Introduction
Oral squamous cell carcinoma (OSCC) accounts for 90%
of all malignant head and neck tumors worldwide (1). Furthermore, metastasis to regional lymph
nodes and distant sites, which occurs in 40 and 10% of all OSCC
cases, respectively, is associated with poor prognosis (2). Although the underlying mechanisms of
metastasis remain unclear, recent studies have demonstrated that a
small subset of tumor cells known as cancer stem cells (CSCs),
which exhibit similar characteristics to normal stem cells
(including self-renewal and pluripotency), may be involved in
cancer invasion and metastasis (3).
Previous studies have revealed that the expression
of four transcription factors [octamer-binding transcription factor
4 (Oct4), sex determining region Y-box 2 (SOX2), avian
myelocytomatosis viral oncogene homolog (c-Myc) and Krüppel-like
factor 4 (KLF4)] is sufficient to reprogram differentiated cells to
pluripotency (4,5). SOX2 and Oct4 are important for
maintaining self-renewal and pluripotency in pluripotent stem cells
(6). KLF4, which is involved in
tissue development, differentiation and maintenance of homeostasis,
may act as either an oncogene or a tumor suppressor in certain
types of cancer, including gastric adenocarcinoma and colon cancer
(7–9).
c-Myc is an oncogenic transcription factor that is involved in cell
proliferation, differentiation and apoptosis (10). In addition, the expression of these
transcription factors is associated with several types of malignant
cancer, including oesophageal (11)
breast (12), bladder (13) and lung cancer (14,15).
However, the role of these genes in CSCs remains unclear.
Recently, the T-box transcription factor brachyury,
which is essential for mesoderm formation during early development
(16,17), has been found to regulate the
epithelial-mesenchymal transition (EMT) and CSC potential in human
salivary carcinoma cells (18–21). In
addition, brachyury expression was found to correlate with lymph
node metastasis in OSCC (22).
However, to date, the association between SOX2, Oct4, KLF4, c-Myc
and brachyury expression in OSCC has not been investigated.
Therefore, the aim of the present study was to determine whether
these transcription factors may represent potential CSC markers and
prognostic factors for OSCC.
Materials and methods
Patients and tumor specimens
A total of 108 OSCC patients who were treated at the
Department of Oral and Maxillofacial Surgery, Kyushu University
Hospital (Fukuoka, Japan) between March 2001 and December 2006 were
retrospectively enrolled in the present study. Pretreatment
biopsies were obtained from 108 patients. Clinicopathological
information, including age, gender, tumor size and location, nodal
status, treatments and the presence or absence of disease
recurrence and metastasis, was obtained from patient records. The
protocol for this study was approved by the Ethics Committee of
Kyushu University.
Histopathology and
immunohistochemistry
Consecutive 4-µm sections were cut from
formalin-fixed paraffin-embedded (FFPE) biopsy samples and
deparaffinized with xylene, rehydrated in a graded alcohol series,
and heat-treated with Target Retrieval Solution (Dako, Carpinteria,
CA, USA) prior to histopathological and immunohistochemical
analyses. Tumors were staged according to the International Union
for Cancer Control tumor-node-metastasis classification system (7th
edition) (23). In addition, tumors
were graded using World Health Organization criteria (24) and Anneroth's multifactorial
classification system (25,26).
Immunohistochemistry was performed to analyze the
expression patterns of SOX2, Oct4, c-Myc, KLF4 and brachyury in
OSCC samples. FFPE sections were treated with 3%
H2O2 and serum-free protein in
phosphate-buffered saline with 0.015 M sodium azide to block
endogenous peroxide activity and nonspecific antibody binding. The
sections were then incubated overnight at 4°C with the following
primary antibodies: Monoclonal rabbit anti-human SOX2 (clone D6D9;
#3579; 1:50; Cell Signaling Technology, Inc., Danvers, MA, USA),
polyclonal rabbit anti-human Oct4 (clone POU5F1; #2750; 1:100; Cell
Signaling Technology, Inc.), polyclonal rabbit anti-brachyury
(clone H-210; #sc-20109; 1:200; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), monoclonal mouse anti-human c-Myc (clone
9E10; #sc-40; 1:200; Santa Cruz Biotechnology, Inc.) and monoclonal
mouse anti-human KLF4 (clone AT4E6; #NBP1-50367; 1:100; Novus
Biologicals, LLC, Littleton, CO, USA). Subsequently, immunostaining
was visualized with the CSA II Biotin-Free Tyramide Signal
Amplification System (Dako), CSA II Rabbit Link amplification
reagent (Dako) and 3,3-diaminobenzidine according to the
manufacturer's instructions. Briefly, the sections were incubated
with horseradish-peroxidase conjugated anti-mouse or rabbit IgG
secondary antibodies (CSA II Biotin-Free Tyramide Signal
Amplification System; Dako) for 15 min at room temperature,
followed by incubation with CSA II amplification reagent (Dako) and
3,3′-diaminobenzidine. Finally, the sections were counterstained
with 0.5% hematoxylin.
The staining pattern was evaluated at three randomly
selected locations along the invasive edge of OSCC tumors using an
optical microscope equipped with a charge-coupled device camera
(BZ-9000; Keyence Corporation, Osaka, Japan). Specifically, the
intensity of staining was quantified as the difference between the
mean pixel density in 10 randomly selected stained carcinoma cells
and that of the background using the BZ-II Analyzer (Keyence
Corporation). To account for staining heterogeneity, the expression
intensity (EI) of a protein was defined as the ratio of the
immunostain density in the nuclei of tumor cells to that of normal
basal epithelial cells in the same OSCC sample (Table IA; Fig.
1), according to the following formula: EI = (mean density of
positive signal in OSCC cells - mean density of background
staining) / (mean density of positive signal in normal cells - mean
density of background staining). The results were classified into
two groups (high or low expression) for each protein according to
the mean value, as shown in Table
IA.
 | Table I.Classification of EI and positive
ER. |
Table I.
Classification of EI and positive
ER.
| A, EI
classification |
|---|
|
|---|
|
| Relative mean pixel
densitya |
|---|
|
|
|
|---|
| Factor | Low | Cut-off | High |
|---|
| SOX2, Oct4, KLF4,
c-Myc, brachyury | < | 1 | ≤ |
|
| B, Positive ER
classification |
|
|
| Positively stained
nuclei, % |
|
|
|
| Factor | Low | Cut-off
(median) | High |
|
| SOX2 | < | 66.57 | ≤ |
| Oct4 | < | 54.74 | ≤ |
| KLF4 | < | 66.72 | ≤ |
| c-Myc | < | 71.92 | ≤ |
| Brachyury | < | 71.86 | ≤ |
The positive expression ratio (ER) was calculated as
the ratio of positively stained nuclei to total number of carcinoma
cells in each field. The results were classified into two groups
(high or low expression) for each protein according to the median
value, as shown in Table IB. All
samples were scored by two independent pathologists who were
blinded to the patient's clinical information and diagnosis.
