Introduction
The increased survival of patients with cancer, the
growing life expectancy and the development of improved diagnostic
techniques have all contributed to the increased frequency of
multiple primary malignancies (1). In
a number of cases, it is difficult to differentiate between two
primary neoplasms or metastatic diseases, although the distinction
is significant as the staging, further management and prognosis are
completely different (2). The
distinction between metastatic and independent tumors is also
notable since it affects staging and prognosis differently
(2). In the present case, the
malignant features of each tumor were synchronously confirmed by
post-operative pathological diagnosis.
Currently, there is no universally accepted,
standard treatment for multiple primary malignancies. The
treatments of choice, depending on the tumor location, include
curative surgical resection of each malignancy, radiotherapy and
chemotherapy (3). The most common
treatment is surgery associated with adjuvant treatment (3). The treatment of synchronous double
cancer generally relies on surgery; however, in surgically
non-resectable tumors, chemotherapy is considered the most
promising form of treatment, targeting each tumor, but
concentrating on the most aggressive (4). In the present case, the treatment of
choice was curative resection of each lesion, and the
post-operative course was uneventful. The patient is followed-up
regularly, with clinical examinations and liver function tests
implemented every 2 months. Additionally, tumor markers [including
α-fetoprotein (AFP), carbohydrate antigen (CA)19-9, CA 125 and
carcinoembryonic antigen (CEA)] have been monitored and abdominal
computed tomography (CT) scans have been performed every 3 months.
The patient is currently healthy, with no evidence of local or
distant recurrence post-surgery.
Case report
A 42-year-old male patient was referred to the
Department of General Surgery of The First Affiliated Hospital of
Henan University of Science and Technology (Luoyang, China) in
August 2014, presenting with poor appetite and abdominal discomfort
in the right upper quadrant for 3 months. In 2004, the patient had
been diagnosed with chronic liver disease secondary to hepatitis B
at The People's Hospital of Yiyang County (Luoyang, China). In
addition, the patient had a previous history of alcohol abuse for
17 years (alcohol intake, 250 g/day) and had smoked for 20 years.
There was no remarkable family history. On admission, the vital
signs were all within the normal ranges (heart rate, 80 beats/min;
normal range, 60–100 beats/min; blood pressure, 130/70 mmHg; normal
range, 60/90–80/140 mmHg, body temperature, 36.5°C; normal range,
36–37.3°C and respiration rate, 20 times/min; normal range, 12–20
times/min). The patient was generally in good health and did not
exhibit any significant weight loss. On physical examination, the
conjunctiva was normal. The abdomen was soft, but tender in the
right upper quadrant; it was noted that there was light resistance
in this area, but no rigidity. The pre-operative serum biochemistry
and complete blood count data on admission were as follows: White
blood cell count, 6.62×109 cells/l (normal range,
4.00–10.00×109 cells/l); hemoglobin count, 132.00 g/l
(normal range, 110.00–160.00 g/l); platelet count,
225.00×109 cells/l (normal range,
100.00–300.00×109 cells/l); blood glucose, 5.20 mmol/l
(normal range, 3.90–6.00 mmol/l); total bilirubin, 17.30 µmol/l
(normal range, 0.00–20.00 µmol/l); aspartate transaminase, 26.00
U/l (normal range, 15.00–45.00 U/l); alanine transaminase, 31.00
U/l (normal range, 9.00–50.00 U/l); alkaline phosphatase, 40.00 U/l
(normal range, 30.00–150.00 U/l); lactic dehydrogenase, 183.00 U/l
(normal range, 90.00–245.00 U/l); serum urea, 5.70 mmol/l (normal
range, 2.60–6.30 mmol/l); serum creatinine, 118.00 µmol/l (normal
range, 40.00–110.00 µmol/l); albumin, 34.80 g/l (normal range,
40.00–55.00 g/l); fibrinogen, 4.67 g/l (normal range, 2.00–4.00
g/l); prothrombin time, 14.20 sec (normal range, 11.00–15.00 sec);
prothrombin time international normalized ratio, 1.19 (normal
range, 0.80–1.20); AFP, 6,050.00 ng/ml (normal range, 0.00–7.00
ng/ml); CEA, 1.86 ng/ml (normal range, 0.00–4.30 ng/ml); CA 19-9,
5.43 U/ml (normal range, 0.00–27.00 U/ml); and CA 125, 36.70 U/ml
(normal range, 0.00–36.00 U/ml). The viral markers were as follows:
Hepatitis B surface antigen, positive; hepatitis B core antibody,
positive; hepatitis Be antibody, positive; and anti-hepatitis C
virus, negative.
