Introduction
In recent years, due to improvements in cancer
diagnosis and treatment, the survival time of cancer patients has
been prolonged considerably. However, the incidence of a second
primary malignancy has also increased. In the majority of cases,
the secondary cancer occurs >6 months after the primary cancer
(1). The precise pathogenesis remains
unknown; however, it may be associated with the radio- and
chemotherapy used to treat the initial tumor (1). The colon is the most frequently involved
organ. Ueno et al identified that colon cancer has the
second highest risk of secondary malignancies (2). The New South Wales Central Cancer
Registry also revealed that following rectal cancer, the risk of
repeat occurrence of colon cancer, prostate and pancreas cancer,
and particularly adenocarcinoma is increased, although the
occurrence of malignant lymphoma following colorectal cancer
surgery is rare (3). Here we report a
case of primary diffuse large B-cell lymphoma (DLBCL) of the colon
in a 66-year-old male, five years after he received rectal cancer
surgery. Written informed consent was obtained from the
patient.
Case report
A 66-year-old male was admitted to the anorectal
department of The First Affiliated Hospital of Zhejiang University
School of Medicine (Hangzhou, China) on October 22, 2013, due to
abdominal pain for a week, and stool changes that had started a few
weeks earlier, and become more noticeable recently. No family
cancer history and no fever were noted. Physical examination
revealed a mass in the right lower quadrant and old surgical scars,
with tenderness on palpation. There was no enlargement of the lymph
nodes and hepatosplenomegaly on palpation. It was noted from the
patient's medical records that he had undergone partial resection
of the rectum for Dukes C colorectal cancer (pT3N2M0, restaging by
the American Joint Committee on Cancer staging system, 7th edition)
in October 2008. The patient had been regularly followed up as
recommended by the National Comprehensive Cancer Network practice
guidelines and was considered to be free of disease from March
2012.
Under the suspicion of colon cancer recurrence, an
abdominal computed tomography (CT) scan was performed. The scan
revealed notable thickening in the middle of the ascending colon
wall. Lumen stenosis as well as a grainy high density shadow in the
