
chemotherapy with 4 cycles of adriamycin (75 mg/m2).
Case 4 was the oldest patient in this study, who developed MFH
transformation in DFSP. The patient received radiotherapy with a
total dose of 4,500 cGy. Case 5 was the youngest patient (33 years)
in the group of patients with sarcomatous transformation in DFSP.
The last case, case 6, was a patient with fibrosarcomatous
transformation in DFSP, which presented with a rapidly growing mass
on the right chest wall. The remaining 4 patients (cases 1, 2, 5
and 6) received only surgical wide excision.
The univariate log-rank analysis identified a large
tumor size and an incorrect first pathological diagnosis as
significant parameters associated with sarcomatous transformation
in DFSP. The mean size of the tumor with DFSP and sarcomatous
transformation was 5.53 cm (range, 1.5–7.5 cm), which was
significantly larger than the mean size of the tumor in the DFSP
patients without sarcomatous transformation (2.97 cm; range, 0.2–10
cm) (P=0.008). The false-negative first pathological diagnosis of
DFSP also showed significant correlation with sarcomatous
transformation in DFSP (P=0.049). As shown in Table III, no statistically significant
correlation was found between sarcomatous transformation and age,
gender, anatomical location, histopathological subtype, recurrence
and surgical margin (at 1 cm).
 | Table III.Correlation between
clinicopathological features and sarcomatous transformation in
DFSP. |
Table III.
Correlation between
clinicopathological features and sarcomatous transformation in
DFSP.
|
Characteristics | DFSP (n=62) | DFSP with
sarcomatous transformation (n=6) | P-value |
|---|
| Age,
yearsa | 39.27±15.64
(3–70) |
47.00±19.59 (33–86) | 0.262 |
| Gender, n |
|
| 0.410 |
|
Male | 28 | 4 |
|
|
Female | 34 | 2 |
|
| Tumor size,
cma |
2.97±2.17 (0.2–10) | 5.53±2.55
(1.5–7.5) | 0.008 |
| Anatomical
location, n |
|
| 0.682 |
| Head
and neck | 18 | 1 |
|
| Upper
extremity | 8 | 1 |
|
| Lower
extremity | 15 | 3 |
|
|
Body | 19 | 1 |
|
|
Genitalia | 2 | 0 |
|
| Histopathological
subtype, n |
|
| 0.075 |
|
Conventional | 52 | 4 |
|
|
Myxoid | 3 | 2 |
|
|
Plaque-like | 3 | 0 |
|
|
Bednar | 4 | 0 |
|
| First pathological
diagnosis, n |
|
| 0.049 |
|
True | 54 | 3 |
|
|
False | 8 | 3 |
|
| Recurrence, n |
|
| 0.145 |
| No | 40 | 2 |
|
|
Yes | 22 | 4 |
|
| Surgical margin
(n=45) |
|
| 0.150 |
| Free
(≥1 cm) | 9 | 2 |
|
| Not
free (<1 cm) | 30 | 4 |
Outcome and recurrence
The patients underwent treatment as described. In
total, 26 cases experienced recurrence. The head and neck region
(11 cases) was the most common anatomical location for tumor
recurrence. The body and extremities exhibited lower risks of
recurrence. As shown in Table IV, no
statistically significant correlation was found between recurrent
DFSP and age, gender, tumor size, anatomical location,
histopathological subtype and sarcomatous transformation.
Conventional surgery was adopted in limited sites where a wide
excision would have been difficult to perform, including the head
and neck region, and genitalia (n=21), which showed a significant
association with recurrent DFSP (P=0.028). Furthermore, the
incorrect first pathological diagnosis remained a highly
significant prognostic factor of the sarcomatous transformation and
recurrence of DFSP (P=0.049 and P<0.001, respectively).
 | Table IV.Correlation between
clinicopathological features and recurrence of DFSP. |
Table IV.
