Introduction
Breast cancer is the leading cause of
cancer-associated mortality in females worldwide, and >1.5
million females are diagnosed with breast cancer annually (1). Although the majority of breast cancer
cases are detected at early stages due to regular screening,
gaining an increased understanding of the molecular biology
underlying breast cancer may aid further improvements to breast
cancer diagnosis and therapy (1).
Microtubules are fibrous cytoskeletal proteins
composed of polymers of α- and β-tubulin heterodimers. α- and
β-tubulins exist as multiple isotypes, and the individual
composition of microtubule fibers varies in specific tissues and
among intracellular functions (2). In
various compositions, microtubules contribute to a number of
cellular mechanisms, including maintenance of cell shape,
intracellular transport and chromosome segregation during mitosis
and meiosis (2). Class III β-tubulin
(βIII-tubulin, TUBB3) is a β-tubulin isotype that has been
suggested to possess a significant role in malignant transformation
and cancer development (3). TUBB3
expression is typically identified in cells of neuronal origin,
where it contributes to the formation of dynamic microtubules,
which are essential for neurite formation and maintenance (3). In addition, low levels of TUBB3
expression have been identified in several extra-neuronal normal
tissues, including the testis, small intestine and placenta
(4). Consequently, although TUBB3
overexpression is frequently observed in brain cancer, variable
levels of expression have also been reported in other types of
solid tumor, including cancer of the lungs, colon, ovary, kidney,
prostate and larynx (4,5). High levels of TUBB3 expression have
additionally been linked to poor clinical outcomes in non-small
cell lung, gastric, breast and ovarian cancer (6–8), and to a
reduced response to taxane-based microtubule-targeting anticancer
drugs in these cancer types (7,9–11).
Additionally, in breast cancer, several clinical
studies have suggested that sensitivity to chemotherapeutic taxane
drugs was significantly decreased in breast cancer cases exhibiting
increased levels of TUBB3 expression, alone or in combination with
other molecular markers (10,12–15).
Discrepancies have been reported with respect to associations
between TUBB3 expression and breast cancer phenotype. For example,
certain studies identified an association between high TUBB3
expression levels and high tumor grade (15,16),
advanced tumor stage (16), negative
hormone receptor state (16), human
epidermal growth factor 2 (HER2) positivity (16,17) and a
triple-negative phenotype (17),
while alternative studies were unable to confirm these results
(10,13,15,17).
Similarly, two studies reported an association between TUBB3
overexpression and reduced overall survival rates (16,18), which
was not identified in a previous study (12). As these previous studies had analyzed
84–314 cases/study, the present study hypothesized that the
analysis of a larger patient cohort may aid the development of an
improved understanding of the prognostic value of TUBB3 expression
in breast cancer. The present study thus analyzed a large breast
cancer prognosis tissue microarray (TMA), containing 2,197
consecutive breast cancer cases, including all histological
subtypes and associated molecular data for TUBB3 expression.
Materials and methods
Breast cancer TMA
The breast cancer TMA utilized in the present study
has been previously described in detail (19). Briefly, 2,197 formalin-fixed (buffered
neutral aqueous 4% solution), paraffin-embedded tumors were
assembled in a TMA format. A custom-made semiautomatic robotic
precision instrument was used to punch out one tissue cylinder
(diameter, 0.6 mm) for each case, from representative tumor areas
of each patient tissue block. The histological grade of each sample
was determined according to a modified scoring system devised by
Elston and Ellis [Bloom-Richardson-Elston (BRE) score] (20). Several examples of molecular data
utilized for the present study were available from previous
studies, including data obtained via immunohistochemistry (IHC) for
estrogen receptor (ER), progesterone receptor (PR) and Ki67
(19,21) expression, as well as amplification
data obtained via fluorescence in situ hybridization (FISH)
for HER2. The use of these tissues for FISH and protein expression
analysis was approved by the ethics committee of the University of
Hamburg (Hamburg, Germany). The median patient age was 62 years
(range, 26–101 years) and median follow-up time was 68 months
(range, 1–176 months). Clinicopathological parameters of the
assayed cancer specimens are described in Table I.
 | Table I.Composition of breast cancer
prognosis TMA and associations between tumor phenotype and TUBB3
immunohistochemistry. |
Table I.