fat space around the colon were observed as well as an increased
lymph shadow. There was no hepatomegaly, splenomegaly or metastasis
(Fig. 1). While evaluating the
evidence of recurrence, colonoscopy was scheduled. An
ulcerofungating mass of 3×3 cm was detected in the cecum, which
extended to the ascending colon. Nonspecific inflammation was
denoted in a pathology specimen taken via endoscopy. The patient
underwent colon resection and enterolysis; he was discharged on the
fifth postoperative day.
The specimen from the laparoscopic right
hemicolectomy demonstrated an ulcerative mass, measuring 8×5 cm.
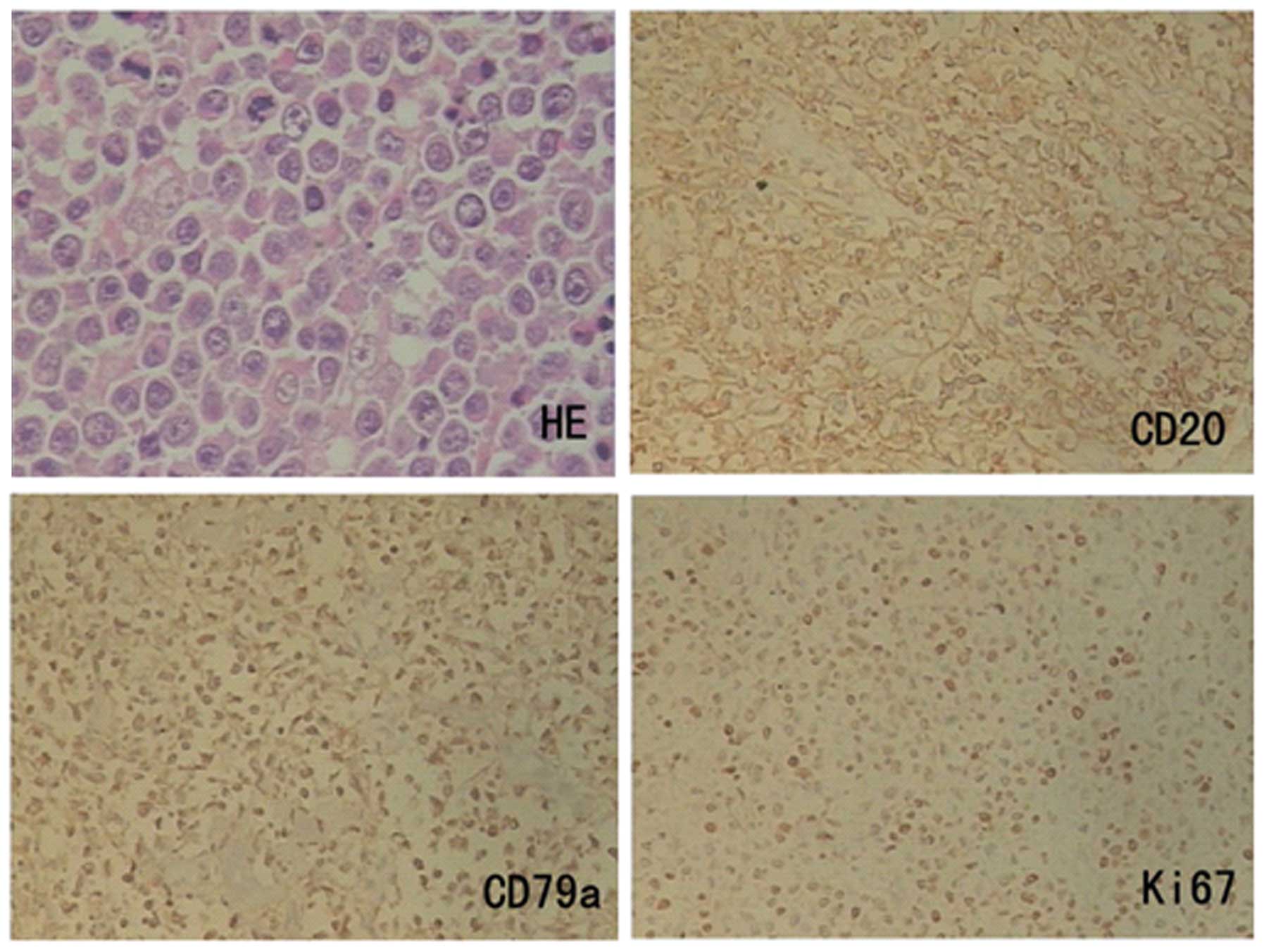
Microscopic examination revealed an ulcerative tumor composed of
sheets of large pleomorphic lymphoid cells with nuclei of different
sizes, nucleoli and mitotic phases visible in most cells. On
histochemical study, the tumor cells were positively stained for
CD45, CD20 and CD79a diffusely, but negative for CD3, CD5, Bcl-2,
Bcl-6 and ALK (Fig. 2). The Ki-67
proliferation index was 40% (Fig. 2),
and in situ hybridization revealed negative Epstein-Barr
virus, indicating DLBCL, non-germinal center B-type. Tumoral
infiltration was detected in two of the 21 lymph nodes dissected
from the intestinal meso.
The patient was admitted to the Department of
Hematology, The First Affiliated Hospital of Zhejiang University.
Hematological examination on admission revealed a white blood cell
count of 7900/m3 with normal differential, a hemoglobin
level of 125 g/l, and a platelet count of 204,000/mm3.
The serum LDH level was 209 U/l (normal), and bone marrow biopsy
reveled no lymphoma involvement. A positron emission tomography
(PET) scan was performed to stage the disease. Increased
flurodeoxyglucose uptake was noted in the distal colon tissue from
the anastomotic site, indicating inflammation, but a neoplasm could
not be excluded. The patient was given four courses of
cyclophosphamide, doxorubicin, vincristine and prednisone and
rituximab (R-CHOP) chemotherapy, then the PET scan was performed
again. No increased flurodeoxyglucose uptake was noted, and another
two courses of R-CHOP were administered. Rituximab was administered
as maintenance therapy every month. The chemotherapy course is now
completed and the patient is being followed up.
