Introduction
Multiple myeloma (MM) is the second most common
hematological cancer, and results from the accumulation of
malignant plasma cells in the bone marrow (1). MM is responsible for ~1% of all cancers
and 10% of hematological cancers (1,2). The
median age at diagnosis of MM is 70 years (3). MM is associated with a number of
complications, which include: Hematological complications, such as
anemia, bone marrow failure and bleeding disorders; bone
complications, such as pathological fractures and lytic bone
lesions; renal insufficiencies; compromised immune function; and
neurological complications, such as spinal cord and nerve root
compression and cranial nerve compression, which are often
associated with base of skull lesions (1). The median survival of patients with MM
was <1 year prior to the introduction of alkylating agents
(4). The introduction of novel
agents, including bortezomib, thalidomide and lenalidomide, for the
treatment of MM patients has significantly improved clinical
outcomes (3,5).
Bortezomib is a proteasome inhibitor that exhibits
significant anti-myeloma activity. The majority of adverse effects
associated with bortezomib are mild or moderate and are generally
manageable, including gastrointestinal symptoms, peripheral
neuropathy and thrombocytopenia (6).
Severe pulmonary complications have rarely been reported. The
present study reports the case of a patient in whom
life-threatening pulmonary adverse effects developed after
receiving bortezomib combined with thalidomide and dexamethasone
for newly diagnosed MM.
Case report
A 62-year-old man was referred to The Second
Affiliated Hospital, Zhejiang University College of Medicine
(Hangzhou, Zhejiang, China) due to dizziness and weakness that had
been apparent for 3 months, since August 2013. The patient had a
9-year history of coronary heart disease, for which the standard
medications (aspirin, 100 mg/day; atorvastatin, 20 mg/day;
metoprolol, 25 mg/day; all for 9 years) were administered, and a
>30-year history of smoking, which the patient quitted after
being diagnosed with coronary heart disease. There was no history
of respiratory diseases. At the cardiological clinic in The Second
Affiliated Hospital, Zhejiang University College of Medicine, the
patient was found to have moderate anemia. Furthermore, serological
tests and bone marrow studies confirmed the pathologic diagnosis of
MM [IgG type; Durie-Salmon, stage III (7)]. The patient was consequently admitted
for chemotherapy.
Treatment with bortezomib (1 mg/m2 on