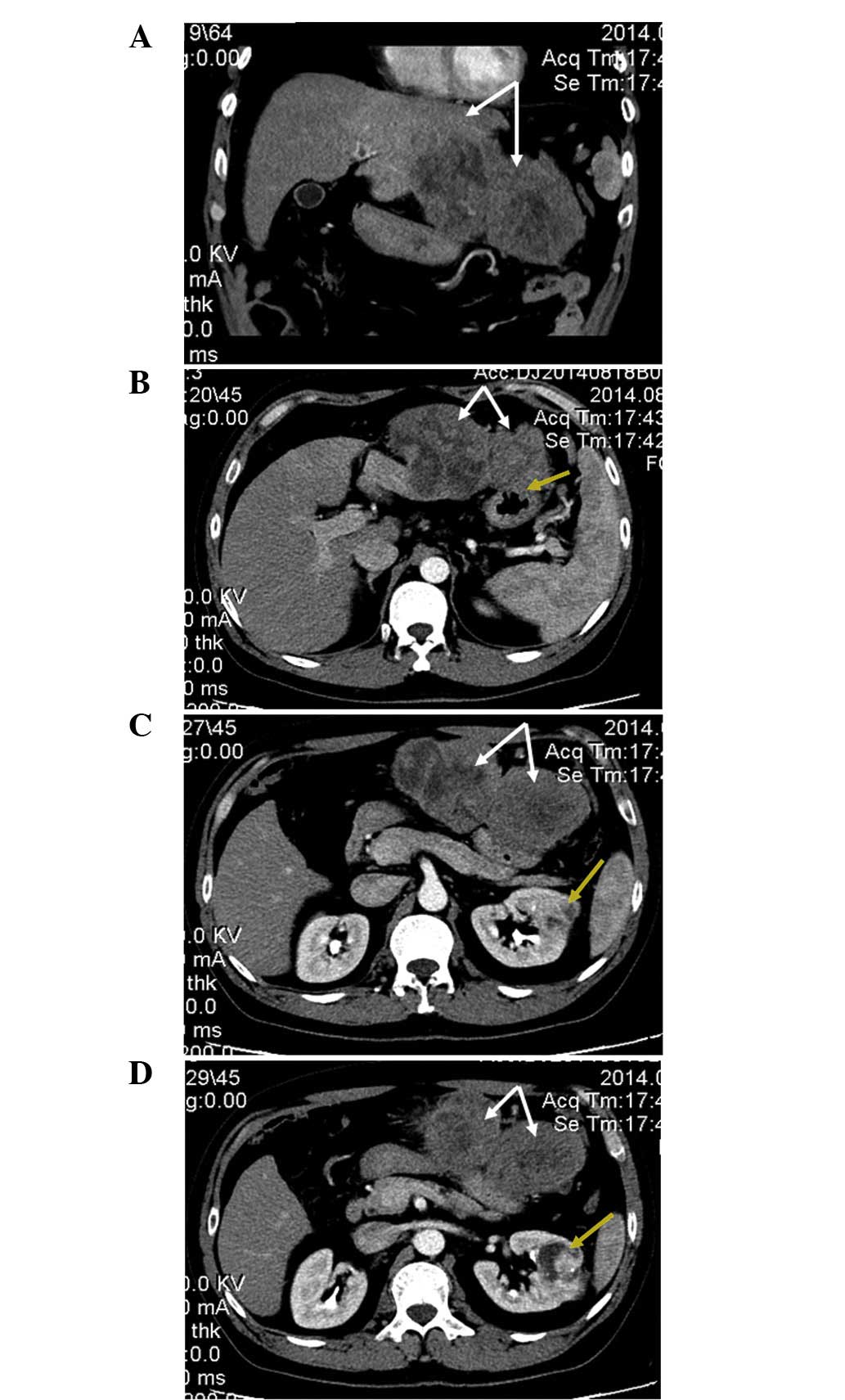
An enhanced CT [TSX-301A; Toshiba Medical Systems
Corp., Tokyo, Japan; contrast medium, iopromide (Bayer AG,
Leverkusen, Germany)] scan of the abdomen revealed a 15.1×7.0-cm,
irregularly-enhanced rim, double-spherical, exogenous, solid tumor
originating from the left lateral hepatic lobe (Fig. 1), which invaded the antrum of the
stomach (Fig. 1B). The CT scan also
revealed a 4.3×4.2-cm, mildly-enhanced, mixed-density mass in the
mid portion of the left kidney (Fig. 1C
and D).