Composition of breast cancer
prognosis TMA and associations between tumor phenotype and TUBB3
immunohistochemistry.
|
|
|
| TUBB3
immunohistochemistry |
|
|---|
|
|
|
|
|
|
|---|
| Clinical
feature | On TMA, n | Analyzable, n | Negative, % | Weak, % | Moderate, % | Strong, % | P-value |
|---|
| All cancers | 2197 | 1652 | 44.3 | 1.9 | 14.5 | 39.3 |
|
| Histology |
|
|
|
|
|
|
|
| No
special type | 1531 | 1211 | 40.3 | 2.2 | 14.7 | 42.8 |
|
| Lobular
carcinoma |
311 |
179 | 65.9 | 0.0 | 17.9 | 16.2 |
<0.0001b |
|
Cribriform carcinoma |
64 |
52 | 53.8 | 1.9 | 11.5 | 32.7 |
|
|
Medullary carcinoma |
57 |
52 | 34.6 | 3.8 | 15.4 | 46.2 |
|
| Tubular
carcinoma |
56 |
29 | 62.1 | 0.0 |
6.9 | 31.0 |
|
|
Papillary carcinoma |
30 |
23 | 56.5 | 4.3 |
8.7 | 30.4 |
|
|
Mucinous carcinoma |
69 |
48 | 41.7 | 0.0 | 10.4 | 47.9 |
|
| Other
rare typesa |
79 |
58 | 50.0 | 0.0 | 10.3 | 39.7 |
|
| Tumor stage |
|
|
|
|
|
|
0.5921 |
| 1 |
804 |
559 | 44.4 | 2.3 | 14.1 | 39.2 |
|
| 2 | 1015 |
803 | 43.6 | 2.0 | 15.3 | 39.1 |
|
| 3 |
124 |
94 | 51.1 | 0.0 | 11.7 | 37.2 |
|
| 4 |
242 |
189 | 44.4 | 1.1 | 13.2 | 41.3 |
|
| BRE grade |
|
|
|
|
|
| <0.0001 |
| 1 |
539 |
383 | 55.4 | 1.3 |
9.7 | 33.7 |
|
| 2 |
839 |
589 | 48.9 | 1.9 | 15.8 | 33.4 |
|
| 3 |
646 |
542 | 31.9 | 2.8 | 16.1 | 49.3 |
|
| Nodal stage |
|
|
|
|
|
|
0.1549 |
| 0 |
936 |
695 | 47.3 | 1.7 | 13.1 | 37.8 |
|
| 1 |
783 |
590 | 41.7 | 1.5 | 16.3 | 40.5 |
|
| 2 |
121 |
102 | 41.2 | 4.9 | 13.7 | 40.2 |
|
| ER status |
|
|
|
|
|
|
|
|
Negative |
474 |
398 | 31.9 | 2.8 | 16.8 | 48.5 | <0.0001 |
|
Positive | 1544 | 1178 | 48.5 | 1.6 | 13.7 | 36.2 |
|
| PR status |
|
|
|
|
|
|
|
|
Negative | 1265 |
984 | 40.3 | 1.7 | 14.8 | 43.1 |
0.0039 |
|
Positive |
661 |
523 | 48.8 | 2.5 | 14.5 | 34.2 |
|
| HER2 FISH |
|
|
|
|
|
|
|
| No
amplification | 1349 | 1051 | 45.4 | 2.1 | 13.8 | 38.7 | <0.0001 |
|
Amplification |
282 |
246 | 29.3 | 2.0 | 19.1 | 49.6 |
|
IHC
Freshly cut TMA sections were subjected to
immunohistochemical analysis. Primary rabbit monoclonal antibody
specific for TUBB3 (dilution, 1:150; cat no. ab68193; Abcam,
Cambridge, MA, USA) was added to the sections, slides were
deparaffinized and subsequently exposed to heat-induced antigen
retrieval for 5 min in an autoclave (Systec 2540 EL; Systec GmbH,
Linden, Germany) at 121°C in pH 7.8 Tris-EDTA-citrate buffer
(Sigma-Aldrich, St. Louis, MO, USA). Following incubation (at 37°C
for 60 min), bound antibody was visualized using the EnVision™ kit
(Dako, Glostrup, Denmark). Internal staining in nerves and axons on
the TMA slide served as a positive control, as previously described
(22). The staining intensity and the
percentage of positively stained tumor cells were recorded for each
tissue spot. Staining intensity was determined by visual inspection
of each TMA spot under a microscope (magnification, ×200; Axioskop
40; Carl Zeiss, Inc., Oberkochen, Germany). Staining intensity
scores were assigned as follows: No visible staining, 0; faint
staining, 1+; medium staining, +2; and strong staining, 3+. In
addition, the fraction of positively stained cells (5, 10, 20, 30,
40, 50, 60, 70, 80, 90 or 100%) was estimated in each spot by
visual inspection. A final score was calculated from these two
parameters according to the following criteria, as previously
described (23): Negative score,
staining intensity of 0; weak score, staining intensity of 1+ in
≤70% of tumor cells, or 2+ in ≤30% of tumor cells; moderate score,
staining intensity of 1+ in >70% of tumor cells, 2+ in 31–70% of
tumor cells, or 3+ in ≤30% of tumor cells; and high score, staining
intensity of 2+ in >70% of tumor cells, or 3+ in >30% of
tumor cells (Fig. 1).