Statistical analysis
The associations between protein expression and
clinicopathological factors were assessed using the χ2
test and Fisher's exact test. Univariate and multivariate logistic
regression analyses were performed to identify independent risk
factors for lymph node and distant metastasis. Overall survival,
disease-specific survival and disease-free survival were analyzed
with the Kaplan-Meier method and the log-rank test. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS 22.0 statistical
software (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
The patient cohort included 69 males and 39 females,
with a median age of 62 years (range, 24–85 years). Primary OSCC
tumors were most frequently identified on the tongue (55/108;
50.9%). Lymph node metastasis occurred in 40/108 patients (37.0%)
and distant metastasis occurred in 9/108 patients (8.3%). The
median follow-up period was 60 months (range, 5–60 months). Further
patient characteristics are shown in Table II.
 | Table II.Association between SOX2, Oct4, KLF4,
c-Myc and brachyury expression intensity and clinicopathological
factors in 108 oral squamous cell carcinoma patients. |
Table II.
Association between SOX2, Oct4, KLF4,
c-Myc and brachyury expression intensity and clinicopathological
factors in 108 oral squamous cell carcinoma patients.
|
|
| SOX2 expression,
n | Oct4 expression,
n | KLF4 expression,
n | c-Myc expression,
n | Brachyury
expression, n |
|---|
|
|
|
|
|
|
|
|
|---|
| Clinicopathological
parameter | Cases, n | Low | High | P-value | Low | High | P-value | Low | High | P-value | Low | High | P-value | Low | High | P-value |
|---|
| Age, years |
|
|
|
0.870 |
|
| 0.341 |
|
| 0.055 |
|
| 0.610 |
|
|
0.190 |
|
<65 | 61 | 25 | 36 |
| 38 | 23 |
| 36 | 25 |
| 40 | 21 |
| 35 | 26 |
|
|
≥65 | 47 | 20 | 27 |
| 25 | 22 |
| 19 | 28 |
| 33 | 14 |
| 21 | 26 |
|
| Gender |
|
|
|
0.919 |
|
| 0.611 |
|
| 0.956 |
|
| 0.784 |
|
|
0.130 |
|
Male | 69 | 29 | 40 |
| 39 | 30 |
| 35 | 34 |
| 46 | 23 |
| 32 | 37 |
|
|
Female | 39 | 16 | 23 |
| 24 | 15 |
| 20 | 19 |
| 27 | 12 |
| 24 | 15 |
|
| Clinical stage |
|
|
|
0.242 |
|
| 0.407 |
|
| 0.097 |
|
| 0.003a |
|
|
0.158 |
| T1 | 18 | 8 | 10 |
| 13 | 5 |
| 12 | 6 |
| 16 | 2 |
| 11 | 7 |
|
| T2 | 46 | 23 | 23 |
| 28 | 18 |
| 26 | 20 |
| 36 | 10 |
| 28 | 18 |
|
| T3 | 21 | 5 | 16 |
| 11 | 10 |
| 10 | 11 |
| 10 | 11 |
| 8 | 13 |
|
| T4 | 23 | 9 | 14 |
| 11 | 12 |
| 7 | 16 |
| 11 | 12 |
| 9 | 14 |
|
| Primary tumor
site |
|
|
|
0.217 |
|
| 0.407 |
|
| 0.001a |
|
| 0.481 |
|
|
0.390 |
| Buccal
mucosa | 8 | 6 | 2 |
| 5 | 3 |
| 1 | 7 |
| 6 | 2 |
| 4 | 4 |
|
| Upper
gingiva | 12 | 5 | 7 |
| 5 | 7 |
| 4 | 8 |
| 9 | 3 |
| 7 | 5 |
|
| Lower
gingiva | 22 | 7 | 15 |
| 9 | 13 |
| 8 | 14 |
| 11 | 11 |
| 7 | 15 |
|
|
Tongue | 55 | 22 | 33 |
| 37 | 18 |
| 34 | 21 |
| 41 | 14 |
| 34 | 21 |
|
| Oral
floor | 10 | 4 | 6 |
| 6 | 4 |
| 8 | 2 |
| 5 | 5 |
| 3 | 7 |
|
|
Palate | 1 | 1 | 0 |
| 1 | 0 |
| 0 | 1 |
| 1 | 0 |
| 1 | 0 |
|
| Lymph node
metastasis |
|
|
|
0.002a |
|
| 0.031a |
|
| 0.003a |
|
| 0.386 |
|
|
0.007a |
|
Positive | 40 | 9 | 31 |
| 18 | 22 |
| 13 | 27 |
| 25 | 15 |
| 14 | 26 |
|
|
Negative | 68 | 36 | 32 |
| 45 | 23 |
| 42 | 26 |
| 48 | 20 |
| 42 | 26 |
|
| Distant
metastasis |
|
|
|
0.051 |
|
| 0.109 |
|
| 0.014a |
|
| 0.322 |
|
|
0.012a |
|
Positive | 9 | 1 | 8 |
| 3 | 6 |
| 1 | 8 |
| 5 | 4 |
| 1 | 8 |
|
|
Negative | 99 | 44 | 55 |
| 60 | 39 |
| 54 | 45 |
| 68 | 31 |
| 55 | 44 |
|
| Tumor
differentiation |
|
|
|
0.263 |
|
| 0.232 |
|
| 0.213 |
|
| 0.057 |
|
|
0.660 |
|
Well | 83 | 37 | 46 |
| 51 | 32 |
| 45 | 38 |
| 60 | 23 |
| 44 | 39 |
|
|
Moderate | 25 | 8 | 17 |
| 12 | 13 |
| 10 | 15 |
| 13 | 12 |
| 12 | 13 |
|
|
Poor | 0 | 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
|
| Anneroth score |
|
|
|
<0.001a |
|
| 0.007a |
|
| 0.496 |
|
| 0.115 |
|
|
<0.001a |
| 1 | 7 | 4 | 3 |
| 6 | 1 |
| 6 | 1 |
| 7 | 0 |
| 5 | 2 |
|
| 2 | 14 | 9 | 5 |
| 8 | 6 |
| 2 | 12 |
| 8 | 6 |
| 10 | 4 |
|
| 3 | 54 | 29 | 25 |
| 37 | 17 |
| 31 | 23 |
| 38 | 16 |
| 34 | 20 |
|
| 4 | 33 | 3 | 30 |
| 12 | 21 |
| 16 | 17 |
| 20 | 13 |
| 7 | 26 |
|
Subcellular localization of SOX2,
Oct4, KLF4, c-Myc and brachyury expression
SOX2, Oct4, c-Myc and brachyury were predominantly
localized to the nucleus of OSCC cells; however, in certain cases,
they were localized to the cytoplasm and nucleus (Fig. 2). KLF4 was primarily localized to the
cytoplasm and nucleus of OSCC cells. All proteins were also
detected in the nucleus of normal basal epithelial cells.