The pre-operative diagnosis was HCC and RCC. A
laparotomy was performed, during which a large dumbbell-shaped
lesion, which originated from the left lateral hepatic lobe, was
observed to occupy the epigastrium and have invaded the antrum of
the stomach. The patient underwent left hemihepatectomy and partial
gastrectomy, in addition to left nephrectomy. Intraoperative
histological examination revealed the presence of HCC, which had
invaded the gastric antrum, alongside RCC. The patient was
subsequently diagnosed with synchronous double primary cancer of
the liver and kidney.
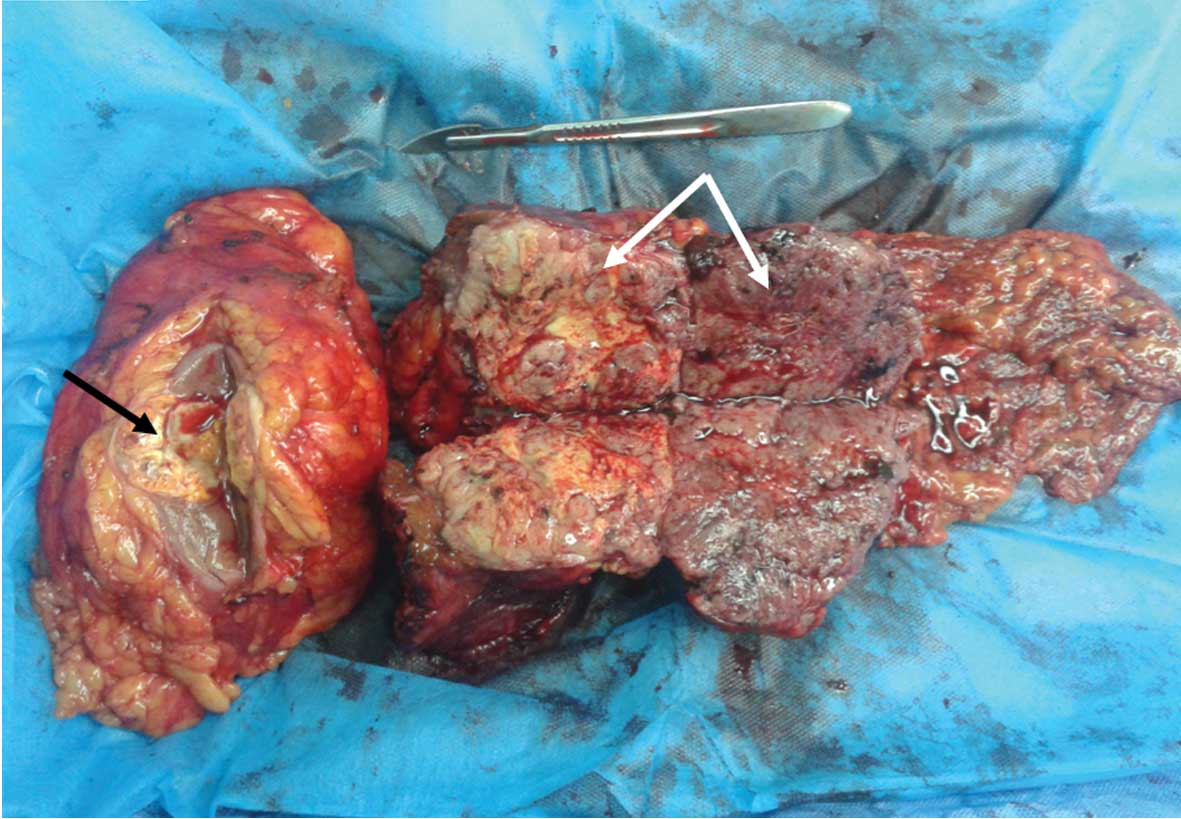
The resected surface of the tumor in the left kidney
revealed a yellow-white, solid lesion that measured 4.0×4.0×4.0 cm
in size (black arrow, Fig. 2). There
was a yellow and light brown, double-spherical, exogenous, solid
tumor that measured 15.0×8.0×7.0 cm in size and originated from the
left lateral lobe of the liver (white arrows, Fig. 2).