Statistical analysis
Statistical calculations were performed using JMP 9
software (SAS Institute Inc., Cary, NC, USA). Contingency tables
and χ2 test were performed to identify associations
between tumor phenotype and molecular parameters. Survival curves
were calculated according to Kaplan-Meier. The Log-Rank test was
applied to identify any significant survival differences between
groups. Cox proportional hazards regression analysis was performed
to investigate statistical independence and significance between
pathological, molecular and clinical features. P<0.05 was
considered to indicate a statistically significant difference.
Results
Technical issues with interpretation
of the TMA
A total of 1,652 (75.2%) tumor samples were
interpretable in the TMA analysis. Reasons for non-interpretable
cases (545 spots; 24.8%) included a lack of tissue samples or an
absence of unequivocal cancer tissue in the TMA spot.
Enhanced TUBB3 expression is
significantly associated with high-grade breast cancer cases,
ER-and PR-negative tumors and the presence of HER2
amplification
TUBB3 immunostaining was localized to the cytoplasm
of the cells. Representative images of positive and negative TUBB3
immunostaining are exhibited in Fig.
1. In total, positive immunostaining was detected in 55.7% of
the 1,652 interpretable breast cancer cases, including 1.9% of
tumors exhibiting weak, 14.5% exhibiting moderate and 39.3%
exhibiting high levels of immunostaining, according to the
aforementioned predefined criteria (23). TUBB3 expression was significantly less
frequent in lobular breast cancer cases (34%) when compared with
other types of breast cancer, including the largest group of breast
cancer cases of no special type (60%; P<0.0001). Increased
levels of TUBB3 expression were significantly associated with
high-grade breast cancer cases (P<0.0001), ER-negative
(P<0.0001) and PR-negative (P=0.0039) tumors and the presence of
HER2 amplification (P<0.0001), although were not associated with
tumor stage (P=0.5921) or presence of nodal metastasis (P=0.1549).
All results are summarized in Table
I. For all subsequent analyses, the small fraction of breast
cancer cases that exhibited weak expression (n=31; 1.9%) was
combined with the subset of breast cancer cases that exhibited
moderate expression (n=239; 14.5%), into a single ‘low expression’
group.
TUBB3 expression is associated with
patient survival
Follow-up data were available for 1,650 breast
cancer cases with interpretable TUBB3 results, including 1,209
cases of no special type. If all breast cancer cases were jointly
analyzed, a statistically significant association existed between
low and high TUBB3 expression and shortened raw survival (P=0.0088;
Fig. 2A), which was less marked
(statistically insignificant) when the analysis was restricted to
the subset of breast cancers of no special type (P=0.1583; Fig. 2B). For all breast cancer cases, this
association was not observed following multivariate analysis
including the established prognosticators of tumor stage stage
(P=0.0039), BRE grade (P<0.0001) and nodal stage (P<0.0001),
in addition to the TUBB3 immunostaining results (P=0.0806; Table II).
 | Table II.Multivariate analysis of overall
survival, including tumor stage, BRE grade, nodal stage and TUBB3
expression. |
Table II.