Correlation between
clinicopathological features and recurrence of DFSP.
|
Characteristics | DFSP without
recurrence (n=42) | DFSP with
recurrence (n=26) | P-value |
|---|
| Age,
yearsa |
39.07±16.86 (3–86) |
41.38±14.73 (4–70) |
0.566 |
| Gender, n |
|
|
0.129 |
|
Male | 17 | 15 |
|
|
Female | 25 | 11 |
|
| Tumor size,
cma |
2.82±2.26 (0.2–10) |
3.81±2.28 (0.6–9) |
0.086 |
| Anatomical
location, n |
|
|
0.061 |
| Head
and neck | 8 | 11 |
|
| Upper
extremity | 7 | 2 |
|
| Lower
extremity | 12 | 6 |
|
|
Body | 15 | 5 |
|
| Genital
organ | 0 | 2 |
|
| Histopathological
subtype, n |
|
|
0.119 |
|
Conventional | 35 | 21 |
|
|
Myxoid | 1 | 4 |
|
|
Plaque-like | 3 | 0 |
|
|
Bednar | 3 | 1 |
|
| First pathological
diagnosis, n |
|
|
<0.001 |
|
True | 42 | 15 |
|
|
False | 0 | 11 |
|
| Sarcomatous
transformation, n |
|
|
0.193 |
| No | 40 | 22 |
|
|
Yes | 2 | 4 |
|
| Surgical margin
(n=45) |
|
|
0.042 |
| Free
(≥1 cm) | 8 | 3 |
|
| Not
free (<1 cm) | 16 | 18 |
|
Discussion
In the current World Health Organization
classification, DFSP is defined as a superficial, low-grade,
locally aggressive fibroblastic neoplasm (1,2). The
present study retrospectively collected data on DFSP that was
uniformly diagnosed based on the expression of CD34 and vimentin by
immunohistochemistry in a single medical institution. DFSP is a
rare disease with a favorable prognosis and relatively low
mortality (1,2). In the present study, the patients ages
ranged from 3–86 years. The anatomical locations that were mostly
affected were the body (n=20), followed by the head and neck
(n=19), and the lower extremities (n=18). The lower extremities
were more commonly affected than the upper extremities. These
findings are in agreement with the majority of previous studies in
the literature (1–4). The first pathological diagnosis of
almost all tumors was DFSP. The overall sensitivity for the
histopathological diagnosis of DFSP was 83.8%. A total of 11
patients were diagnosed firstly with other pathologies, including
dermatofibroma in 5 cases, neurofibroma in 4 cases, fibromatosis in
1 case and schwannoma in 1 case. The components of these tumors are
composed of spindle cells, which can mimic DFSP and its variants,
particularly the myxoid histopathological subtype. Once
pathologists re-evaluated the tumor and additional CD34
immunohistochemical study was performed, the pathological diagnosis
was changed to DFSP.
The immunohistochemical markers identified for DFSP
are CD34 and vimentin (1–4). However, CD34 immunohistochemical
positivity is also expressed by other spindle cell tumors,
including solitary fibrous tumors, sclerotic fibroma, superficial
acral fibromyxoma, cellular digital fibroma, dermatofibroma and
nuchal-type fibroma. Additional immunohistochemical staining of
factor XIIIa, stromelysin III, CD44, CD163 and D2–40 have been
found to be positive in dermatofibroma and negative in DFSP
(1–4,6–10). Spindle tumor cells are immunonegative
for S100 protein, smooth muscle actin, desmin, cytokeratin and
epithelial membrane antigen (1–4).
Wide excision remains the cornerstone of the
treatment of DFSP (10–12). However, the response from patients
remains poor and locoregional recurrence has been observed during
follow-up (10–12). The natural history of DFSP is
low-grade malignancy, with a 26–60% local recurrence rate
attributed to incomplete excision due to poor circumscription and
irregular boundaries (13). The
lesion typically infiltrates well beyond its grossly visible margin
into the surround tissue. Certain studies have advocated the use of
Mohs micrographic surgery with incremental excision until normal
tissue is obtained, as documented by repeat frozen sections
(13). Several studies have
demonstrated a significant correlation between a wide excision and
a low recurrence rate (9,13). In the present study, the recurrence of
DFSP occurred in 26 cases (38.2%). Locoregional recurrence after
such excision appears to be associated with anatomical tumor
location, adequacy of surgical margin and a corrected first
pathological diagnosis. The main site of tumor recurrence was
mostly the head and neck, which is the common site of recurrent
tumor in this study. We hypothesize that this may be due to the
difficulty of performing a resection with free surgical margins in
this anatomical location. An adequate surgical margin cannot be
completely obtained in these regions. The periosteum, pericranium
and cervical fascia of the head and neck are the limited anatomical
sites for complete wide surgical excision. In conclusion, the major
factor predicts the recurrence of tumor was inadequate surgical
margin.