For microscopic examination, the gross samples were
sectioned as a tissue mass measuring 1.5×1.5×0.2 cm in size, and
were fixed in 10% formalin at room temperature for 24 h. The
samples were then processed through the Leica ASP300 S Fully
Enclosed Tissue Processor (Leica Microsystems GmbH, Wetzlar,
Germany) and paraffin-embedded by the Tissue Embedding System TES
99 (Medite GmbH, Burgdorf, Germany). The paraffin-embedded tissues
were sectioned into 4 µm slices ready for examination using the HM
325 Rotary Microtome (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). On microscopic examination, the tumor in the mid portion of
the left kidney exhibited diffusely distributed tumor cells with
transparent cytoplasm, karyopyknosis and hyperchromatism. The
dividing lines of the malignant cells were clear and reacted
positively to cluster of differentiation (CD)10 (mouse anti-human
monoclonal antibody; catalog no., MAB-0668; Fuzhou Maixin Biotech
Co., Ltd., Fuzhou, China) and epithelial membrane antigen (mouse
anti-human monoclonal antibody; catalog no., Kit-0011; Fuzhou
Maixin Biotech Co., Ltd.) by immunostaining. The tumor originating
from the left lateral hepatic lobe exhibited cell clusters with a
flake-like distribution, which were separated by a sinusoid blood
vessel. The dividing line of the malignant cells was not clear, and
their cytoplasm was eosinophilic. In addition, the cells exhibited
large, heteromorphic, eosinophilic nucleoli. Immunostaining
demonstrated a positive reaction for hepatocyte-specific antigens
(mouse anti-human monoclonal antibody; catalog no., MAB-0249;
Fuzhou Maixin Biotech Co., Ltd.) and CD34 (mouse anti-human
monoclonal antibody; catalog no., Kit-0004; Fuzhou Maixin Biotech
Co., Ltd.). The left kidney tumor was diagnosed as
moderately-differentiated ccRCC, and the left hepatic lobe tumor
was diagnosed as poorly-differentiated HCC (Fig. 3).
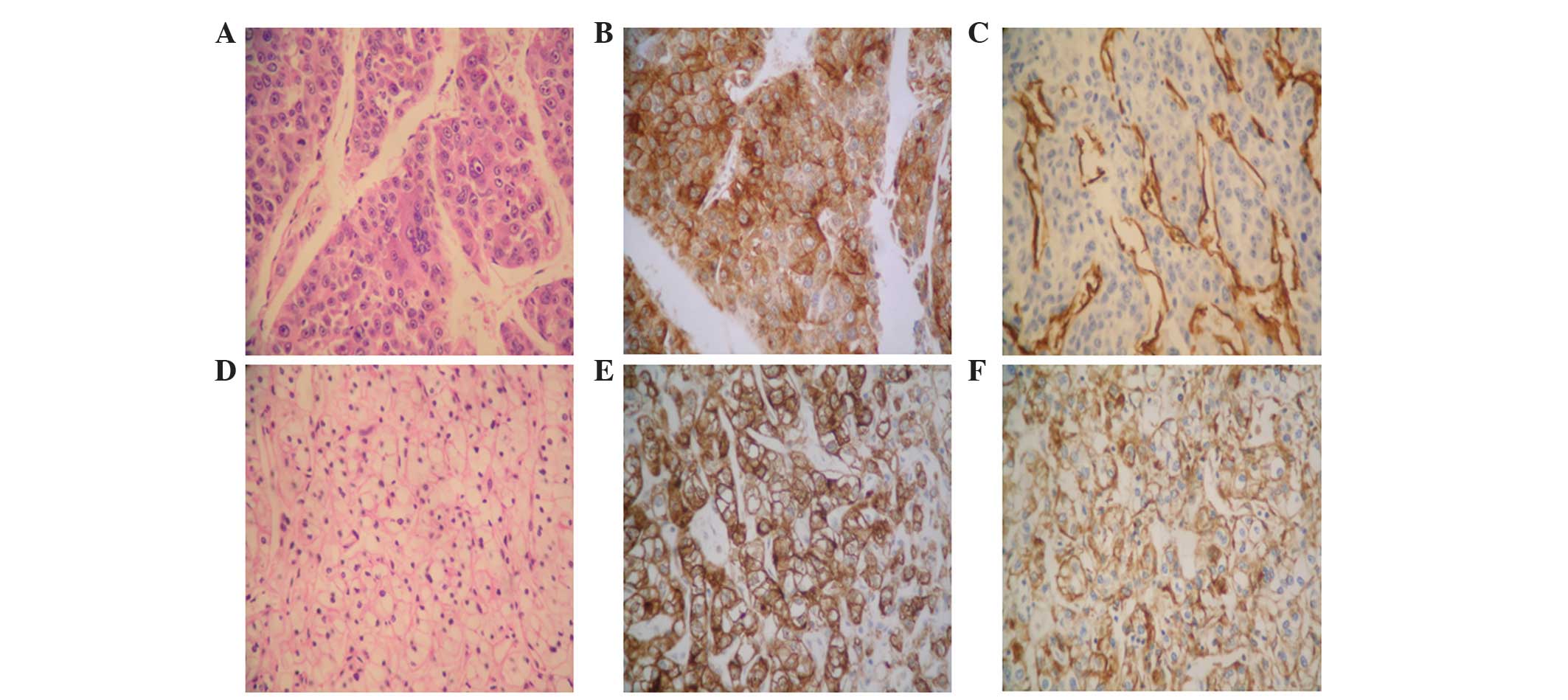 | Figure 3.(A-C) Histological analysis of the
resected HCC tumor tissues. (A) Poorly-differentiated HCC
exhibiting cell clusters with a flake-like distribution, which were
separated by a sinusoid blood vessel. The dividing line of the
malignant cells was not clear, and their cytoplasm was
eosinophilic. In addition, the cells exhibited large,
heteromorphic, eosinophilic nucleoli (staining, H&E). (B)
Brown/yellow color indicates positive staining for
hepatocyte-specific antigens in the cytoplasm and cellular
membrane. (C) Brown/yellow color indicates positive staining for
CD34 in the vascular endothelial cells. (D-F) Histological analysis
of the resected ccRCC tumor tissues. (D) Moderately-differentiated
ccRCC. The tumor cells were hyperchromatic and presented diffuse
distribution, transparent cytoplasm and karyopyknosis. The dividing
lines of the malignant cells were clear (staining, H&E).
Brown/yellow color indicates positive staining for (E) CD10 and (F)
epithelial membrane antigen in the cytoplasm and cellular membrane.
