Introduction
Multiple myeloma (MM) accounts for ~1% of all tumors
and 13% of hematological malignancy cases (1). The median age of patients at myeloma
diagnosis is 66 years, with diagnosis being particularly rare in
individuals <40 years of age (1).
The diagnosis of normal MM is commonly determined through a
combination of clinical, pathological and radiological techniques
and the subsequent findings (2). MM
is often associated with extensive skeletal destruction, anemia,
hypercalcemia, infections and renal failure (2). However, certain cases presenting with
atypical features may be challenging for hematopathologists to
diagnose (3). The current study
describes a case of immunoglobulin G (IgG) myeloma presenting with
small lymphoid cells. Diagnosis of the case based on morphology
alone proved to be challenging, with flow cytometric
immunophenotyping confirming the cells as monoclonal myeloma cells
with myeloid antigen expression. The patient obtained complete
remission following combination chemotherapy and received
thalidomide as maintenance therapy. Several months later, the
patient suffered a relapse and eventually succumbed to the
disease.
Case report
In May 2012, a 49-year-female was admitted to the
Department of Hematology, Affiliated Hospital of Nanjing University
of Traditional Chinese Medicine (Nanjing, China), presenting with
dizziness, episodic tiredness and a lack of motivation. Physical
examination was unremarkable, with the exception of a cough, chest
tightness and pallor. The hemoglobin and red blood cell counts were
72 g/l (normal range, 110–150 g/l) and 2.34×1012 cells/l
(normal range, 3.5×1012−5.0×1012 cells/l)
respectively, whilst the white blood cell and platelet counts were
within normal ranges. Immunology tests indicated that the IgG κ
paraprotein and β-2-microglobulin levels were 66 g/l (normal range,
6.94–16.20 g/l) and 3,879 µg/l (normal range, 1,035–1,945 µg/l),
respectively. Additionally, on serum free light chain evaluation,
the κ light chain concentration measured 7,960 mg/l (normal range,
629–1,350 mg/dl) and the λ light chain level measured 30 mg/l
(normal range, 313–723 mg/dl), with a κ/λ light chain ratio of
265.33. Osteoporosis was revealed by computed tomography and X-ray
examination.
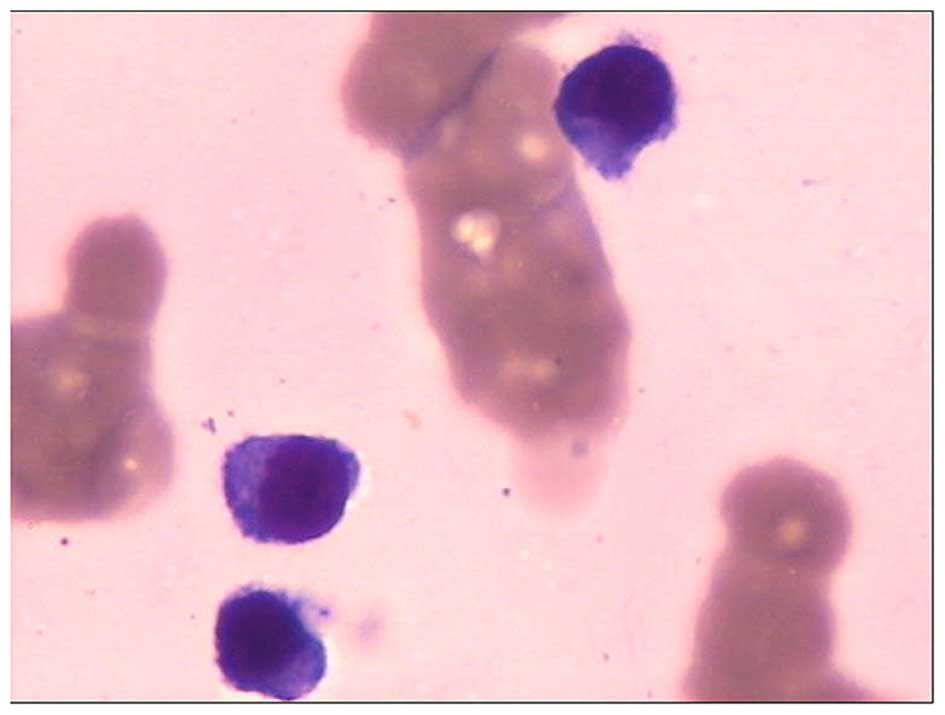
A bone marrow aspiration was performed and the
sample underwent cytological study. Small lymphoid or
lymphoplasmacytic-like plasma cells were observed, and accounted
for 22.5% of all nucleated cells in the bone marrow. The cells had
round nuclei of a uniform size, the chromatin was dense and coarse,
and the cytoplasm was slightly basophilic and scanty, wrapping
closely around the nucleus (Fig. 1).
