Introduction
Lung cancer is the most frequent cause of
cancer-associated mortality among men and women globally (1). The skeletal system is one of the most
common sites of metastasis. A large number of lung cancer patients
do not have the option to undergo surgery at the time of initial
diagnosis due to the presence of metastases. In total, 30–40% of
patients exhibiting advanced lung cancer go on to develop skeletal
metastases, which may cause skeletal-related events (SREs),
including spinal cord compression, severe bone pain, pathological
fractures and potentially life-threatening hypercalcemia of
malignancy, requiring treatment with consecutive orthopedic surgery
or palliative radiotherapy (2,3). In
general, the majority of these patients are treated using systemic
therapy or a symptom-based palliative approach. Overall, median
survival rates are poor, ranging between 4 and 11 months (4). However, ~7% of patients exhibiting
metastatic non-small cell lung cancer (NSCLC) will present with
solitary metastasis following a complete evaluation (5).
According to the guidelines for NSCLC published by
the National Comprehensive Cancer Network (6), the therapeutic strategies for solitary
brain or adrenal metastasis are easily identified. Synchronous
brain or adrenal metastasectomy is the recommended treatment if the
tumor in the lung can be totally resected (7–9). However,
we could not identify any suggestions for the appropriate
synchronous treatment of solitary bone metastasis and NSCLC. A
limited number of case reports concerning a synchronous bone
metastasectomy and lung tumor resection were identified in Japanese
studies. In these studies, patients achieved long-term survival
following synchronous bone metastasectomies and lung tumor
resections (10–12). Bae et al (13) demonstrated that although solitary bone
metastasis occurred much less frequently than metastasis at two or
more sites, it was a predictor of a good prognosis.
The present study attempted to assess the safety and
effectiveness of a synchronous complete lung cancer resection and
solitary bone metastasectomy. Perioperative indicators and
progression-free survival (PFS) were analyzed. In addition,
post-operative survival time was observed.
Materials and methods
Patient clinical characteristics
A total of 5 NSCLC patients exhibiting solitary bone
metastasis were enrolled into the present study between October
2009 and November 2011. Prior to surgery, patients underwent a
series of standard clinical examinations, including
fluorodeoxyglucose-positron emission tomography-computed tomography
(18FDG-PET-CT) or bone scintigraphy and brain magnetic
resonance imaging, in order to demonstrate the presence of solitary
bone metastasis and to exclude the presence of metastasis in other
organs. The study was approved by the ethics committee of Shanghai
Sixth People's Hospital (Shanghai, China) and written informed
consent was obtained from all patients.
Treatment planning
Bone metastases were assessed using PET-CT and
isotope bone scans by orthopedists and identified as being
resectable. Lung tumors were additionally observed to be completely
resectable. Metastases/tumors were considered resectable if they
were isolated, without invasion of the vital organs, and could be
removed completely with wide resection margins. All patients were
informed of the risks and significance of synchronous surgeries and
accepted the therapeutic schedule. For primary lung tumors,
thoracotomy or video-assisted thoracoscopic surgery (VATS)
lobectomies and systematic mediastinal lymph node resections were
performed. For bone metastases, limb metastases were resected and
reconstructed using prosthetic replacement. Spine metastases were
resected and also underwent an internal fixation or interventional
embolization.
Following tumor resection, all patients were
administered four 4-week cycles of standard first-line chemotherapy
(75 mg/m2 docetaxel, day 1; 75 mg/2
cisplatin, day 1) and bisphosphonate (4 mg Zometa) treatment.
Perioperative indicators, including time of thoracic drainage,
incidence of post-operative complications, length of hospital stay
and PFS, were observed. Data is expressed as the median ± standard
deviation.
Results
Patient clinical characteristics
Table I summarizes the
detailed clinical characteristics of each patient enrolled in the
present study. The histologies of the lung cancer cases included 4
patients with adenocarcinoma and 1 patient with large cell
carcinoma. A total of 2 patients had lung masses located in the
right upper lobe and 3 had lung masses located in the middle lobe.