 | Figure 2.EI of SOX2, Oct4, KLF4, c-Myc and
brachyury in oral squamous cell carcinoma tissue. Photomicrographs
show representative examples of normal epithelium (left column) and
low (middle column) or high (right column) EI of (A-C) SOX2, (D-E)
Oct4, (G-I) KLF4, (J-L) c-Myc and (M-O) brachyury. Scale bar, 50
µm. EI, expression intensity; SOX2, sex determining region Y-box 2;
Oct4, octamer-binding transcription factor 4; c-Myc, avian
myelocytomatosis viral oncogene homolog; KLF4, Krüppel-like factor
4. |
Association between SOX2, Oct4, KLF4,
c-Myc and brachyury expression and clinicopathological factors
The median ERs of SOX2, Oct4, KLF4, c-Myc and
brachyury, which were used as the cut-off values for low or high
expression, were 66.6, 54.7, 66.7, 71.9 and 71.9%, respectively
(Fig. 3). The EIs and ERs of these
transcription factors were found to be significantly associated
with several clinicopathological factors (Tables II–IV). For example, c-Myc EI was significantly
associated with clinical tumor stage (P=0.003), while SOX2, Oct4,
KLF4 and brachyury EIs were significantly associated with lymph
node metastasis (P=0.002, P=0.031, P=0.003 and P=0.007,
respectively) (Table II). KLF4 and
brachyury EIs were also significantly associated with distant
metastasis (P=0.014 and P=0.012, respectively). However, no
significant differences were identified between the EIs of these
proteins and the degree of tumor differentiation. Notably, the EIs
of SOX2, Oct4 and brachyury were significantly associated with
Anneroth scores (P<0.001, P=0.007 and P<0.001, respectively).
χ2 tests revealed that the EIs of SOX2, KLF4 and
brachyury in tumors with an Anneroth score of 3 were significantly
associated with lymph node metastasis (P=0.015, P=0.005 and
P=0.025, respectively) (Table V).
However, no significant differences were identified between EIs of
SOX2, KLF4 and brachyury in tumors with Anneroth scores of 1, 2 or
4.
 | Figure 3.Positive ER of SOX2, Oct4, KLF4,
c-Myc and brachyury in oral squamous cell carcinoma tissue.
Photomicrographs show representative examples of low (left column)
or high (right column) positive ER for (A and B) SOX2, (C and D)
Oct4, (E and F) KLF4, (G and H) c-Myc, and (I and J) brachyury.
Scale bar, 50 µm. ER, expression ratio; SOX2, sex determining
region Y-box 2; Oct4, octamer-binding transcription factor 4;
c-Myc, avian myelocytomatosis viral oncogene homolog; KLF4,
Krüppel-like factor 4. |
 | Table IV.Predictive factors for lymph node and
distant metastasis in oral squamous cell carcinoma patients. |
Table IV.
Predictive factors for lymph node and
distant metastasis in oral squamous cell carcinoma patients.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Type of
metastasis | Comparison | OR | P-value | 95% CI | OR | P-value | 95% CI |
|---|
| Lymph node |
|
|
|
|
|
|
|
|
SOX2 | Low vs. high
EI |
3.875 | 0.003 | 1.604–9.359 | 4.526 | 0.011 |
1.404–14.588 |
|
Oct4 | Low vs. high
EI |
2.391 | 0.033 | 1.074–5.323 | 1.148 | 0.795 | 0.405–3.255 |
|
KLF4 | Low vs. high
EI |
3.355 | 0.004 | 1.474–7.639 | 4.851 | 0.004 |
1.667–14.116 |
|
c-Myc | Low vs. high
EI |
1.440 | 0.387 | 0.631–3.288 | 0.559 | 0.284 | 0.193–1.622 |
|
Brachyury | Low vs. high
EI |
3.000 | 0.008 | 1.330–6.766 | 0.999 | 0.998 | 0.312–3.193 |
| Distant |
|
|
|
|
|
|
|
|
SOX2 | Low vs. high
EI |
6.400 | 0.086 |
0.771–53.123 | 3.766 | 0.314 |
0.285–49.820 |
|
Oct4 | Low vs. high
EI |
3.077 | 0.127 |
0.727–13.030 | 1.003 | 0.997 | 0.188–5.359 |
|
KLF4 | Low vs. high
EI |
9.600 | 0.036 |
1.157–79.673 | 9.607 | 0.053 |
0.974–94.804 |
|
c-Myc | Low vs. high
EI |
1.755 | 0.425 | 0.441–6.987 | 0.579 | 0.494 | 0.121–2.775 |
|
Brachyury | Low vs. high
EI | 10.000 | 0.033 |
1.205–83.005 | 3.301 | 0.360 |
0.256–42.542 |
 | Table V.Association between SOX2, Oct4, KLF4,
c-Myc and brachyru protein expression intensity and metastasis in
oral squamous cell carcinoma patients according to Anneroth
score. |
Table V.
Association between SOX2, Oct4, KLF4,
c-Myc and brachyru protein expression intensity and metastasis in
oral squamous cell carcinoma patients according to Anneroth
score.