The antibody expression in the cells was measured using a
multiparameter flow cytometer (FC500 Flow Cytometer; Beckman
Coulter, Inc., Brea, CA, USA). The results demonstrated that the
cells (Fig. 2A) expressed cluster of
differentiation (CD)38 (Fig. 2B) and
CD33 (Fig. 2C). There was also κ
light chain restriction (κ/λ = 13.1) (Fig. 2D, E and F). The majority of the
CD138+ myeloma cells expressed CD19, but lacked CD56
expression (Fig. 2G and H). The
abnormal cells were negative for CD117, CD10, CD20, CD22, CD34 and
CD13 expression (data not presented). Interphase fluorescence in
situ hybridization (FISH) analysis on the bone marrow aspirate
smear, using a probe specific for cyclin D1
(CCND1)/IgH, did not demonstrate a fusion signal, and
chromosome analysis revealed a normal karyotype (data not
presented). According to the aforementioned findings, the patient
of the present case was diagnosed with IgG myeloma. Subsequently,
the patient was treated with a
vincristine-doxorubicin-dexamethasone regimen that consisted of 1
mg vincristine (days 1–4), 10 mg doxorubicin (days 1–4) and 20 mg
dexamethasone (days 1–4, 9–12 and 17–20 for the first cycle and on
days 1–4 for the next three cycles). Thalidomide (75 mg per night)
was administered as maintenance therapy during each cycle of
chemotherapy. The general conditions of the patient improved
following the treatment. The lab tests revealed the following
results: Bone marrow, 2.5% plasma cells (normal range, 0–2.0%); red
blood cells, 3.12×1012 cells/l (normal range,
3.5×1012−5.0×1012 cells/l); hemoglobin, 101
g/l (normal range, 110–150 g/l); IgG κ paraprotein, 15.8 g/l
(normal range, 6.94–16.20 g/l), β-2-microglobulin, 2,373 µg/l
(normal range, 1,035–1,945 µg/l); κ light chain, 1,580 mg/dl
(normal range, 629–1,350 mg/dl); λ light chain, 331 mg/dl (normal
range, 313–723 mg/dl); and κ/λ ratio, 4.77. Subsequently,
consolidate therapy was discontinued by the patient. In May 2013,
the patient was admitted to the Department of Hematology,
Affiliated Hospital of Nanjing University of Traditional Chinese
Medicine complaining of serious bone pain following an injury. The
lab tests revealed the following results: white blood cells,
2.4×109 cells/l; hemoglobin, 105 g/l; blood urea
nitrogen, 5.19 mmol/l (normal range, 1.70–8.30 mmol/l); creatinine,
47.2 µmol/l (normal range, 44–110 mmol/l); β-2-microglobulin, 3,518
µg/l; IgG, 70.7 g/l; and bone marrow, 18.5% plasma cells. The
patient was treated with the DECP regimen, which consisted of 20 mg
dexamethasone, 100 mg etoposide, 0.6 g cyclophosphamide and 30 mg
cisplatinum for 4 days. However, the patient succumbed 18 days
later without remission.
 | Figure 2.Flow cytometry dot plots presenting
the cells of interest in (A) gate B (abnormal cells 20.2%) on
CD45/SS dot plot, with low SS and negative CD45 expression; (B and
C) potent expression of CD38 and expression of CD33; (D, E and F)
restricted expression of κ light chain (cells of gate N were normal
B cells); (G and H) expression of CD138 and CD19, with
CD56-negativity. CD, cluster of differentiation; SS, side scatter;
Lin, linear scale; PE, phycoerythrin; PC5, phycoerythrin cyanin 5;
FITC, fluorescein isothiocyanate; ECD, electron-coupled dye. |
Discussion
Diagnosis of typical MM is not often difficult.
However, in a small number of cases, unusual morphological variants
may pose a challenge when determining a morphological diagnosis,
particularly in plasma cell leukemia (2,3). In the
current case, the neoplastic cells presented with round nuclei and
dense, coarse chromatin. The cytoplasm was slightly basophilic and
scanty, wrapping closely around the nucleus. All of the
aforementioned features were similar to those of neoplastic cells,
in particular small lymphoid or lymphoplasmacytic cells. The
utilization of flow cytometry demonstrated that the cells had a low
side scatter and were CD45−. The cells were also
CD138+, CD38+, CD19+,
CD33+ and CD56−. Plasma cell myelomas
generally lack surface Ig, but express monotypic Ig (4). Like normal plasma cells, plasma cell
myelomas also commonly express CD138, CD79α and potent CD38;
however, in contrast to normal plasma cells, they are frequently
CD19− (5). Additionally,
CD56 is abnormally expressed in 67–79% of cases (6,7).
In patients with myeloma, it has been observed that
disease aggression correlates with the absence of CD56;
CD56− patients were found to exhibit higher levels of
β-2-microglobulin, alongside a higher incidence of Bence Jones
protein, extramedullary disease, thrombocytopenia and renal
insufficiency when compared with CD56+ patients
(7,8).
In addition to CD56, it has been reported that CD117 is expressed
in the malignant plasma cells of certain patients with myeloma
(4). Furthermore, Bataille et
al (4) noted that a lack of CD117
was associated with aggressive disease and a significantly shorter
survival time compared with CD117+ cases. Pozdnyakova
et al (9) reported that the
simultaneous assessment of CD56 and CD117 by flow cytometry
identified cytogenetically distinct groups of plasma cell myeloma,
and CCND1 rearrangement was almost exclusively observed in
cases demonstrating CD117 and CD56 negativity.