In addition, 2 patients possessed metastasis to the spine and
exhibited an SRE of spinal cord compression. The remaining 3
patients demonstrated no SREs, however, exhibited a solitary
metastasis to the right scapula, left humerus and right femur,
respectively. In total, 2 patients underwent thoracotomy
procedures, and 3 underwent VATS. Systematic mediastinal lymph node
resection is essential for lobectomy, however, lymph node
positivity was not identified in the patients in the present
study.
 | Table I.General clinical characteristics of
patients. |
Table I.
General clinical characteristics of
patients.
|
| Disease location | Type of
resection |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Patient no. | Primary | Metastasis | Primary | Metastasis | Pathology | Lymph node
positivity | PFS, months | Survival time,
months |
|---|
| 1 | RUL | R scapula | VATS lobectomy | BR | Adenocarcinoma | No | 26 |
35+a |
| 2 | ML | T9 spine | Lobectomy | TR+IF | Adenocarcinoma | No | 6 | 8 |
| 3 | ML | T4 spine | Lobectomy | TR+IF | LCC | No | 10 | 12 |
| 4 | RUL | L humerus | VATS lobectomy |
TR+PR | Adenocarcinoma | No | 14 |
14+a |
| 5 | ML | R femur | VATS lobectomy |
TR+PR | Adenocarcinoma | No | 10 |
10+a |
Imaging results