|
|
| SOX2 expression,
n | Oct4 expression,
n | KLF4 expression,
n | c-Myc expression,
n | Brachyury
expression, n |
|---|
|
|
|
|
|
|
|
|
|---|
| Parameter | Cases, n | Low | High | P-value | Low | High | P-value | Low | High | P-value | Low | High | P-value | Low | High | P-value |
|---|
| Anneroth
score=1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lymph
node metastasis |
|
|
| – |
|
| – |
|
| – |
|
| – |
|
| – |
|
Positive | 0 | 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
|
|
Negative | 7 | 4 | 3 |
| 6 | 1 |
| 6 | 1 |
| 7 | 0 |
| 5 | 2 |
|
| Distant
metastasis |
|
|
| – |
|
| – |
|
| – |
|
| – |
|
| – |
|
Positive | 0 | 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
|
|
Negative | 7 | 4 | 3 |
| 6 | 1 |
| 6 | 1 |
| 7 | 0 |
| 5 | 2 |
|
| Anneroth
score=2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lymph
node metastasis |
|
|
| 0.545 |
|
| 0.594 |
|
| 0.495 |
|
| 0.594 |
|
| 0.689 |
|
Negative | 10 | 6 | 4 |
| 6 | 4 |
| 2 | 8 |
| 6 | 4 |
| 7 | 3 |
|
| Distant
metastasis |
|
|
| 0.110 |
|
| 0.165 |
|
| 0.725 |
|
| 0.165 |
|
| 0.066 |
|
Positive | 2 | 0 | 2 |
| 0 | 2 |
| 0 | 2 |
| 0 | 2 |
| 0 | 2 |
|
|
Negative | 12 | 9 | 3 |
| 8 | 4 |
| 2 | 10 |
| 8 | 4 |
| 10 | 2 |
|
| Anneroth
score=3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lymph
node metastasis |
|
|
| 0.015a |
|
| 0.095 |
|
| 0.005a |
|
| 0.537 |
|
| 0.025a |
|
Positive | 17 | 5 | 12 |
| 9 | 8 |
| 5 | 12 |
| 11 | 6 |
| 7 | 10 |
|
|
Negative | 37 | 24 | 13 |
| 28 | 9 |
| 26 | 11 |
| 27 | 10 |
| 27 | 10 |
|
| Distant
metastasis |
|
|
| 0.716 |
|
| 0.535 |
|
| 0.177 |
|
| 0.509 |
|
| 0.608 |
|
Positive | 2 | 1 | 1 |
| 1 | 1 |
| 0 | 2 |
| 1 | 1 |
| 1 | 1 |
|
|
Negative | 52 | 28 | 24 |
| 36 | 16 |
| 31 | 21 |
| 37 | 15 |
| 33 | 19 |
|
| Anneroth
score=4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lymph
node metastasis |
|
|
| 0.384 |
|
| 0.947 |
|
| 0.393 |
|
| 0.727 |
|
| 0.652 |
|
Positive | 19 | 1 | 18 |
| 7 | 12 |
| 8 | 11 |
| 12 | 7 |
| 4 | 15 |
|
|
Negative | 14 | 2 | 12 |
| 5 | 9 |
| 8 | 6 |
| 8 | 6 |
| 3 | 11 |
|
| Distant
metastasis |
|
|
| 0.600 |
|
| 0.612 |
|
| 0.187 |
|
| 0.331 |
|
| 0.277 |
|
Positive | 5 | 0 | 5 |
| 2 | 3 |
| 1 | 4 |
| 4 | 1 |
| 0 | 5 |
|
|
Negative | 28 | 3 | 25 |
| 10 | 18 |
| 15 | 13 |
| 16 | 12 |
| 7 | 21 |
|
In addition, clinical tumor stage was significantly
associated with Oct4 and KLF4 ERs (P=0.048 and P=0.028,
respectively) (Table III). Lymph
node metastasis was significantly associated with Oct4 ER (P=0.046)
and distant metastasis was significantly associated with SOX2
(P=0.016). Anneroth scores were significantly associated with SOX2,
Oct4, KLF4, c-Myc and brachyury ERs (P=0.005, P=0.019, P=0.003,
P=0.019 and P=0.010, respectively); however, only Oct4 expression
was significantly associated with tumor differentiation
(P=0.012).
 | Table III.Association between SOX2, Oct4, KLF4,
c-Myc and brachyury positive expression ratio and
clinicopathological factors in 108 oral squamous cell carcinoma
patients. |
Table III.
Association between SOX2, Oct4, KLF4,
c-Myc and brachyury positive expression ratio and
clinicopathological factors in 108 oral squamous cell carcinoma