Multivariate analysis of overall
survival, including tumor stage, BRE grade, nodal stage and TUBB3
expression.
| Clinicopathological
parameter | Hazard ratio | 95% Confidence
interval | P-value |
|---|
| Tumor stage |
|
|
0.0039 |
| pT2 vs.
pT1 | 1.3 | 1.0–1.7 |
|
| pT3 vs.
pT2 | 0.9 | 0.6–1.3 |
|
| pT4 vs.
pT3 | 1.6 | 1.1–2.5 |
|
| BRE grade |
|
| <0.0001 |
| G2 vs.
G1 | 1.3 | 0.9–1.7 |
|
| G3 vs.
G2 | 2.1 | 1.7–2.6 |
|
| Nodal stage |
|
| <0.0001 |
| pN1 vs.
pN0 | 2.3 | 1.8–3.0 |
|
| pN2 vs.
pN1 | 2.7 | 2.1–3.6 |
|
| TUBB3 |
|
|
0.0806 |
| Low vs.
negative | 1.4 | 1.0–1.8 |
|
| High
vs. low | 0.8 | 0.6–1.1 |
|
TUBB3 expression is significantly
associated with triple negative breast cancer
ER, PR and HER2 data were combined to identify a
triple-negative phenotype in 177 (12.7%) cases, an ER- and/or
PR-positive phenotype in 973 (69.7%) cases and a HER2-positive
(presence of HER2 amplification) phenotype in 246 cases (17.6%) of
1,396 breast cancer cases with interpretable data for TUBB3
expression. TUBB3 expression was significantly associated with
subsets of breast cancer exhibiting HER2 amplification or a
triple-negative phenotype: 70% each of triple-negative tumors and
HER2-amplified breast cancer cases were classified as
TUBB3-positive, compared with only 51% of ER/PR-positive specimens
(Fig. 3).
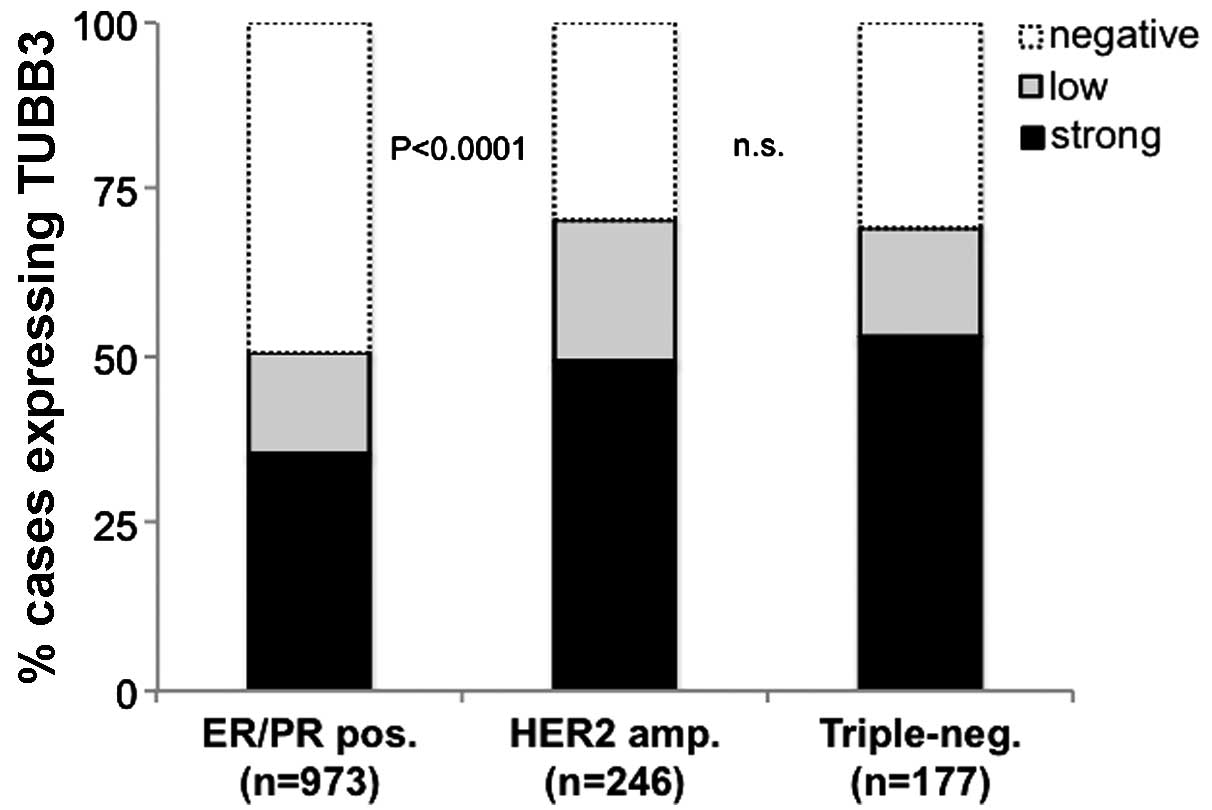 | Figure 3.Association between TUBB3
immunohistochemical analysis results and subsets of breast cancer
according to ER/PR state (ER and/or PR positive, HER2 negative),
presence of HER2 amplification (HER2 positive irrespective of ER/PR
state) and total lack of ER/PR/HER2 positivity (triple-negative).