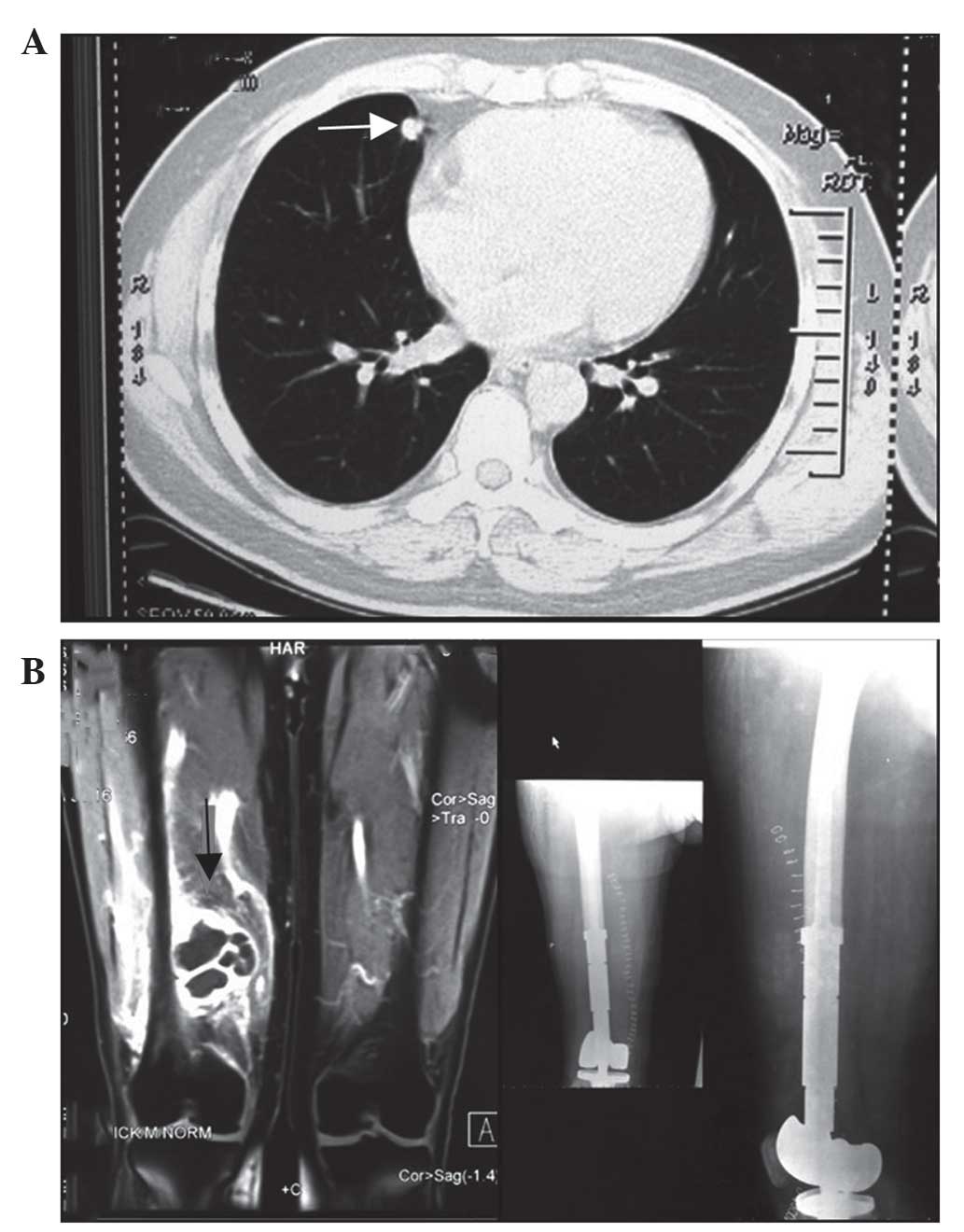
Fig. 1 shows images
captured following metastasectomy in a 47-year-old male patient
presenting with a middle lobe nodule and right femur metastasis.
The patient underwent a synchronous lobectomy and metastasectomy,
and reconstruction with a prosthetic replacement in April 2011. A
total of two lesions identified in the patient were pathologically
proven to be adenocarcinoma. Additional distant bone metastases
were identified in the patient 10 months after the initial surgery,
therefore further chemotherapy (75 mg/m2 docetaxel, day
1; 75 mg/2 cisplatin, day 1; four 4-week cycles) was
administered and the patient remains under follow-up.
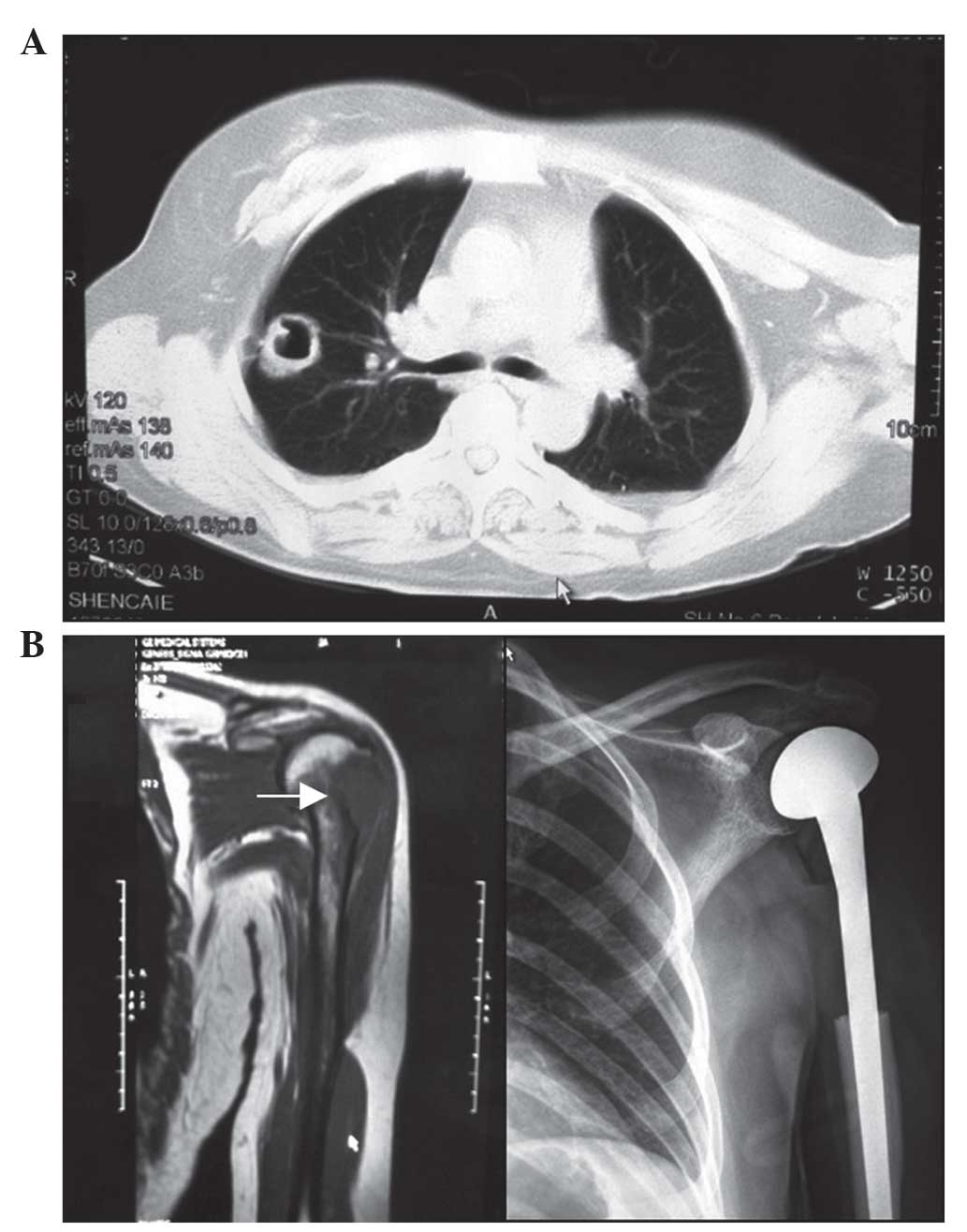
Fig. 2 shows images
captured following metastasectomy in a 47-year-old woman presenting
with a right upper lobe mass and left humeral lesion. The patient
underwent a synchronous surgery and reconstruction with prosthetic
replacement in September 2011. The lesions identified in the
patient were pathologically proven to be adenocarcinoma. For
cancers that begin in glandular secretory cells, a
well-differentiated low grade tumor resembles the normal glandular
structure. Poorly-differentiated high grade adenocarcinomas do not