days 1, 4, 8 and 11), thalidomide (100 mg/day) and dexamethasone
(20 mg on days 1, 2, 4, 5, 8, 9, 11 and 12) was employed in a
21-day cycle. The initial chest high-resolution computed tomography
(HRCT) scan was normal. The first cycle was effective and
uneventful. However, on the eve of the second cycle, the patient
developed a moderate fever (38.5°C) and a mild dry cough. Despite
the fact that the leukocyte count and lung auscultation were
normal, moxifloxacin (400 mg/day) was prescribed to prevent
probable pneumonia. The fifth and sixth agents of bortezomib were
administered on schedule. After the sixth agent, the patient's
temperature ascended to 39.1°C, and auscultation revealed crackles
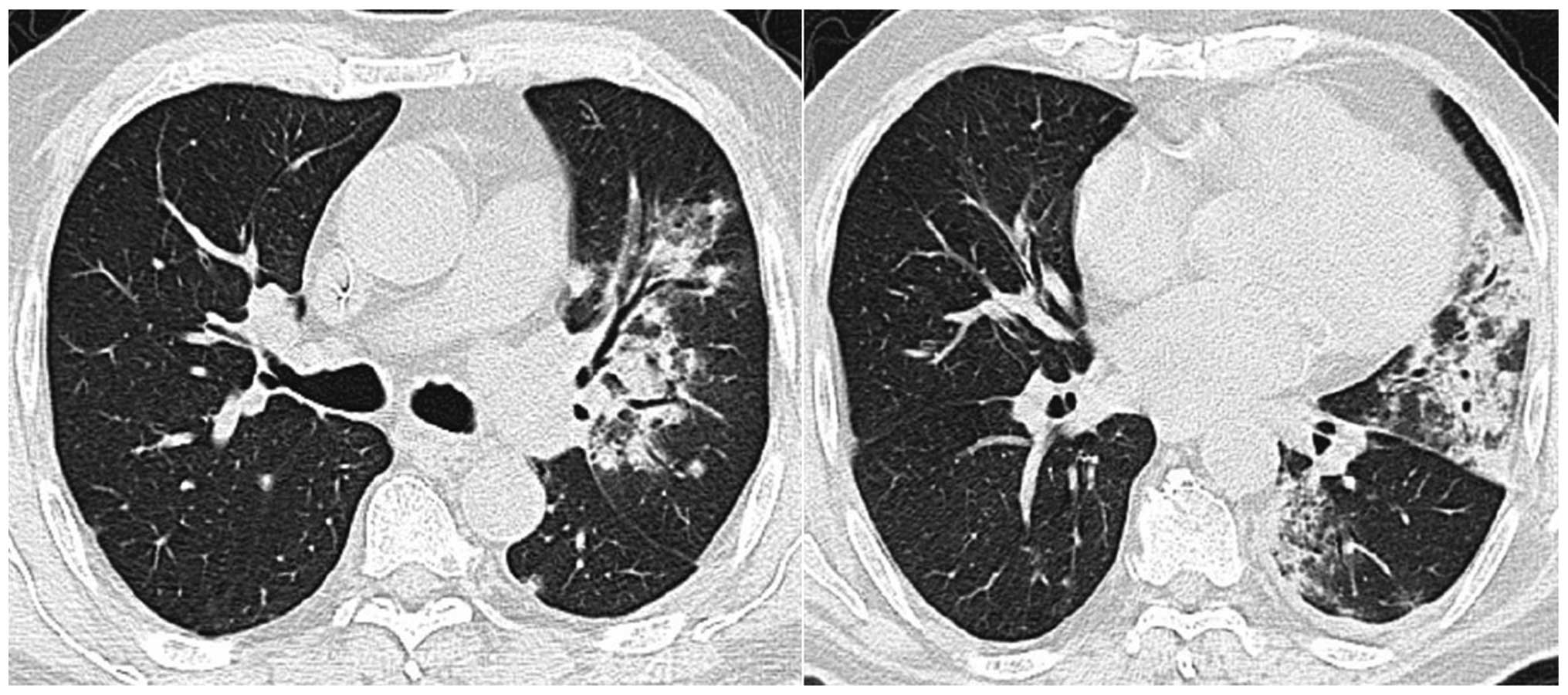
in the left lung. The chest HRCT scan demonstrated a newly
developed consolidation in the upper lobe and infiltration in the
lower lobe of the left lung (Fig. 1).
Pneumonia was initially considered due to the immunosuppressive
status of the patient, and intravenous broad-spectrum antibiotics
(including 500 mg imipenem/cilastatin every 8 h, 1,000 mg
vancomycin every 12 h and 200 mg voriconazole every 12 h) were
administered.
However, the patient's clinical condition worsened
following the treatment. The next day, asthma-like symptoms and
mild dyspnea were noted, and the patient began to expectorate
bloody sputum. The high fever remained (maximum temperature, 39°C).
Acute respiratory distress (SpO2, 85%) on room air
developed 3 days later. The chest HRCT scan showed bilateral
diffuse alveolar infiltrations, multiple subpleural lesions and
slight pleural effusion (Fig. 2). The
chemotherapy was discontinued due to the patient's poor clinical
condition. Infection by virus and/or Pneumocystis jiroveci
could not be definitively excluded; therefore, ganciclovir (300 mg
every 12 h for 3 days) and trimethoprim/sulfamethoxazole (960 mg
every 12 h for 3 days) were added into the therapy. Intravenous
immunoglobulin (10 g/day for 6 days; then 5 g/day for 3 days) was
also administered to strengthen the patient's immunity. The patient
was then transferred into the Intensive Care Unit.
Despite receiving a large number of powerful
antibiotics, the patient's clinical status did not improve. The
patient retained an SpO2 level of 92% when masked with a
reservoir bag, and a flow rate of 15 l/min oxygen. Serological
tests, including repeated C-reactive protein (CRP) and
procalcitonin (PCT) analysis, were normal. The bacteriological and
mycological cultures of the sputum and blood samples were negative.