Magnification, ×400. HCC, hepatocellular carcinoma; H&E,
hematoxylin and eosin; CD, cluster of differentiation; ccRCC, clear
cell renal cell carcinoma. |
The post-operative course was uneventful, and the
patient was discharged on day 22 post-surgery. Adjuvant
chemoradiation therapy was advised, as the HCC tumor was
particularly large (diameter, >10.0 cm) with adjacent viscera
invasion. However, this was not administered, as the patient
declined further treatment. The patient has been followed-up
regularly with clinical examination and liver function tests
implemented every 2 months. Additionally, tumor markers (including
AFP, CA 19-9, CA 125 and CEA) have been monitored and abdominal CT
scans have been performed every 3 months. The patient is currently
healthy, with no evidence of local or distant recurrence
post-surgery.
Discussion
The occurrence of multiple primary malignant tumors
in a single patient is particularly rare, with a literature review
of 1,104,269 patients with cancer reporting the incidence of
multiple primary malignancies as 0.73–11.70% (5). The following diagnostic criteria have
been proposed for the accurate diagnosis of multiple primary
malignancies: i) Each tumor must be distinct; ii) each tumor must
exhibit marked features of malignancy; and iii) the probability of
one lesion being a metastasis of the other must be excluded
(6). Multiple primary malignancies
may be synchronous or metachronous, often depending on the length
of the interval between diagnoses (7). Synchronous multiple malignancies are
secondary lesions that present simultaneously or within 6 months
following the development of the initial malignancy, while
metachronous multiple malignancies are secondary lesions that
present >6 months following the development of the initial
malignancy (8). In the present case,
histopathological analysis confirmed the malignant features of each
tumor. The tumors were pathologically established as different
types of cancer and had developed within different systems, with
the tumor in the kidney confirmed as moderately-differentiated
ccRCC and the tumor in the liver confirmed as poorly-differentiated
HCC. These findings support the notion that these two types of
cancer occurred in a random and synchronous manner. HCC is
understood to be pathogenically associated with chronic hepatitis
virus infection, abuse of alcohol and liver cirrhosis (9). Although the mechanisms underlying the
occurrence of multiple primary malignancies are not fully
understood, certain factors have been implicated, including genetic
factors, carcinogenic viruses, immunological and environmental
factors, and chemical and radiological treatments (10). With regards to the current case,
chronic hepatitis B virus infection may have served a crucial role
in the development of HCC. The prognosis of patients with multiple
primary malignancies may be determined independently by the stage
of each malignancy (11). In the
present case, the treatment of choice was curative resection of
each lesion.