TUBB3, class III β-tubulin; ER, estrogen receptor; PR, progesterone
receptor; HER2, human epidermal growth factor 2; pos, positive;
amp, amplified; neg, negative; n.s., not significant. |
High TUBB3 expression is associated
with cell proliferation
Immunohistochemical data regarding Ki67 expression
were available for 1,276 breast cancer cases possessing
interpretable TUBB3 results. A high proliferation index (Ki67
labeling index; i.e., the % of Ki67-positive cancer cells per high
power field) was associated with high TUBB3 immunostaining if all
types of breast cancer were jointly analyzed (P<0.0001);
however, subset analysis revealed that this association was
primarily driven by BRE grade, as there was no statistically
significant increase in cell proliferation rates within the
subgroups of breast cancer cases of identical BRE grade (Fig. 4).
Discussion
The results of the present study demonstrated that
TUBB3 is frequently expressed in breast cancer cases, and is
associated with adverse prognostic features, including a
triple-negative hormone receptor status and the presence of HER2
amplification.
Positive TUBB3 staining was detected in 55.7% of the
1,652 breast cancer samples that were successfully analyzed in the
present study. A total of 40% of cancer cases evaluated exhibited
high immunostaining according to the predefined criteria. These
data were concurrent with the results of earlier IHC studies
investigating TUBB3 expression, which had analyzed between 46 and
1,205 cases of breast cancer (10,13,15,16,18).
Despite variable thresholds for TUBB3 expression levels, these
previous studies reported TUBB3 positivity in 34–62% of samples. In
the present study, utilization of a TMA enabled the analysis of a
markedly larger cohort of cancer cases, enabling evaluation of the
potential biological and clinical roles of TUBB3 expression.
Although the analysis was limited to a single tumor specimen of 0.6
mm in diameter per patient, the breast cancer prognosis TMA
utilized in the present study has previously been proven to be
effective in identifying associations between biomarkers and
clinicopathological parameters of breast cancer (19,21,24–26).
A comparison of immunostaining results with the
phenotype of the assayed types of cancer revealed that increased
TUBB3 expression levels were associated with certain features of
aggressive breast cancer, including advanced tumor grade, loss of
ER/PR expression, HER2 amplification and triple-negative
phenotypes; however, TUBB3 expression levels exhibited no
significant associations with tumor stage and metastatic growth.
These results appear to disprove a major role for TUBB3 in tumor
progression or metastasis. The results of the present study are
supported by those of previous studies, which reported increased
levels of TUBB3 expression in grade 3 tumors, compared with those
of grade 1 and 2 tumors (15,16), in hormone receptor-negative cancer
compared with hormone receptor-positive cancer (16), in HER2 amplified cancer (16,17) and in
triple-negative cancer (17). The
fact that none of these previous studies identified all of these
associations is potentially due to the comparatively small sample
sets used, ranging between 84 and 314 tumors. The fact that all
associations were able to be clearly visualized in the present
study, which analyzed >1,600 breast cancer cases, emphasizes the
importance of analyzing as large as possible a tumor set for
candidate biomarker validation.