patients.
|
|
| SOX2 expression,
n | Oct4 expression,
n | KLF4 expression,
n | c-Myc expression,
n | Brachyury
expression, n |
|---|
|
|
|
|
|
|
|
|
|---|
| Clinicopathological
parameter | Cases, n | Low | High | P-value | Low | High | P-value | Low | High | P-value | Low | High | P-value | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.56 |
|
| 0.846 |
|
| 0.846 |
|
| 0.332 |
|
| 0.560 |
|
<65 | 61 | 29 | 32 |
| 31 | 30 |
| 31 | 30 |
| 33 | 28 |
| 32 | 29 |
|
|
≥65 | 47 | 25 | 22 |
| 23 | 24 |
| 23 | 24 |
| 21 | 26 |
| 22 | 25 |
|
| Gender |
|
|
| 0.317 |
|
| 0.161 |
|
| 0.317 |
|
| 0.548 |
|
| 0.071 |
|
Male | 69 | 32 | 37 |
| 31 | 38 |
| 32 | 37 |
| 33 | 36 |
| 30 | 39 |
|
|
Female | 39 | 22 | 17 |
| 23 | 16 |
| 22 | 17 |
| 21 | 18 |
| 24 | 15 |
|
| Clinical stage |
|
|
| 0.550 |
|
| 0.048a |
|
| 0.028a |
|
| 0.845 |
|
| 0.420 |
| T1 | 18 | 11 | 7 |
| 13 | 5 |
| 12 | 6 |
| 9 | 9 |
| 11 | 7 |
|
| T2 | 46 | 24 | 22 |
| 22 | 24 |
| 27 | 19 |
| 25 | 21 |
| 25 | 21 |
|
| T3 | 21 | 10 | 1 |
| 6 | 15 |
| 9 | 12 |
| 9 | 12 |
| 8 | 13 |
|
| T4 | 23 | 9 | 14 |
| 13 | 10 |
| 6 | 17 |
| 11 | 12 |
| 10 | 13 |
|
| Primary tumor
site |
|
|
| 0.030a |
|
| 0.465 |
|
| 0.329 |
|
| 0.091 |
|
| 0.045a |
| Buccal
mucosa | 8 | 6 | 2 |
| 4 | 4 |
| 6 | 2 |
| 6 | 2 |
| 5 | 3 |
|
| Upper
gingiva | 12 | 8 | 4 |
| 5 | 7 |
| 6 | 6 |
| 5 | 7 |
| 8 | 4 |
|
| Lower
gingiva | 22 | 9 | 13 |
| 11 | 11 |
| 8 | 14 |
| 12 | 10 |
| 10 | 12 |
|
|
Tongue | 55 | 29 | 26 |
| 30 | 25 |
| 28 | 27 |
| 29 | 26 |
| 29 | 26 |
|
| Oral
floor | 10 | 1 | 9 |
| 3 | 7 |
| 6 | 4 |
| 1 | 9 |
| 2 | 8 |
|
|
Palate | 1 | 1 | 0 |
| 1 | 0 |
| 0 | 1 |
| 1 | 0 |
| 0 | 1 |
|
| Lymph node
metastasis |
|
|
| 1.000 |
|
| 0.046a |
|
| 0.425 |
|
| 0.111 |
|
| 0.425 |
|
Positive | 40 | 20 | 20 |
| 15 | 25 |
| 18 | 22 |
| 16 | 24 |
| 18 | 22 |
|
|
Negative | 68 | 34 | 34 |
| 39 | 29 |
| 36 | 32 |
| 38 | 30 |
| 36 | 32 |
|
| Distant
metastasis |
|
|
| 0.016a |
|
| 0.244 |
|
| 0.081 |
|
| 0.500 |
|
| 0.081 |
|
Positive | 9 | 1 | 8 |
| 3 | 6 |
| 2 | 7 |
| 4 | 5 |
| 2 | 7 |
|
|
Negative | 99 | 53 | 46 |
| 51 | 48 |
| 52 | 47 |
| 50 | 49 |
| 52 | 47 |
|
| Tumor
differentiation |
|
|
| 0.254 |
|
| 0.012a |
|
| 0.820 |
|
| 0.110 |
|
| 0.110 |
|
Well | 83 | 44 | 39 |
| 47 | 36 |
| 42 | 41 |
| 45 | 38 |
| 45 | 38 |
|
|
Moderate | 25 | 10 | 15 |
| 7 | 18 |
| 12 | 13 |
| 9 | 16 |
| 9 | 16 |
|
|
Poor | 0 | 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
|
| Anneroth score |
|
|
| 0.005a |
|
| 0.019a |
|
| 0.003a |
|
| 0.019a |
|
| 0.010a |
| 1 | 7 | 3 | 4 |
| 6 | 1 |
| 5 | 2 |
| 4 | 3 |
| 5 | 2 |
|
| 2 | 14 | 12 | 2 |
| 6 | 8 |
| 9 | 5 |
| 10 | 4 |
| 10 | 4 |
|
| 3 | 54 | 30 | 24 |
| 31 | 23 |
| 31 | 23 |
| 29 | 25 |
| 27 | 27 |
|
| 4 | 33 | 9 | 24 |
| 11 | 22 |
| 9 | 24 |
| 11 | 22 |
| 12 | 21 |
|
Predictive factors for lymph node and
distant metastasis
As the results of the present study indicated that
lymph node and distant metastases were more significantly
associated with EI than ER, whether SOX2, Oct4, KLF4, c-Myc and
brachyury EIs are significant predictive factors for lymph node and