Discussion
Colorectal lymphoma constitutes 6–12% of all
gastrointestinal lymphomas (4). The
involvement of most colorectal lymphomas is normally secondary to
widespread diseases. Primary colorectal lymphoma is extremely rare,
constituting only 0.2% of all malignant tumors arising from the
colorectal region, with the cecum, ascending colon and rectum most
commonly affected (5). Our case
fulfilled Dawson's criteria which was used for labeling primary
gastrointestinal lymphoma, but his history of colorectal cancer
made it an even rarer disease. According to the literature, there
have only been three cases of malignant lymphoma following
colorectal cancer. Ikeda et al reported a case of peripheral
T-cell lymphoma developing at the ileocolonic anastomosis site two
years after colectomy for adenocarcinoma (6), and Liao et al reported on a
patient who received concurrent chemoradiotherapy for sigmoid
adenocarcinoma and developed mantle cell lymphoma in the duodenal
bulb 20 months later (7). Shaheen and
Guddati reported a case of secondary mucosa-associated lymphoid
tissue lymphoma of the colon (8).
However, no cases of DLBCL following colorectal cancer have been
reported until now. The etiology is unknown, but the risk factors
involved may include tobacco and alcohol intake, infections and
immunosuppression, genetic predisposition, and toxic effects
related to treatment by chemotherapy or radiotherapy (9).
Colorectal lymphoma predominantly affects males in
their 40s to 60s, and presents as abdominal pain, loss of weight,
palpable abdominal mass or lower gastrointestinal bleeding
(4,5).
Obstruction and perforation are relatively rare in patients with
colorectal lymphoma (10). Lymphoma
of the colorectal region is mostly of B-cell lineage, as with other
sites of the gastrointestinal tract. Endoscopically, lymphoma
appears as fungating, ulcerative, infiltrative, ulcerofungating or
ulcer infiltrative type, with fungating and ulcerofungating types
being most common (11). The
radiological appearance of colorectal lymphoma is variable and
significantly overlaps with other benign and malignant conditions
of the colorectal region. It was therefore difficult to
differentiate it from adenocarcinoma recurrence in our case.
Treatment for colorectal lymphoma usually involves
surgery and chemotherapy. Currently, due to the introduction of new
active drugs as monoclonal antibodies like rituximab as part of
chemotherapy treatment (12), the
role of surgery is debatable. Certain authors propose that surgery
may be beneficial to prevent perforation or bleeding (13), and Huang et al proposed that
radical surgery could significantly increase patients' overall
survival rate, compared with chemotherapy alone and palliative
surgery (14). However, other authors
have suggested that early diagnosis and chemotherapy might avoid a
surgical procedure. Pascual et al suggested that surgery may
be beneficial in patients at risk of complications including
hemorrhage, obstruction and perforation, but it should be
associated with postoperative chemotherapy (15). In our case, the patient underwent
surgery and suffered serious postoperative diarrhea. Unfortunately,
there are no controlled trials providing evidence of the optimal
therapeutic approach in patients with primary colorectal
lymphoma.
The survival rate of colorectal lymphoma is poor.
The nonspecific symptoms make this entity difficult to diagnose
and, in the majority of cases, the disease is advanced at the time
of the first treatment. Histological grade and need for emergency
surgery appear to be factors affecting survival.
In conclusion, lymphoma of the colon and rectum is a
rare tumor. A second DLBCL following successful resection of the
primary adenocarcinoma of the rectum has never been previously
reported. A combination of chemotherapy and surgery appears to be
the most effective treatment for the majority of patients. However,
the optimal management of lymphoma of the colon and rectum has not
been established. This case illustrates the need for heightened
awareness of the possibility of development of secondary
malignancies, particularly lymphoma, following hemicolectomy, and
not only recurrence of the original cancer.