Notably, the subtype of lobular cancer demonstrated
significantly lower TUBB3 expression compared with that of other
breast cancer types. Given that loss of E-cadherin function is the
primary characteristic feature of lobular breast cancer (27), it is possible that TUBB3 expression
does not provide a selective advantage in an E-cadherin-negative
molecular background, or that E-cadherin signaling may be involved
in regulating TUBB3 expression. E-cadherin maintains epithelial
integrity by binding to the cytoplasmic α- and β-catenin proteins,
which link cadherin to the actin cytoskeleton (28). Loss of E-cadherin induces epithelial
mesenchymal transition, a significant event in the development of
cancer (27). It may be hypothesized
that loss of E-cadherin function in lobular breast cancer cases
results in such marked changes to the cytoskeleton, that the
increase in microtubule dynamics induced by TUBB3 overexpression
may exert little or no additional effects. However, a recent study
identified that drug-induced microtubule disassembly in
vitro resulted in aberrant expression of E-cadherin (29), supporting a direct molecular link
between tubulin turnover and E-cadherin expression. To the best of
our knowledge, only one study, including 44 breast cancer cases of
no special type and 40 lobular breast cancer cases (13), had previously investigated the
variations in TUBB3 expression between these histological subtypes,
however this study identified no significant differences, in
contrast to the results of the present study.
In the present study, TUBB3 expression was
significantly associated with shortened survival when all 1,649
cancer cases were jointly analyzed. In a multivariate analysis
including established prognosticators, the hazard ratio for overall
survival was increased by expression of TUBB3, however, this was
not dependent on the expression level in multivariate analysis.
These results suggest that TUBB3 may not be an optimal prognostic
marker for routine application in breast cancer diagnosis. This may
be explained by the fact that TUBB3 overexpression was linked to
certain adverse prognostic features, including HER2 amplification
and a triple-negative phenotype, however not to other significant
prognostic factors, including tumor stage and metastatic growth.
For example, the fraction of TUBB3-positive cases was identical in
early (pT1) and late (pT4) stage cancer, or in nodal-negative (pN0)
and nodal-positive (pN+) tumors, and was not unequivocally linked
to tumor cell proliferation in the present study. The results of
the present study support those of a previous study, which reported
that TUBB3 messenger RNA expression was associated with reduced
survival, although the authors did not identify a significant
association when TUBB3 expression was determined by TUBB3 IHC
analysis (16). Furthermore, in
concordance with the results of the present study, Horak et
al (17) reported an association
between TUBB3 overexpression and HER2-enriched and basal-like
cancer types, as well as a triple-negative phenotype. Increased
levels of TUBB3 have additionally been linked to adverse phenotypes
and poor prognosis in various other types of solid cancer,
including colon (3), prostate
(5), lung (9,30), ovarian
(31,32) and neurological cancer (33). The adverse effects of TUBB3 may be
linked to its significant role in rendering microtubules dynamic.
High microtubule plasticity is required for cellular processes that
also have significant roles in cancer cells, including cell
motility, mitotic spindle formation and cell division. Microtubules
containing α/TUBB3-heterodimers are more dynamic than those
assembled from other isotypes, for example α/β class II, which
results in the generation of less flexible microtubules (34,35). It is
thought that the TUBB3 isotype is responsible for generating the
highly dynamic microtubules required for neurite formation and
motility in neuronal tissues (33).
Several studies have suggested that TUBB3 may be a
clinically relevant biomarker for the prediction of responses to
drugs targeting microtubules, including vinca alkaloids, taxanes
and epothilone analogues in breast (10,13–15), lung,
ovarian, prostate, breast, stomach and pancreatic tumors (36). These drugs constitute a significant
class of chemotherapeutic agent, which impair microtubule assembly
and activity and inhibit cell division via inhibition of the
mitotic spindle and induction of apoptosis, in solid tumors and
hematological malignancies (37,38). It
has thus been suggested that the increase in microtubule dynamics
conferred by TUBB3 may provide resistance to microtubule-targeting
drugs (39). However, the two largest
studies of TUBB3 and breast cancer, including 314 specimens
analyzed by the Hellenic Cooperative Oncology Group and >1,200
specimens from the BCIRG001 trial, were unable to identify an
association between TUBB3 expression levels and the benefit of
inclusion of Paclitaxel into the treatment regimen (16,18). It is
possible that the discrepant conclusions arising from the present
and previous studies may be associated with the study size and/or
composition of the cancer specimens, with respect to histological
subtypes and alterations in clinically relevant molecular pathways,
including ER or HER2 signaling. The results of the present study
contribute to the ongoing discussion, indicating that it may be
possible that lobular breast cancer, which demonstrated the lowest
levels of TUBB3 expression amongst all histological subtypes
included in the present study, may benefit more from
tubulin-targeting agents, compared with alternative histological
subtypes of cancer, provided that TUBB3 expression levels posses
predictive value for responses to therapy.