distant metastases was investigated. Univariate analyses revealed
that high SOX2, Oct4, KLF4 and brachyury EIs were significantly
associated with lymph node metastasis [odds ratios (ORs), 3.875,
2.391, 3.355 and 3.000, respectively], and high KLF4 and brachyury
EIs were associated with distant metastasis (ORs, 9.600 and 10.000,
respectively) (Table IV).
Multivariate analysis also revealed that high SOX2 and KLF4 EIs
were significantly associated with lymph node metastasis (ORs,
4.526 and 4.851, respectively).
Correlation between SOX2, Oct4, KLF4,
c-Myc and brachyury expression and survival in OSCC patients
No significant associations between the five-year
overall survival rates of OSCC patients and the EIs of SOX2, Oct4,
KLF4, c-Myc or brachyury were identified (Fig. 4A). However, the five-year
disease-specific survival rates of OSCC patients with high SOX2 and
brachyury expression were significantly decreased when compared
with those exhibiting low expression [SOX2, 87.3% vs. 100%,
respectively (P=0.015; χ2=5.891); brachyury, 86.5% vs.
98.2%, respectively (P=0.023; χ2=5.201); Fig. 4B]. In addition, the five-year
disease-free survival rates of OSCC patients with high Oct4 and
KLF4 expression were significantly decreased when compared with
those exhibiting low expression [Oct4, 62.2% vs. 84.1%,
respectively (P=0.006; χ2=7.519); KLF4, 66.0% vs. 83.6%,
respectively (P=0.029; χ2=4.758); Fig. 4C].
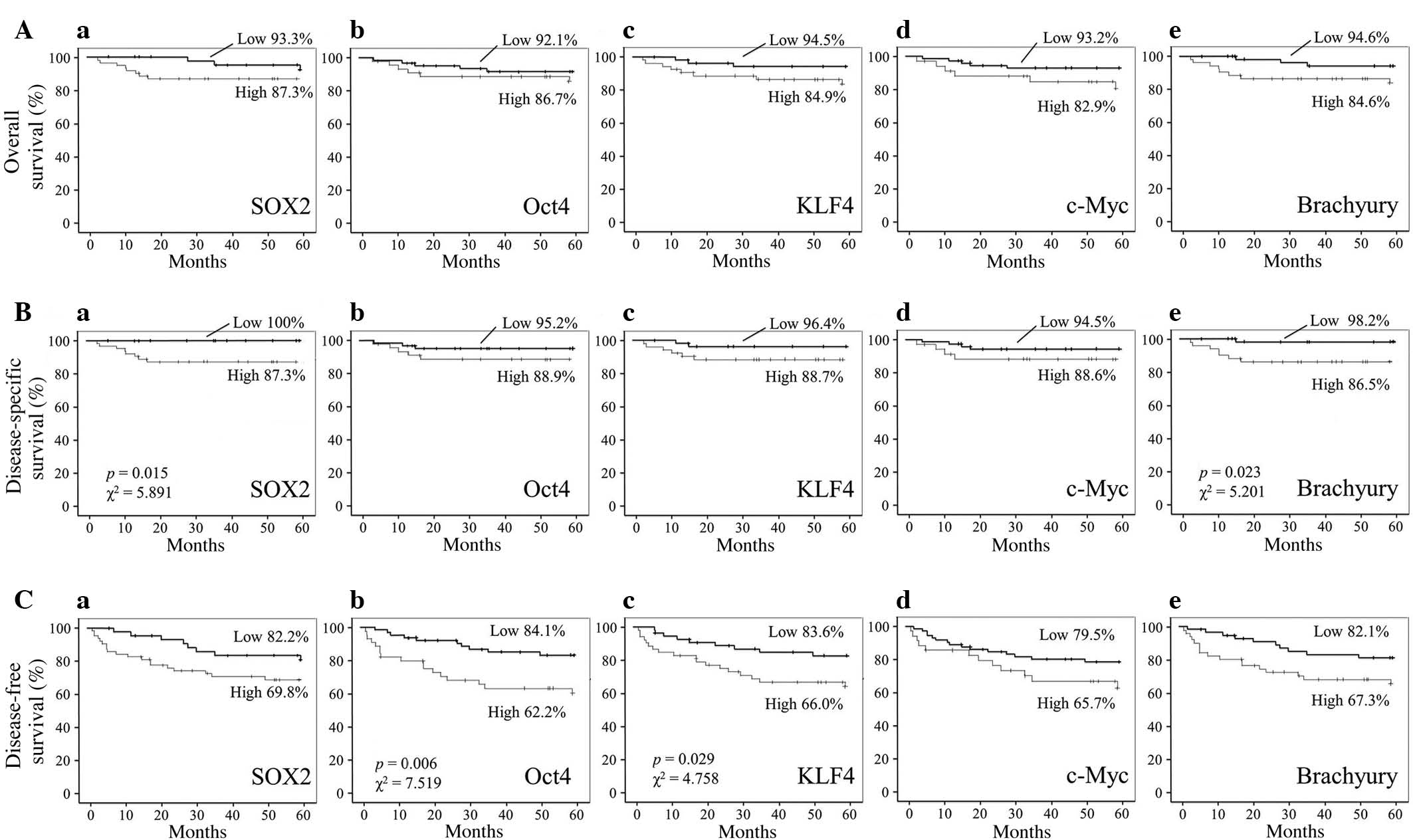 | Figure 4.Correlation between SOX2, Oct4, KLF4,
c-Myc and brachyury expression and survival in OSCC patients. (A)
Overall, (B) disease-specific and (C) disease-free survival of OSCC
patients with high or low expression intensity of (a) SOX2, (b)
Oct4, (c) KLF4, (d) c-Myc and (e) brachyury. P-values and
χ2 statistics are shown in plots with statistically
significant differences. OSCC, oral squamous cell carcinoma; SOX2,
sex determining region Y-box 2; Oct4, octamer-binding transcription
factor 4; c-Myc, avian myelocytomatosis viral oncogene homolog;
KLF4, Krüppel-like factor 4. |
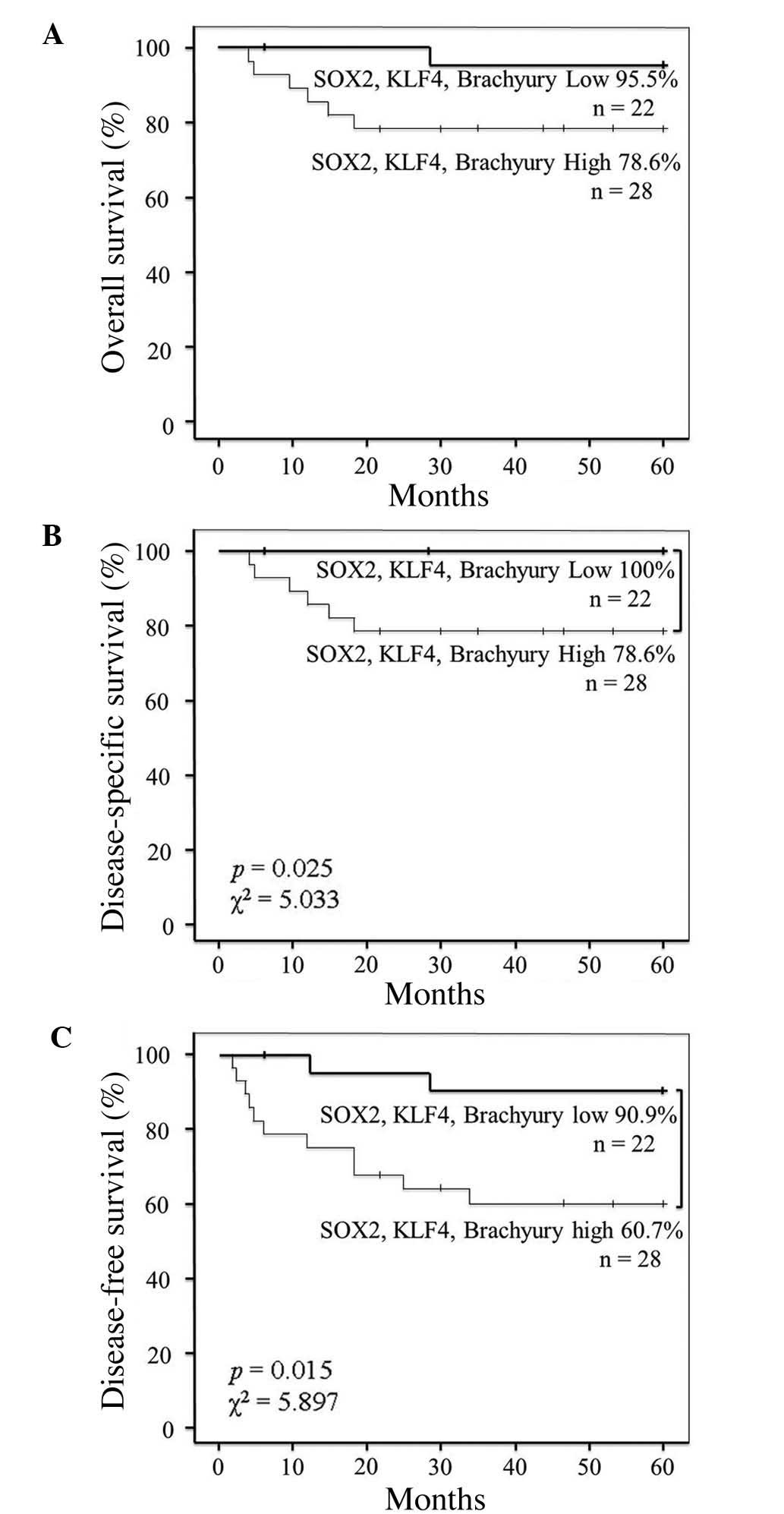
As SOX2, KLF4 and brachyury EIs were found to be
associated with lymph node and distant metastasis in this study,
the association between patient survival and the co-expression of
SOX2, KLF4 and brachyury was also investigated. The results
revealed that the co-expression of SOX2, KLF4 and brachyury was not
significantly associated with overall survival (Fig. 5A). However, the five-year
disease-specific survival rate of patients with high co-expression
of these proteins was decreased when compared with that of patients
exhibiting low co-expression (78.6% vs. 100%, respectively;
P=0.025; χ2=5.033) (Fig.
5B). Similarly, the five-year disease-free survival rate of
patients with high co-expression was decreased compared with that
of patients exhibiting low co-expression (60.7% vs. 90.9%,
respectively; P=0.015; χ2=5.897) (Fig. 5C).
Discussion
The self-renewal and pluripotent properties of CSCs,
which are hypothesized to enable primary tumors to metastasize
(27,28), indicate that their identification in
tumor samples may be important for cancer diagnosis and treatment.