resemble normal glands and may be detected by positive staining for
mucin, which the glands produce. Adenocarcinoma may also be
distinguished by positive staining for transcription termination
factor, RNA polymerase I, a cell marker for adenocarcinoma. In the
present study, the cancer cells formed matured tubular, acinus
structure or columnar cells lining the papillary structure, or the
lesions exhibited mucus secretion. Diagnosis of the lesions was
based on the observation that the cancerous tissues possessed
characteristics of adenoid differentiation. The patient survived
for 14 months with no evidence of recurrence and remains under
follow-up.
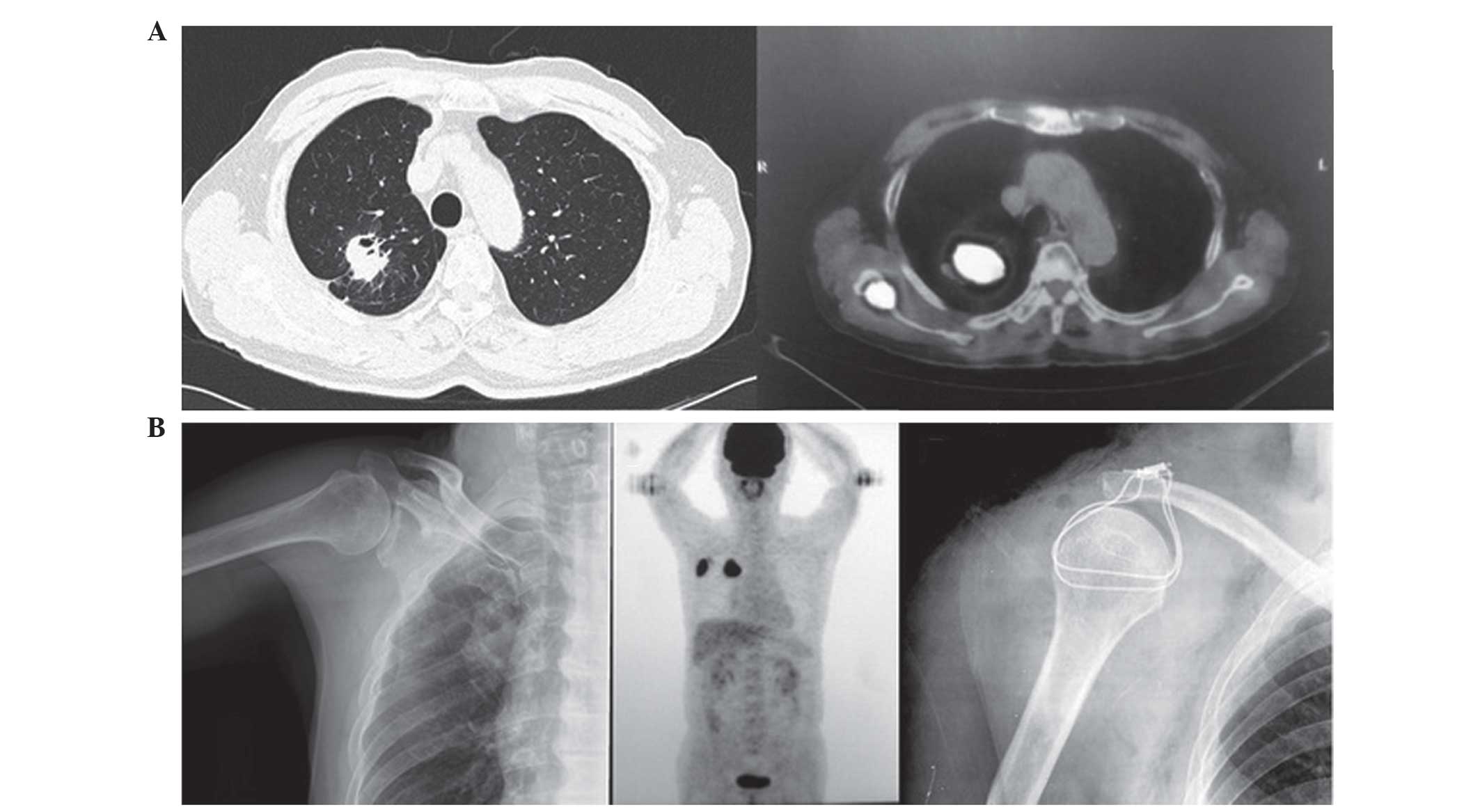
Fig. 3 shows images
captured from a 72-year-old man presenting with a right upper lobe
mass and right scapula metastasis. It was difficult to identify
metastasis using X-ray imaging, however, these difficulties were
simplified by using 18FDG-PET-CT scanning. The patient
underwent a VATS lobectomy and right scapula resection in September
2009, and had a PFS time of 26 months. The patient remains alive
following the administration of multimodal therapy with thymosin (4
mg, twice a week) for 3 months and Traditional Chinese Medicine for
6 months. 18FDG-PET-CT demonstrates increased diagnostic
value (sensitivity and specificity) for the diagnosis of bone
metastasis from lung cancer compared with alternative imaging
methods (14).
Surgical outcomes
Additional perioperative data is summarized in
Table II. The median patient age was
54.6 years, with a range of 47–72 years. All procedures were
performed safely, and without the occurrence of serious
complications. The median thoracic drainage time was 4.6±1.1 days,
and the length of post-operative hospitalization was 8.8±2.2 days.
The PFS time of the patients was 13.2±7.7 months. All patients were
administered standard first-line chemotherapy and bisphosphonate
treatment following surgery. The 2 patients who initially exhibited
spinal metastasis, demonstrated malignant pericardial and thoracic
effusion without paralysis ~1 year subsequent to the initial
surgery and rapidly succumbed to the disease. The remaining 3
patients exhibiting limb bone metastases experienced an increased
average PFS time of ~16 months. These patients have survived for
>2 years following the initial surgery and remain under
follow-up. The post-operative survival time of all patients was
15.8±10.9 months.
 | Table II.Surgical features of patients
(n=5). |
Table II.