References
|
1
|
Morton LM, Swerdlow AJ, Schaapveld M,
Ramadan S, Hodgson DC, Radford J and van Leeuwen FE: Current
knowledge and future research directions in treatment-related
second primary malignancies. EJC Suppl. 12:5–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ueno M, Muto T, Oya M, Ota H, Azekura K
and Yamaguchi T: Multiple primary cancer: an experience at the
Cancer Institute Hospital with special reference to colorectal
cancer. Int J Clin Oncol. 8:162–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cooperberg MR and Fiedler PN: Ki-1
anaplastic large-cell lymphoma occurring at the site of ileocolonic
anastomosis in a patient treated surgically for colonic
adenocarcinoma: case report and review of the literature. Ann Diagn
Pathol. 5:162–167. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dionigi G, Annoni M, Rovera F, Boni L,
Villa F, Castano P, Bianchi V and Dionigi R: Primary colorectal
lymphomas: review of the literature. Surg Oncol. 16(Suppl 1):
S169–S171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stanojevic GZ, Nestorovic MD, Brankovic
BR, Stojanovic MP, Jovanovic MM and Radojkovic MD: Primary
colorectal lymphoma: an overview. World J Gastrointest Oncol.
3:14–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ikeda J, Yamauchi A, Hoshida Y, Okamura S,
Hashimoto K, Aozasa K and Morii E: Peripheral T-cell lymphoma
developing at ileocolonic anastomosis site after colectomy for
adenocarcinoma. Pathol Res Pract. 206:376–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liao MT, Cheng MF, Chang WC, Wu YC, Lee HS
and Tsai SH: Duodenal mantle cell lymphoma in a patient with
advanced sigmoid adenocarcinoma. South Med J. 102:429–431. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shaheen S and Guddati AK: Secondary
mucosa-associated lymphoid tissue (MALT) lymphoma of the colon. Med
Oncol. 30:5022013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui Y, Liu T, Zhou Y, Ji Y, Hou Y, Jin W
and Feng Y: Five cases report of solid tumor synchronously with
hematologic malignancy. Cancer Res Treat. 44:63–68. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonzalez QH, Heslin MJ, Dávila-Cervantes
A, Alvarez-Tostado J, de los Monteros AE, Shore G and Vickers P:
Primary colonic lymphoma. Am Surg. 74:214–216. 2008.PubMed/NCBI
|
|
11
|
Myung SJ, Joo KR, Yang SK, Jung HY, Chang
HS, Lee HJ, Hong WS, Kim JH, Min YI, Kim HC, et al:
Clinicopathologic features of ileocolonic malignant lymphoma:
analysis according to colonoscopic classification. Gastrointest
Endosc. 57:343–347. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salar A, Domingo-Domenech E, Estany C,
Canales MA, Gallardo F, Servitje O, Fraile G and Montalbán C:
Combination therapy with rituximab and intravenous or oral
fludarabine in the first-line, systemic treatment of patients with
extranodal marginal zone B-cell lymphoma of the mucosa-associated
lymphoid tissue type. Cancer. 115:5210–5217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bairey O, Ruchlemer R and Shpilberg O:
Non-Hodgkin's lymphomas of the colon. Isr Med Assoc J. 8:832–835.
2006.PubMed/NCBI
|
|
14
|
Huang S, Zheng ZX, Xu Q and Yuan XH:
Diagnosis and treatment of primary colorectal non-Hodgkin's
lymphoma: Analysis of 52 cases. Zhonghua Zhong Liu Za Zhi.
35:305–308. 2013.(In Chinese). PubMed/NCBI
|
|
15
|
Pascual M, Sánchez-González B, García M,
Pera M and Grande L: Primary lymphoma of the colon. Rev Esp Enferm
Dig. 105:74–78. 2013. View Article : Google Scholar : PubMed/NCBI
|