No invasive investigations, such as bronchoalveolar lavage or
transbronchial biopsy, were performed due to thrombocytopenia and
the critical condition of the patient. A suspected diagnosis of a
bortezomib-induced severe pulmonary complication was formed, and
120 mg/day methylprednisolone was instigated. The respiratory
failure was relieved 5 days later and a normal temperature was
recorded. The dose of methylprednisolone was tapered (120 mg/day
for 2 days; 80 mg/day for 2 days; 40 mg/day for 2 days; 30 mg/day
for 2 days; 20 mg/day for 2 days; then discontinued), the patient's
clinical condition improved gradually and the results on chest HRCT
scan improved synchronously (Fig. 3).
Another 18 days later, the patient was discharged without any
respiratory symptoms.
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Discussion
The first study of MM patients who developed
life-threatening pulmonary complications after receiving bortezomib
therapy reported the cases of 4 Japanese patients (6). Since this time, 16 cases have been
described as MM with bortezomib-induced pulmonary complications
(6,8–15). The
patient in the present study also developed severe pulmonary
complications during bortezomib treatment. The differential
diagnosis included infectious pneumonia and drug-induced pulmonary
toxicity. The clinical course in this case was similar to those
previously reported cases. The patient developed symptoms that
included a fever, a cough, bloody sputum, gradual dyspnea, and
ultimately, respiratory failure. The chest HRCT scan showed diffuse
infiltration and consolidation of the bilateral lungs, accompanied
with slight pleural effusion. The inflammatory markers, CRP and
PCT, were normal. All the cultures of the blood and sputum were
negative. Powerful broad-spectrum antibiotics were inefficacious.
Therefore, the patient was diagnosed with bortezomib-induced severe
pulmonary complications. Although no histopathological evaluation
was obtained to prove the diagnosis, a good response to
corticosteroids favored the diagnosis, as observed in previous
reports (9,12,15).
The clinical characteristics of all 16 reported
patients are listed in Table I. The
cases consisted of 10 male patients (62.5%) and 6 female patients
(37.5%). The mean age was 62.3 years (range, 47–74 years). Only 3
patients (18.8%) had a previous history of respiratory diseases
[including invasive pulmonary aspergillosis (6), pulmonary embolism (6) and chronic obstructive pulmonary disease
(9)], while 11 (68.8%) had not, and 2
had an unknown history. In total, 9 patients (56.3%) were of IgG
type, 2 (12.5%) of IgD type, 1 (6.3%) of IgA type, 1 (6.3%) of IgC
type and 3 (18.8%) of unknown type. The cases consisted of 10
relapsed patients (62.5%), 4 newly diagnosed patients (25%) and 2
refractory patients (12.5%). A total of 6 patients (37.5%) had
received autologous peripheral blood stem cell transplantation
(auto-PBSCT) prior to treatment with bortezomib, 1 (6.3%) had
received an unrelated bone marrow transplantation, 4 (25%) had no
transplantation history and 5 (31.3%) had an unknown
transplantation history. Therefore, the male gender, IgG type,
relapse state and previous history of auto-PBSCT may be risk
factors for bortezomib-induced severe pulmonary complications in
MM.
 | Table I.Characteristics of 16 MM patients with
bortezomib-induced severe pulmonary complications. |
Table I.
Characteristics of 16 MM patients with
bortezomib-induced severe pulmonary complications.
| First author,
year | Age, years | Gender | MM type | Previous history of
lung injury | Status of MM prior to
bortezomib therapy | Prior
transplantation | Ref. |
|---|
| Miyakoshi et
al, 2006 | 47 | F | IgG | Invasive pulmonary
aspergillosis | Relapse | Auto-PBSCT | (6) |
|
| 59 | F | IgA | Pulmonary
embolism | Relapse | Auto-PBSCT |
|
|
| 48 | F | IgD | No | Relapse | Unrelated bone marrow
transplantation |
|
|
| 53 | F | Unknown | No | Relapse | Auto-PBSCT |
|
| Boyer et al,
2006 | 66 | M | Unknown | No | Relapse | No | (8) |
| Zappasodi et
al, 2007 | 66 | M | Unknown | COPD | Refractory | Unknown | (9) |
| Dun et al,
2010 | 72 | M | IgG | No | Relapse | Unknown | (10) |
|
| 51 | M | IgD | No | Refractory | Unknown |
|
|
| 72 | F | IgG | No | New | No |
|
|
| 68 | F | IgG | No | Relapse | Unknown |
|
|
| 72 | M | IgG | No | Relapse | Unknown |
|
| Kang et al,
2010 | 67 | M | IgG | Unknown | New | No | (11) |
| Yamaguchi et
al, 2012 | 64 | M | IgG | No | Relapse | Auto-PBSCT | (12) |
| Vandeix et al,
2012 | 74 | M | IgC | No | New | No | (13) |
| Chew et al,
2007 | 66 | M | IgG | Unknown | New | Auto-PBSCT | (14) |
| Pitini et al,
2007 | 51 | M | IgG | No | Relapse | Auto-PBSCT | (15) |
In the 16 cases, the mean number of bortezomib
injections administered prior to severe pulmonary complications
appearing was 6.9 (range, 1–32), and the mean time from the first
administration of bortezomib to the appearance of severe pulmonary
complications was 31.3 days (range, 1–161 days) (Table II).