However, to date, few CSC markers in OSCC have been identified
(29,30). The results of the current study
indicate that SOX2, KLF4 and brachyury may present clinically
useful CSC markers, and their expression levels may be prognostic
factors for OSCC. The expression levels of these transcription
factors were quantified in terms of EI and ER, which reflect the
level of protein expression and the number of cells expressing a
protein, respectively. As high expression levels of CSC-related
transcription factors may promote tumorigenesis (31,32), EI
may also be a measure of tumor invasiveness and local metastasis.
Similarly, as large numbers of CSCs increase the chance that some
will maintain stemness when they disseminate to other sites
(33), ER may be a measure of the
likelihood of distant metastasis. Thus, the EI and ER of
CSC-related transcription factors may be associated with survival
outcomes in cancer patients.
The results of the present study revealed that SOX2
EI and ER were significantly associated with lymph node metastasis
and distant metastasis, respectively, indicating that SOX2
expression is involved in OSCC metastasis. In addition, the
significant association between high SOX2 expression and reduced
five-year disease-specific survival rate indicates that SOX2 may be
a prognostic factor in OSCC patients. These results are consistent
with those of previous reports, which have revealed that SOX2 is
associated with poor prognosis in several types of cancer (12,13,15,34,35).
Furthermore, SOX2 regulates stemness (36) and upregulates CSC-related gene
expression in skin squamous cell carcinoma, and helps maintain
neural stem cells (37).
Similarly, a significant association between KLF4 EI
and lymph node metastasis was identified in the present study;
however, this association was not observed between KLF4 ER and
distant metastasis, which indicates that KLF4 may be less important
for metastasis than SOX2. Furthermore, the association identified
between high KLF4 EI and decreased five-year disease-free survival
rate in OSCC patients is consistent with the association between
increased nuclear expression of KLF4 and poor prognosis in breast
cancer and head and neck cancer patients, which has been reported
in previous studies (38,39).
In the present study brachyury EI was found to
significantly correlate with lymph node metastasis, distant
metastasis and Anneroth scores, which indicates that brachyury is
also involved in OSCC metastasis. These results are consistent with
those of previous studies, which revealed that silencing brachyury
expression inhibits tumor formation and metastasis in human adenoid
cystic carcinoma cells (19,20). In addition, a previous study revealed
that brachyury expression is associated with EMT and lymph node
metastasis in OSCC patients (22).
The results of the present study found that c-Myc EI
and ER were not associated with lymph node or distant metastasis.
By contrast, Oct4 EI and ER were significantly associated with
lymph node metastasis and Anneroth scores, which suggests that Oct4
may be involved in tumor metastasis. c-Myc EI was only
significantly associated with clinical tumor stage, which is
consistent with its reported association with tumorigenesis and
sustained tumor growth (40).
Two conclusions may be drawn from the results of the
present study. Firstly, the EI of CSC markers is a better indicator
of metastasis and survival than ER in OSCC patients. This may be
due to the relative uniformity of ER in normal and tumor cells in
biopsy specimens (data not shown). In addition, the normal
expression level of the transcription factors examined in this
study was higher in OSCC tissue samples than in noncancerous tissue
samples. Secondly, high SOX2, KLF4 and brachyury expression is
significantly associated with tumor invasion and metastasis, as
well as decreased disease-specific survival and disease-free
survival, in OSCC patients. Thus, these transcription factors may
be involved in tumor progression, and may represent clinically
useful prognostic markers in OSCC.
In conclusion, the expression of SOX2, KLF4 and
brachyury may present novel prognostic factors in OSCC and thus,
the combined use of these factors and classical prognostic factors,
such as Anneroth score, may improve the accuracy of metastasis
prediction. Therefore, future prospective studies investigating
clinical intervention in OSCC patients with positive SOX2, KLF4 and
brachyury expression are required.
Acknowledgements
This study was supported by Grants-in-Aid from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan (no. 23390465 to Professor Tsuyoshi Sugiura; no. 25861958 to
Dr Yosuke Kobayashi; and no. 25893174 to Dr Satomi Chigita).
References
|
1
|
Slootweg PJ and Eveson JW: Tumours of the
oral cavity and oropharynx. World Health Organization
Classification of Tumours. Pathology & Genetics of Head and
Neck Tumours. Barnes L, Eveson JW, Reichart P and Sidransky D:
(Lyon). IARC Press. 166–167. 2005.
|
|
2
|
Genden EM, Ferlito A, Bradley PJ, Rinaldo
A and Scully C: Neck disease and distant metastases. Oral Oncol.
39:207–212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaichi S, Hasegawa K, Takaya T, et al:
Cell line-dependent differentiation of induced pluripotent stem
cells into cardiomyocytes in mice. Cardiovasc Res. 88:314–323.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chambers I and Tomlinson SR: The
transcriptional foundation of pluripotency. Development.
136:2311–2322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evans PM and Liu C: Roles of Krüppel-like
factor 4 in normal homeostasis cancer and stem cells. Acta Biochim
Biophys Sin (Shanghai). 40:554–564. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu LS, Chan CP, Chen CJ, Lin SH, Lai MT,
Hsu JD, Yeh KT and Soon MS: Decreased Krüppel-like factor 4 (KLF4)
expression may correlate with poor survival in gastric
adenocarcinoma. Med Oncol. 30:6322013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel NV, Ghaleb AM, Nandan MO and Yang
VW: Expression of the tumor suppressor Krüppel-like factor 4 as a
prognostic predictor for colon cancer. Cancer Epidemiol Biomarkers
Prev. 19:2631–2638. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adhikary S and Eilers M: Transcriptional
regulation and transformation by Myc proteins. Nat Rev Mol Cell
Bio. 6:635–645. 2005. View
Article : Google Scholar
|
|
11
|
He W, Li K, Wang F, Qin YR and Fan QX:
Expression of OCT4 in human esophageal squamous cell carcinoma is
significantly associated with poorer prognosis. World J
Gastroenterol. 18:712–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neumann J, Bahr F, Horst D, Kriegl L,
Engel J, Luque RM, Gerhard M, Kirchner T and Jung A: SOX2
expression correlates with lymph-node metastases and distant spread
in right-sided colon cancer. BMC Cancer. 11:5182011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruan J, Wei B, Xu Z, Yang S, Zhou Y, Yu M,
Liang J, Jin K, Huang X, Lu P and Cheng H: Predictive value of SOX2
expression in transurethral resection specimens in patients with T1
bladder cancer. Med Oncol. 30:4452013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Han B, Huang J, Zheng B, Geng Q,
Aziz F and Dong Q: Prognostic significance of OCT4 expression in
adenocarcinoma of the lung. Jpn J Clin Oncol. 40:961–966. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sholl LM, Barletta JA, Yeap BY, Chirieac
LR and Hornick JL: SOX2 protein expression is an independent poor
prognostic indicator in stage I lung adenocarcinoma. Am J Surg
Pathol. 34:1193–1198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vidricaire G, Jardine K and McBurney MW:
Expression of the brachyury gene during mesoderm development in
differentiating embryonal carcinoma cell cultures. Development.