Surgical features of patients
(n=5).
| Surgical feature | Value |
|---|
| Median age (range),
years | 54.6 (47–72) |
| Gender |
|
| Male | 4 |
|
Female | 1 |
| Thoracic drainage,
days | 4.6±1.1 |
| Post-operative
hospital stay, days | 8.8±2.2 |
| PFS, months | 12.8±7.7 |
| Post-operative
survival, months | 15.8±10.9 |
Discussion
Following more than a decade of investigation, the
treatment of synchronous single-organ metastatic NSCLC remains
under debate. Considering the low incidence of this particular
presentation of NSCLC, it remains unlikely that large prospective
randomized studies will be conducted. Therefore, knowledge has to
be constructed from the results of retrospective studies (5). Bone metastasis identified at the initial
diagnosis of NSCLC typically indicates the possibility of
consequent tumor spread and a poor patient prognosis (15). Decroisette et al (16) reported a poor prognosis among NSCLC
patients exhibiting bone metastatic disease. In the study, the
median survival time was observed to be 5.8 months, and the 1- and
2-year survival rates were 22 and 7%, respectively. There was no
significant difference observed in overall survival between the
patients with and without SRE at enrollment (16). In a study performed by Sugiura et
al (17), the cumulative survival
rate following bone metastasis was observed to be 59.9% for 6-month
survival, 31.6% for 1-year survival and 11.3% for 2-year survival.
The mean survival time was 9.7 months (17).
Among the patients in the present study, a PFS time
of 13.2±7.7 months was obtained. This meant that patients remained
tumor-free for >1 year after surgery. We propose that it is
important to prolong the overall patient survival time, and that
this has a positive effect on patient psychology. In addition, the
average post-operative survival time of 15.8±10.9 months in the
present study was an improvement on the times in aforementioned
studies. In a study performed by Weiss and Wedin (18), patients exhibiting spinal metastases
possessed a significantly poorer prognosis in a patient cohort with
skeletal metastasis. However, 16/31 patients exhibiting spinal
metastases demonstrated a considerable improvement in neurological
function following surgery. In the present study, the 2 patients
exhibiting spinal metastases possessed reduced PFS and
post-operative survival times, however, demonstrated no paralysis
or neurological symptoms prior to mortality. In the present study,
the 2 patients exhibiting extremity metastases complained of pain
prior to surgery, which was subsequently relieved, without loss of
limb function. Orthopedic surgeries may reserve the majority of
function of the extremities and reduce pain, decreasing the rate of
disability and pathological fracture, and improving patient quality
of life (19).
There is currently a general consensus concerning
synchronous metastasectomy of solitary adrenal or brain metastasis
in NSCLC. We propose that solitary bone metastasis should be
considered to have a surgically treatable status. Due to the low
occurrence rate of solitary bone metastasis in NSCLC patients, the
sample investigated in the present study was small, and it was
difficult to achieve a definitive conclusion. However, the present
study demonstrated an acceptable PFS time, and overall survival
results were more positive compared with those achieved with
conservative therapy. In conclusion, an aggressive regimen of
synchronous metastasectomy and primary tumor resection may be
beneficial for selected patients, leading to prolonged survival
times and an improved quality of life.
Acknowledgements
The abstract of the present study was previously
published in the proceedings of the 15th World Conference on Lung
Cancer, Oct 27–31, 2013, in Sydney, and was published as abstract
PL03.01 in J Thorac Oncol 8 (Suppl 2) 2013.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosen LS, Gordon D, Tchekmedyian NS,
Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, De Souza P, Zheng
M, Urbanowitz G, et al: Long-term efficacy and safety of zoledronic
acid in the treatment of skeletal metastases in patients with
nonsmall cell lung carcinoma and other solid tumors: A randomized,
Phase III, double-blind, placebo-controlled trial. Cancer.