 | Table II.Clinical details, treatments and
outcomes of 16 multiple myeloma patients with bortezomib-induced
severe pulmonary complications. |
Table II.
Clinical details, treatments and
outcomes of 16 multiple myeloma patients with bortezomib-induced
severe pulmonary complications.
| First author,
year | Bortezomib doses
prior to symptoms | Time after first
bortezomib administration, days | Combined with
corticosteroid | Clinical
manifestations | Chest radiological
findings | Pathological
findings | Initial
corticosteroid type and dose | Outcome | Ref. |
|---|
| Miyakoshi et
al, 2006 | 8 | 35 | No | Cough, asthma-like
symptoms, fever, dyspnea, respiratory failure | Bilateral
infiltrates | Organizing diffuse
alveolar damage | Methylprednisolone,
1,000 mg | Mortality | (6) |
|
| 4 | 18 | No | Dyspnea, fever,
asthma-like symptoms, respiratory failure | Right pleural
effusion and pulmonary edema | No | Methylprednisolone,
1,000 mg | Alive |
|
|
| 4 | 11 | No | High-grade fever,
respiratory failure | Bilateral
infiltrates with pleural effusions | No | Methylprednisolone,
500 mg | Alive |
|
| 1 | 1 | No | Dyspnea, wheezing,
low-grade fever, respiratory failure | Bilateral
infiltrates | No | Methylprednisolone,
1,000 mg | Mortality |
|
| Boyer et al,
2006 | 9 | 43 | No | Cough, dyspnea,
fever, acute respiratory failure | Bilateral
infiltrates and consolidations | Fibrosis with
admixed chronic inflammatory cells | Prednisone, 60
mg | Alive | (8) |
| Zappasodi | 8 | 42 | Yes | Respiratory
failure | Bilateral
consolidations ground glass opacities, bilateral pleural
effusion | No | Methylprednisolone,
500 mg | Alive | (9) |
| Dun et al,
2010 | 7 | 29 | Yes | Fever, cough,
pursiness, dyspnea, respiratory failure | Bilateral pleural
effusion, pulmonary infiltrates | No | Methylprednisolone,
160–640 mg | Mortality | (10) |
|
| 2 | 5 | Yes | Breath lessness,
fever, dyspnea, respiratory failure | Pulmonary
infiltrates, ground glass opacities | No | Methylprednisolone,
160–640 mg | Mortality |
|
|
| 3 | 8 | Yes | Dyspnea | Left pleural
effusion, pulmonary edema | No | Methylprednisolone,
160–640 mg | Mortality |
|
|
| 2 | 4 | Yes | Breath lessness,
fever, dyspnea, respiratory failure | Pulmonary
edema | No | Methylprednisolone,
160–640 mg | Mortality |
|
|
| 8 | 32 | Yes | High fever, acute
respiratory failure | Pulmonary
edema | No | Methylprednisolone,
160–640 mg | Mortality |
|
| Kang et al,
2010 | 4 | 21 | Yes | Dyspnea | Ground glass
appearance | Diffuse
interstitial fibrosis | Unknown | Alive | (11) |
| Yamaguchi et
al, 2012 | 2 | 13 | Yes | Dry cough, fever,
dyspnea | Bilateral ground
glass, opacities peripheral | No | Prednisolone, 1
mg/kg | Alive | (12) |
| Vandeix et
al, 2012 | 32 | 161 | Yes | Cough, dyspnea,
acute respiratory distress | Bilateral
interstitial alveolar infiltration, right pleural effusion | Bronchiolitis
obliterans with organizing fibroblastic polyps in alveoli | Methylprednisolone,
1 mg/kg | Alive | (13) |
| Chew et al,
2007 | 8 | 35 | Yes | Severe dyspnea,
respiratory failure | Extensive bilateral
acute pulmonary injury | No | Methylprednisolone,
dose unknown | Mortality | (14) |
| Pitini et
al, 2007 | 9 | 43 | Unknown | Dyspnea, acute
respiratory failure | Bilateral multiple
pulmonary infiltrates | No | Methylprednisolone,
1,000 mg | Alive | (15) |
The most common clinical symptoms of
bortezomib-induced severe pulmonary complications were dyspnea
(13/16; 81.3%), respiratory failure (13/16; 81.3%), a fever (10/16;
62.5%) and a cough (5/16; 31.3%). The most common radiological
findings were pulmonary infiltration (9/16; 56.3%), pleural
effusion (6/16; 37.5%), pulmonary edema (4/16; 25%) and
ground-glass opacities (4/16; 25%) (Table II).