120:115–122. 1994.PubMed/NCBI
|
|
17
|
Kispert A, Herrmann BG, Leptin M and
Reuter R: Homologs of the mouse brachyury gene are involved in the
specification of posterior terminal structures in Drosophila,
Tribolium and Locusta. Genes Dev. 8:2137–2150. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishii K, Shimoda M, Sugiura T, Seki K,
Takahashi M, Abe M, Matsuki R, Inoue Y and Shirasuna K: Involvement
of epithelial-mesenchymal transition in adenoid cystic carcinoma
metastasis. Int J Oncol. 38:921–931. 2011.PubMed/NCBI
|
|
19
|
Shimoda M, Sugiura T, Imajyo I, Ishii K,
Chigita S, Seki K, Kobayashi Y and Shirasuna K: The T-box
transcription factor brachyury regulates epithelial-mesenchymal
transition in association with cancer stem-like cells in adenoid
cystic carcinoma cells. BMC Cancer. 12:3772012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayashi Y, Sugiura T, Imajyo I, Shimoda
M, Ishii K, Akimoto N, Yoshihama N and Mori Y: Knockdown of the
T-box transcription factor brachyury increases sensitivity of
adenoid cystic carcinoma cells to chemotherapy and radiation in
vitro, Implications for a new therapeutic principle. Int J Oncol.
44:1107–1117. 2014.PubMed/NCBI
|
|
21
|
Fernando RI, Litzinger M, Trono P,
Hamilton DH, Schlom J and Palena C: The T-box transcription factor
brachyury promotes epithelial-mesenchymal transition in human tumor
cells. J Clin Invest. 120:533–544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Imajyo I, Sugiura T, Kobayashi Y, Shimoda
M, Ishii K, Akimoto N, Yoshihama N, Kobayashi I and Mori Y: T-box
transcription factor brachyury expression is correlated with
epithelial-mesenchymal transition and lymph node metastasis in oral
squamous cell carcinoma. Int J Oncol. 41:1985–1995. 2012.PubMed/NCBI
|
|
23
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours (7th). Hoboken, NJ:
Wiley-Blackwell. 2009.
|
|
24
|
Pindborg JJ, Reichart PA, Smith CJ and van
der Waal I: World Health Organization International Histological
Classification of Tumours. Histological Typing of Cancer and
Precancer of the Oral Mucosa (Berlin). Springer-Verlag. 1997.
View Article : Google Scholar
|
|
25
|
Anneroth G, Hansen LS and Silverman S Jr:
Malignancy grading in oral squamous cell carcinoma. I. Squamous
cell carcinoma of the tongue and floor of mouth Histologic grading
in the clinical evaluation. J Oral Pathol. 15:162–168. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anneroth G, Batsakis J and Luna M: Review
of the literature and a recommended system of malignancy grading in
oral squamous cell carcinomas. Scand J Dent Res. 95:229–249.
1987.PubMed/NCBI
|
|
27
|
Nomura A, Banerjee S, Chugh R, Dudeja V,
Yamamoto M, Vickers SM and Saluja AK: CD133 initiates tumors,
induces epithelial-mesenchymal transition and increases metastasis
in pancreatic cancer. Oncotarget. 6:8313–8322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
González-Moles MA, Scully C, Ruiz-Ávila I
and Plaza-Campillo JJ: The cancer stem cell hypothesis applied to
oral carcinoma. Oral Oncol. 49:738–746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu CC, Hu FW, Yu CH and Chou MY: Targeting
CD133 in the enhancement of chemosensitivity in oral squamous cell
carcinoma-derived side population cancer stem cells. Head Neck Dec.
24:2014.(Epub ahead of print).
|
|
30
|
Patel SS, Shah KA, Shah MJ, Kothari KC and
Rawal RM: Cancer stem cells and stemness markers in oral squamous
cell carcinomas. Asian Pac J Cancer Prev. 15:8549–8556. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu K, Lin B, Zhao M, Yang X, Chen M, Gao
A, Liu F, Que J and Lan X: The multiple roles for Sox2 in stem cell
maintenance and tumorigenesis. Cell Signal. 25:1264–1271. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen S, Xu Y, Chen Y, Li X, Mou W, Wang L,
Liu Y, Reisfield RA, Xiang R, Xiang R, Lv D and Li N: SOX2 gene
regulates the transcriptional network of oncogenes and affects
tumorigenesis of human lung cancer cells. PLoS One. 7:e363262012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Forghanifard MM, Khales Ardalan S,
Javdani-Mallak A, Rad A, Farshchian M and Abbaszadegan MR: Stemness
state regulators SALL4 and SOX2 are involved in progression and
invasiveness of esophageal squamous cell carcinoma. Med Oncol.
31:9222014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lengerke C, Fehm T, Kurth R, Neubauer H,
Scheble V, Müller F, Schneider F, Petersen K, Wallwiener D, Kanz L,
et al: Expression of the embryonic stem cell marker SOX2 in
early-stage breast carcinoma. BMC Cancer. 11:422011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kitamura H, Torigoe T, Hirohashi Y,
Asanuma H, Inoue R, Nishida S, Tanaka T, Fukuta F, Masumori N, Sato
N and Tsukamoto T: Prognostic impact of the expression of ALDH1 and
SOX2 in urothelial cancer of the upper urinary tract. Mod Pathol.
26:117–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boumahdi S, Driessens G, Lapouge G, Rorive
S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E,
et al: SOX2 controls tumour initiation and cancer stem-cell
functions in squamous-cell carcinoma. Nature. 511:246–250. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Episkopou V: SOX2 functions in adult
neural stem cells. Trends Neurosci. 28:219–221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tai SK, Yang MH, Chang SY, Chang YC, Li
WY, Tsai TL, Wang YF, Chu PY and Hsieh SL: Persistent Krüppel-like
factor 4 expression predicts progression and poor prognosis of head
and neck squamous cell carcinoma. Cancer Sci. 102:895–902. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pandya AY, Talley LI, Frost AR, Fitzgerald
TJ, Trivedi V, Chakravarthy M, Chhieng DC, Grizzle WE, Engler JA,
Krontiras H, et al: Nuclear localization of KLF4 is associated with
an aggressive phenotype in early-stage breast cancer. Clin Cancer
Res. 10:2709–2719. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen BJ, Wu YL, Tanaka Y and Zhang W:
Small molecules targeting c-Myc oncogene, Promising anti-cancer
therapeutics. Int J Biol Sci. 10:1084–1096. 2014. View Article : Google Scholar : PubMed/NCBI
|