CD33 is a glycoprotein expressed on myeloid cell
surfaces and has a mass of 67-kDa. A small number of studies have
observed the expression of CD33 on plasma cell surfaces; however,
the reactivity of the marker has been noted in 6.5–12% of patients
with myeloma (10,11). The expression of CD33 in such patients
is reported to be associated with certain clinical parameters; the
CD33+ patient group had a lower survival rate compared
to the CD33− patient group, thus indicating the
clinicopathological significance of CD33 expression (12).
Certain cases of CCND1+ myeloma are
associated with the t(11;14)(q13;q32) rearrangement, involving the
CCND1 gene (13). Such cases
have been linked with a lymphoplasmacytic morphological appearance
and may therefore be misdiagnosed, particularly if CCND1 and CD138
immunohistochemical staining has not been performed (13). However, in the present case, the FISH
analysis for CCND1/IgH and the chromosome analysis
appeared normal (data not presented). Due to the low frequency of
aberrant antigen expression and the unclear association between
morphology and myeloid antigenic expression, the possible
prognostic indications of these features require further
investigation.
In conclusion, forming a conclusive diagnosis for MM
patients with unusual morphological appearances, based on
morphology only, presents a challenge. In the present study, the
flow cytometry technique allowed doctors to ascertain the nature of
the neoplastic cells with atypical morphology. The present case
possessed small-lymphoid cells and with myeloid antigen expression.
Therefore, flow cytometry immunophenotyping may aid the diagnosis
and the monitoring of minimal residual disease, particularly for
patients that demonstrate no cytogenetical evidence.
References
|
1
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 64:1046–1060. 2011. View Article : Google Scholar
|
|
2
|
Lorsbach RB, Hsi ED, Dogan A and Fend F:
Plasma cell myeloma and related neoplasms. Am J Clin Pathol.
136:168–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heerema-McKenney A, Waldron J, Hughes S,
Zhan F, Sawyer J, Barlogie B and Shaughnessy JD Jr: Clinical,
immunophenotypic, and genetic characterization of small
lymphocyte-like plasma cell myeloma: A potential mimic of mature
B-cell lymphoma. Am J Clin Pathol. 133:265–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bataille R, Jégo G, Robillard N,
Barillé-Nion S, Harousseau JL, Moreau P, Amiot M and
Pellat-Deceunynck C: The phenotype of normal, reactive and
malignant plasma cells. Identification of ‘many and multiple
myelomas’ and of new targets for myeloma therapy. Haematologica.
91:1234–1240. 2006.PubMed/NCBI
|
|
5
|
Lin P, Owens R, Tricot G and Wilson CS:
Flow cytometric immunophenotypic analysis of 306 cases of multiple
myeloma. Am J Clin Pathol. 121:482–488. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pellat-Deceunynck C, Barillé S, Jego G,
Puthier D, Robillard N, Pineau D, Rapp MJ, Harousseau JL, Amiot M
and Bataille R: The absence of CD56 (NCAM) on malignant plasma
cells is a hallmark of plasma cell leukemia and of a special subset
of multiple myeloma. Leukemia. 12:1977–1982. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sahara N, Takeshita A, Shigeno K, Fujisawa
S, Takeshita K, Naito K, Ihara M, Ono T, Tamashima S, Nara K, et
al: Clinicopathological and prognostic characteristics of
CD56-negative multiple myeloma. Br J Haematol. 117:882–885. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sahara N and Takeshita A: Prognostic
significance of surface markers expressed in multiple myeloma: CD56
and other antigens. Leuk Lymphoma. 45:61–65. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pozdnyakova O, Morgan EA, Li B, Shahsafaei
A and Dorfman DM: Patterns of expression of CD56 and CD117 on
neoplastic plasma cells and association with genetically distinct
subtypes of plasma cell myeloma. Leuk Lymphoma. 53:1905–1910. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Almeida J, Orfao A, Ocqueteau M, Mateo G,
Corral M, Caballero MD, Blade J, Moro MJ, Hernandez J and Miguel
San JF: High-sensitive immunophenotyping and DNA ploidy studies for
the investigation of minimal residual disease in multiple myeloma.
Br J Haematol. 107:121–131. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robillard N, Wuillème S, Lodé L,
Magrangeas F, Minvielle S and Avet-Loiseau H: CD33 is expressed on
plasma cells of a significant number of myeloma patients, and may
represent a therapeutic target. Leukemia. 19:2021–2022. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sahara N, Ohnishi K, Ono T, Sugimoto Y,
Kobayashi M, Takeshita K, Shigeno K, Nakamura S, Naito K, Tamashima
S, et al: Clinicopathological and prognostic characteristics of
CD33-positive multiple myeloma. Eur J Haematol. 77:14–18. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rajkumar SV: Multiple myeloma: 2013 update
on diagnosis, risk-stratification, and management. Am J Hematol.
88:226–235. 2013. View Article : Google Scholar : PubMed/NCBI
|