An incorrect first pathological diagnosis was
significantly associated with sarcomatous transformation and the
recurrence of DFSP (P=0.049 and P<0.001, respectively). DFSP is
a distinct tumor entity that often presents a diagnostic challenge.
In the present study, the false-negative pathological diagnoses
included dermatofibroma, neurofibroma, schwannoma and fibromatosis,
which are all benign conditions. Initial misdiagnosis led to a
delay in treatment, with the tumor producing significant local
extension and destruction. An incorrect first pathological
diagnosis may lead to a negative morbidity outcome as serious as
failure to treat a missed case of low-grade sarcoma, DFSP.
Successful DFSP treatment begins with an accurate pathological
diagnosis.
Sarcomatous change in DFSP represents a form of
tumor progression, which occurs in 10–15% of DFSP and is associated
with a prognosis worse than ordinary DFSP (1,14–18). In the present study, sarcomatous
transformation occurred in 6/68 (8.8%) of DFSP cases. These
sarcomatous transformations included fibrosarcoma (3/6), followed
by MFH (2/6) and undifferentiated sarcoma (1/6). The factors
predicting sarcomatous transformation in this study were a large
tumor size and an incorrect first pathological diagnosis (P=0.008
and P=0.049, respectively).
The characteristic cytogenetic features of DFSP are
a supernumerary ring chromosome and a reciprocal chromosomal
translocation t(17;22)(q22;q13), causing a fusion of the
platelet-derived growth factor β-chain (PDGFB) gene at 22q13
and the collagen type 1α1 (COL1A1) at 17q22 (1). This rearrangement results in the
constitutive activation of the PDGF as a consequence of deregulated
ligand expression (19). PDGFB
copy number status may become a useful diagnostic marker since the
gene is a potential target of treatment in patients with DFSP.
Imatinib, the target therapy for PDGFB, was administered to
1 patient in the present study for 4 months. The patient
experienced disease-free survival for >103 months. Further
molecular study in DFSP patients is warranted and has important
implications for the study of the pathogenesis of disease.
In conclusion, the present study found that the
factors that predict the sarcomatous transformation of DFSP are a
larger tumor size and an incorrect first pathological diagnosis.
The factors that predict the recurrence of DFSP are an incorrect
first pathological diagnosis and an inadequate surgical margin. In
patients who have the spindle cell tumors arranged in a storiform
pattern, CD34 immunohistochemical staining provides the
pathological diagnosis of DFSP. However, a combination of
clinicopathological features, immunohistochemistry and, in specific
cases, molecular or cytogenetic testing, is essential for
definitive diagnosis. The exact histopathological categorization is
important for selecting the appropriate treatment and for
predicting the clinical outcome.
References
|
1
|
Mentzel T, Peeutour F, Lazar A and Coindre
JM: Dermatofibrosarcoma protuberans. World Health Organization
(WHO) Classification of Tumours of Soft tissue and Bone. Pathology
and Genetics. Fletcher CDM, Bridge JA, Hogendoorn P and Martens F:
5:(4th). (Lyon). IARC Press. 77–79. 2013.
|
|
2
|
Weyers W, Mentzel T, Kasper RC, Tosti A,
Iorizzo M, Zelger B and Caputo R: In: World Health Organization
Classification of Tumours. Pathology and Genetics Skin Tumours.