To the best of our knowledge, the present case is
the first of its kind to describe the occurrence of synchronous
double primary cancer of the kidney and liver. When treating
patients with malignant tumors, the possibility of developing a
secondary primary malignancy should be considered. The incidence of
multiple primary malignancies does not necessarily signify an
unfavorable prognosis, as long as satisfactory diagnosis and
effective treatment are performed.
In summary, the present case of synchronous double
primary cancer of the kidney and liver was confirmed by
pathological and immunohistochemical analyses. Imaging findings may
be helpful to assist with achieving a correct preoperative
diagnosis. If in doubt, intraoperative frozen section analysis may
be necessary to select the correct surgical approach. Simultaneous
removal of multiple primary cancers should be attempted, and
adjuvant treatment (radio/chemotherapy) should also be considered.
Healthcare workers should consider that the appearance of an
additional tumor in a cancer patient may be either a metastatic or
novel lesion, and the possibility of a metachronous or a
synchronous malignancy should be investigated. Furthermore,
prolonged follow-up after surgery should be performed.
References
|
1
|
Spratt JS Jr and Hoag MG: Incidence of
multiple primary cancers per man-year of follow up: 20-year review
from the Ellis Fischel State Cancer Hospital. Ann Surg.
164:775–784. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sheu BC, Lin HH, Chen CK, Chao KH, Shun CT
and Huang SC: Synchronous primary carcinomas of the endometrium and
ovary. Int J Gynaecol Obstet. 51:141–146. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Irimie A, Achimas-Cadariu P, Burz C and
Puscas E: Multiple primary malignancies - epidemiological analysis
at a single tertiary institution. J Gastrointestin Liver Dis.
19:69–73. 2010.PubMed/NCBI
|
|
4
|
Kourie HR, Markoutsaki N, Roussel H, Rahmi
G, Van der Stiegel M, Palazzo L, Fabre M, Cuenod CA, Dubreuil O,
Landi B, et al: Double pancreatic and gastric adenocarcinomas: A
rare association. Clin Res Hepatol Gastroenterol. 37:e137–e140.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Demandante CG, Troyer DA and Miles TP:
Multiple primary malignant neoplasms: Case report and a
comprehensive review of the literature. Am J Clin Oncol. 26:79–83.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warren S and Gates O: Multiple primary
malignant tumors: A survey of the literature and a statistical
study. Am J Cancer. 16:1358–1414. 1932.
|
|
7
|
Suzuki T, Takahashi H, Yao K, Inagi K,
Nakayama M, Makoshi T, Nagai H and Okamoto M: Multiple primary
malignancies in the head and neck: A clinical review of 121
patients. Acta Otolaryngol. (Suppl 122): 88–92. 2002. View Article : Google Scholar
|
|
8
|
Yun HR, Yi LJ, Cho YK, Park JH, Cho YB,
Yun SH, Kim HC, Chun HK and Lee WY: Double primary malignancy in
colorectal cancer patients - MSI is the useful marker for
predicting double primary tumors. Int J Colorectal Dis. 24:369–375.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohwada S, Yoshihiro O, Iwazaki S,
Tanahashi Y, Sawada T, Takeyoshi I, Kawashima Y, Nakaura S, Iino Y
and Morishita Y: Double cancer in different hepatic lobes:
Hepatocellular and cholangiocellular carcinoma.
Hepatogastroenterology. 42:411–414. 1995.PubMed/NCBI
|
|
10
|
Tamura M, Shinagawa M and Funaki Y:
Synchronous triple early cancers occurring in the stomach, colon
and gallbladder. Asian J Surg. 26:46–49. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshino K, Asanuma F, Hanatani Y, Kumai K
and Ishibiki K: Statistical studies on multiple primary cancers
including gastric cancers. Gan No Rinsho. 30(Suppl 12):
S1514–S1523. 1984.(In Japanese).
|