Due to the poor clinical condition of the patients,
only 4 (25%) achieved a pathological diagnosis [organizing diffuse
alveolar damage (6), fibrosis with
admixed chronic inflammatory cells (8), diffuse interstitial fibrosis (11) and bronchiolitis obliterans with
organizing fibroblastic polyps in alveoli (13), respectively] that totally accounted
for the changes of the pulmonary interstitium. The inner mechanism
of bortezomib-induced severe pulmonary complications remains
unclear. However, the following pathogenetics have been involved in
the development of lung injury: i) Activation nuclear
factor-κB-related proinflammatory factors, including tumor necrosis
factor-α, interleukin (IL)-6, IL-1β and IL-11; and ii) accumulation
of bortezomib and/or its metabolites, altering multiple signaling
pathways (6,10,13).
All patients received corticosteroid therapy
following the occurrence of severe pulmonary complications,
however, while half of these patients experienced improvement, the
other half succumbed. Among the 7 patients in whom corticosteroid
types or doses were not precisely mentioned, 6 patients (85.7%)
succumbed and 1 patient (14.3%) improved (Table II).
The first hypothesis of proinflammatory factors
would explain the good response to corticosteroid therapy in
certain patients (9,13); however, other patients developed
severe pulmonary complications even when receiving dexamethasone in
combination with bortezomib. In all 16 reported patients, 10
(62.5%) were administered corticosteroids concurrently with
bortezomib, while 5 (31.3%) were not and in 1 case this was
unknown. Among the 10 cases that received bortezomib combined with
corticosteroids, 4 (40%) showed improvement and 6 (60%) succumbed
when retreated with corticosteroids. However, among the 5 cases
that received bortezomib without corticosteroids, 3 (60%) showed
improvement and 2 (40%) succumbed. Despite the fact that
retreatment with corticosteroids remained efficacious, it appeared
that outcomes of patients who received corticosteroids combined
with bortezomib were worse than those treated with bortezomib only.
This would suggest the existence of another unknown complex
pathogenesis. Further investigations are required to elucidate the
underlying reasons.
In a number of the early published studies (6,9,15), the patients mainly received high-dose
corticosteroids (500–1,000 mg/day methylprednisolone), but the
outcomes were not totally satisfactory. Among the 6 patients who
received high-dose corticosteroids, 4 (66.7%) showed improvement
and 2 (33.3%) succumbed. However, low-dose corticosteroids (1
mg/kg/day methylprednisolone/prednisolone) showed a good response
in several cases (8,12,13). All 3
of the patients who received low-dose steroids showed improvement.
In the present case, success was also achieved with 120 mg
methylprednisolone initially.
In two particular cases reported by Miyakoshi et
al (6) and Chew et al
(14), the patients experienced two
episodes of drug-induced pulmonary adverse effects in their whole
course of bortezomib therapy. The first pulmonary complications
improved spontaneously or through treatment with corticosteroid.
However, severe pulmonary complications developed again and the
patients ultimately succumbed when retreated with bortezomib. As a
result, we hypothesize that retreating patients with bortezomib
after a ‘sentinel’ episode of lung injury, a history of pulmonary
complications during the previous treatment with bortezomib, could
be fatal and should be avoided.