Fibrous, fibrohistiocytic and histiocytic tumours. Le Boit PE, Burg
G, Weedon D and Sarasin A: (Lyon). IARC Press. 259–261. 2002.
|
|
3
|
Kempson RL, Fletcher CDM, Evans HL,
Hendrickson MR and Sibley RK: Atlas of Tumor Pathology. Tumors of
Soft Tissues. 3rd series. Fascicle 30. Armed Forces Institute of
Pathology (Washington, DC). 138–148. 2001.
|
|
4
|
Goldblum JR, Folpe AL and Weiss SW:
Fibrohistocytic tumors of intermediate malignancy. Enzinger and
Weisss soft tissue tumors (6th). (Philadelphia, PA). Elsevier
Saunders. 387–400. 2013.
|
|
5
|
Darier J and Ferrand M: Dermatofibromes
progressifs et récidivants ou fibrosarcomes de la peau. Ann
Dermatol Syphiligr (Paris). 5:545–562. 1924.
|
|
6
|
Prieto VG, Reed JA and Shea CR: CD34
immunoreactivity distinguishes between scar tissue and residual
tumor in re-excisional specimens of dermatofibrosarcoma
protuberans. J Cutan Pathol. 21:324–329. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Llombart B, Serra-Guillén C, Monteagudo C,
López Guerrero JA and Sanmartín O: Dermatofibrosarcoma protuberans:
A comprehensive review and update on diagnosis and management.
Semin Diagn Pathol. 30:13–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Erdem O, Wyatt AJ, Lin E, Wang X and
Prieto VG: Dermatofibrosarcoma protuberans treated with wide local
excision and followed at a cancer hospital: Prognostic significance
of clinicopathologic variables. Am J Dermatopathol. 34:24–34. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stivala A, Lombardo GA, Pompili G, Tarico
MS, Fraggetta F and Perrotta RE: Dermatofibrosarcoma protuberans:
Our experience of 59 cases. Oncol Lett. 4:1047–1055.
2012.PubMed/NCBI
|
|
10
|
Archontaki M, Korkolis DP, Arnogiannaki N,
Konstantinidou C, Georgopoulos S, Dendrinos P, Zarkadas G and
Kokkalis G: Dermatofibrosarcoma protuberans: A case series of 16
patients treated in a single institution with literature review.
Anticancer Res. 30:3775–3779. 2010.PubMed/NCBI
|
|
11
|
Fiore M, Miceli R, Mussi C, Lo Vullo S,
Mariani L, Lozza L, Collini P, Olmi P, Casali PG and Gronchi A:
Dermatofibrosarcoma protuberans treated at a single institution: A
surgical disease with a high cure rate. J Clin Oncol. 23:7669–7675.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
DuBay D, Cimmino V, Lowe L, Johnson TM and
Sondak VK: Low recurrence rate after surgery for
dermatofibrosarcoma protuberans: A multidisciplinary approach from
a single institution. Cancer. 100:1008–1016. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lemm D, Mügge LO, Mentzel T and Höffken K:
Current treatment options in dermatofibrosarcoma protuberans. J
Cancer Res Clin Oncol. 135:653–665. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Connelly JH and Evans HL:
Dermatofibrosarcoma protuberans. A clinicopathologic review with
emphasis on fibrosarcomatous areas. Am J Surg Pathol. 16:921–925.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato N, Kimura K and Tomita Y: Recurrent
dermatofibrosarcoma protuberans with myxoid and fibrosarcomatous
changes paralleled by loss of CD34 expression. J Dermatol.
22:665–672. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mentzel T, Beham A, Katenkamp D, Dei Tos
AP and Fletcher CD: Fibrosarcomatous (‘high-grade’)
dermatofibrosarcoma protuberans: Clinicopathologic and
immunohistochemical study of a series of 41 cases with emphasis on
prognostic significance. Am J Surg Pathol. 22:576–587. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldblum JR, Reith JD and Weiss SW:
Sarcomas arising in dermatofibrosarcoma protuberans: A reappraisal
of biologic behavior in eighteen cases treated by wide local
excision with extended clinical follow up. Am J Surg Pathol.
24:1125–1130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abbott JJ, Oliveira AM and Nascimento AG:
The prognostic significance of fibrosarcomatous transformation in
dermatofibrosarcoma protuberans. Am J Surg Pathol. 30:436–443.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McArthur G: Dermatofibrosarcoma
protuberans: Recent clinical progress. Ann Surg Oncol.
14:2876–2886. 2007. View Article : Google Scholar : PubMed/NCBI
|