100:2613–2621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Price N: Bisphosphonates to prevent
skeletal morbidity in patients with lung cancer with bone
metastases. Clin Lung Cancer. 5:267–269. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Groome PA, Bolejack V, Crowley JJ, Kennedy
C, Krasnik M, Sobin LH and Goldstraw P: IASLC International Staging
Committee; Cancer Research and Biostatistics; Observers to the
Committee; Participating Institutions: The IASLC Lung Cancer
Staging Project: Validation of the proposals for revision of the T,
N, and M descriptors and consequent stage groupings in the
forthcoming (seventh) edition of the TNM classification of
malignant tumours. J Thorac Oncol. 2:694–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pfannschmidt J and Dienemann H: Surgical
treatment of oligometastatic non-small cell lung cancer. Lung
Cancer. 69:251–258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ettinger DS, Wood DE, Akerley W, Bazhenova
LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, D'Amico TA,
Demmy TL, et al: Non-small cell lung cancer, version 6.2015. J Natl
Compr Canc Netw. 13:515–524. 2015.PubMed/NCBI
|
|
7
|
Hu C, Chang EL, Hassenbusch SJ III, Allen
PK, Woo SY, Mahajan A, Komaki R and Liao Z: Nonsmall cell lung
cancer presenting with synchronous solitary brain metastasis.
Cancer. 106:1998–2004. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raviv G, Klein E, Yellin A, Schneebaum S
and Ben-Ari G: Surgical treatment of solitary adrenal metastases
from lung carcinoma. J Surg Oncol. 43:123–124. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reyes L, Parvez Z, Nemoto T, Regal AM and
Takita H: Adrenalectomy for adrenal metastasis from lung carcinoma.
J Surg Oncol. 44:32–34. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Agarwala AK and Hanna NH: Long-term
survival in a patient with stage IV non-small-cell lung carcinoma
after bone metastasectomy. Clin Lung Cancer. 6:367–368. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirano Y, Oda M, Tsunezuka Y, Ishikawa N
and Watanabe G: Long-term survival cases of lung cancer presented
as solitary bone metastasis. Ann Thorac Cardiovasc Surg.
11:401–404. 2005.PubMed/NCBI
|
|
12
|
Ono K, Nagashima A, Yokoyama E, Nose N and
Yasumoto K: Long-term survival after surgical resection of bone
metastasis from lung cancer. Kyobu Geka. 63:216–219. 2010.(In
Japanese). PubMed/NCBI
|
|
13
|
Bae HM, Lee SH, Kim TM, Kim DW, Yang SC,
Wu HG, Kim YW and Heo DS: Prognostic factors for non-small cell
lung cancer with bone metastasis at the time of diagnosis. Lung
Cancer. 77:572–577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qu X, Huang X, Yan W, Wu L and Dai K: A
meta-analysis of 18FDG-PET-CT, 18FDG-PET, MRI
and bone scintigraphy for diagnosis of bone metastases in patients
with lung cancer. Eur J Radiol. 81:1007–1015. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lopes G, Piedade A, Goes L, Alves M and
Balu S: Diagnoses and treatment patterns for Non-small cell lung
cancer (Nsclc) within the private health system in Brazil. Value
Health. 18:A8252015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Decroisette C, Monnet I, Berard H, Quere
G, Le Caer H, Bota S, Audigier-Valette C, Geriniere L, Vernejoux JM
and Chouaid C: Groupe Français de Pneumo-Cancérologie 0601 Team.
Epidemiology and treatment costs of bone metastases from lung
cancer: A French prospective, observational, multicenter study
(GFPC 0601). J Thorac Oncol. 6:576–582. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugiura H, Yamada K, Sugiura T, Hida T and
Mitsudomi T: Predictors of survival in patients with bone
metastasis of lung cancer. Clin Orthop Relat Res. 466:729–736.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weiss RJ and Wedin R: Surgery for skeletal
metastases in lung cancer. Acta Orthop. 82:96–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bao DM, Li N and Xia L: Risk assessment
and decision-making for patients undergoing orthopedic surgery. J
Orthop Surg Res. 10:1692015. View Article : Google Scholar : PubMed/NCBI
|