In conclusion, clinicians should be aware of
potential severe pulmonary complications in patients receiving
bortezomib, even when combined with dexamethasone, and particularly
in patients who are male, of IgG type, with a relapse status and a
history of auto-PBSCT. In the literature review, it was noted that
when severe pulmonary complications were present, corticosteroids
remained the only effective drug. It appears that the outcomes of
the patients who received corticosteroids combined with bortezomib
were worse than those for patients treated with bortezomib only.
Considering the adverse effects associated with high-dose
corticosteroids, a relatively low dose could be more suitable. At
the same time, bortezomib should be discontinued immediately.
Retreating patients with bortezomib after a ‘sentinel’ episode of
lung injury could be fatal and should be avoided.
Acknowledgements
The authors would like to thank Dr Lili Yang and Dr
Rongwang Yang (The Children's Hospital, Zhejiang University College
of Medicine) for copy-editing the original manuscript.
Glossary
Abbreviations
Abbreviations:
|
MM
|
multiple myeloma
|
|
HRCT
|
high-resolution computed
tomography
|
|
CRP
|
C-reactive protein
|
|
PCT
|
procalcitonin
|
|
auto-PBSCT
|
autologous peripheral blood stem cell
transplantation
|
References
|
1
|
Smith L, McCourt O, Henrich M, Paton B,
Yong K, Wardle J and Fisher A: Multiple myeloma and physical
activity: A scoping review. BMJ Open. 5:e0095762015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kyle RA and Rajkumar SV: Multiple myeloma.
Blood. 111:2962–2972. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gozzetti A, Candi V, Papini G and Bocchia
M: Therapeutic advancements in multiple myeloma. Front Oncol.
4:2412014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy
MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust
JA, et al: Improved survival in multiple myeloma and the impact of
novel therapies. Blood. 111:2516–2520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fulciniti M, Munshi NC and Martinez-Lopez
J: Deep response in multiple myeloma: A critical review. Biomed Res
Int. 2015:8320492015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyakoshi S, Kami M, Yuji K, Matsumura T,
Takatoku M, Sasaki M, Narimatsu H, Fujii T, Kawabata M, Taniguchi
S, et al: Severe pulmonary complications in Japanese patients after
bortezomib treatment for refractory multiple myeloma. Blood.
107:3492–3494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hari PN, Zhang MJ, Roy V, Pérez WS, Bashey
A, To LB, Elfenbein G, Freytes CO, Gale RP, Gibson J, et al: Is the
International Staging System superior to the Durie-Salmon staging
system? A comparison in multiple myeloma patients undergoing
autologous transplant. Leukemia. 23:1528–1534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boyer JE, Batra RB, Ascensao JL and
Schechter GP: Severe pulmonary complication after bortezomib
treatment for multiple myeloma. Blood. 108:11132006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zappasodi P, Dore R, Castagnola C, Astori
C, Varettoni M, Mangiacavalli S, Lazzarino M and Corso A: Rapid
response to high-dose steroids of severe bortezomib-related
pulmonary complication in multiple myeloma. J Clin Oncol.
25:3380–3381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dun XY, Yuan ZG, Fu WJ, Zhang CY and Hou
J: Severe pulmonary complications after bortezomib treatment in
multiple myeloma. Hematol Oncol. 28:49–52. 2010.PubMed/NCBI
|
|
11
|
Kang W, Kim JS, Cho SH, Kim SK, Chang J
and Park MS: Nonspecific interstitial pneumonitis after bortezomib
and thalidomide treatment in a multiple myeloma patient. Yonsei Med
J. 51:448–450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamaguchi T, Sasaki M and Itoh K:
Bortezomib-induced pneumonitis during bortezomib retreatment in
multiple myeloma. Jpn J Clin Oncol. 42:637–639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vandeix E, Favard F, Pichon N,
Delage-Corre M, Melloni B and Clavel M: Bortezomib-induced
bronchiolitis obliterans organizing pneumonia. Case Rep Pulmonol.
2012:4301412012.PubMed/NCBI
|
|
14
|
Chew E, Filsher R and Wei A: Development
of fatal bortezomib induced acute lung injury despite concurrent
therapy with high-dose dexamethasone. Leuk Lymphoma. 48:212–213.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pitini V, Arrigo C, Altavilla G and Naro
C: Severe pulmonary complications after bortezomib treatment for
multiple myeloma: An unrecognized pulmonary vasculitis? Leuk Res.
31:1027–1028. 2007. View Article : Google Scholar : PubMed/NCBI
|