In conclusion, the present study emphasized the
significant role for TUBB3 in breast cancer, based on its
association with prognostic adverse molecular subtypes, including
HER2 positive and triple-negative tumors. In addition, the results
of the present study demonstrated variations in TUBB3 expression
levels between the two most frequent histological subtypes of
breast cancer, lobular cancer and tumors of no special type.
However, the comparatively low prognostic power of TUBB3
measurement by IHC appears to disprove a potential role for this
factor as a clinically relevant prognostic biomarker in breast
cancer.
Acknowledgements
The authors would like to thank Miss. Christina
Koop, Miss. Janett Lütgens, Miss. Sünje Seekamp and Mrs. Inge
Brandt from the tissue microarray facility at the Institute of
Pathology, University Medical Center Hamburg - Eppendorf (Hamburg,
Germany) for technical support.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Orr GA, Verdier-Pinard P, McDaid H and
Horwitz SB: Mechanisms of Taxol resistance related to microtubules.
Oncogene. 22:7280–7295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katsetos CD, Herman MM and Mörk SJ: Class
III beta-tubulin in human development and cancer. Cell Motil
Cytoskeleton. 55:77–96. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leandro-García LJ, Leskelä S, Landa I,
Montero-Conde C, López-Jiménez E, Letón R, Cascón A, Robledo M and
Rodríguez-Antona C: Tumoral and tissue-specific expression of the
major human beta-tubulin isotypes. Cytoskeleton (Hoboken).
67:214–223. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsourlakis MC, Weigand P, Grupp K, Kluth
M, Steurer S, Schlomm T, Graefen M, Huland H, Salomon G, Steuber T,
et al: βIII-tubulin overexpression is an independent predictor of
prostate cancer progression tightly linked to ERG fusion status and
PTEN deletion. Am J Pathol. 184:609–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrandina G, Zannoni GF, Martinelli E,
Paglia A, Gallotta V, Mozzetti S, Scambia G and Ferlini C: Class
III beta-tubulin overexpression is a marker of poor clinical
outcome in advanced ovarian cancer patients. Clin Cancer Res.
12:2774–2779. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hetland TE, Hellesylt E, Flørenes VA,
Tropé C, Davidson B and Kærn J: Class III β-tubulin expression in
advanced-stage serous ovarian carcinoma effusions is associated
with poor survival and primary chemoresistance. Hum Pathol.
42:1019–1026. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koh Y, Jang B, Han SW, Kim TM, Oh DY, Lee
SH, Kang CH, Kim DW, Im SA, Chung DH, et al: Expression of class
III beta-tubulin correlates with unfavorable survival outcome in
patients with resected non-small cell lung cancer. J Thorac Oncol.
5:320–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang HL, Ruan L, Zheng LM, Whyte D, Tzeng
CM and Zhou XW: Association between class III β-tubulin expression
and response to paclitaxel/vinorebine-based chemotherapy for
non-small cell lung cancer: A meta-analysis. Lung Cancer. 77:9–15.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X, Wu J, Lu H, Huang O and Shen K:
Measuring β-tubulin III, Bcl-2, and ERCC1 improves pathological
complete remission predictive accuracy in breast cancer. Cancer
Sci. 103:262–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng WE, Chen H, Yuan SF, Wu LL, Zhang W,
Sun HY and Chen WJ: Overexpression of βIII-tubulin and survivin
associated with drug resistance to docetaxel-based chemotherapy in
advanced gastric cancer. J BUON. 17:284–290. 2012.PubMed/NCBI
|
|
12
|
Galmarini CM, Treilleux I, Cardoso F,
Bernard-Marty C, Durbecq V, Gancberg D, Bissery MC, Paesmans M,
Larsimont D, Piccart MJ, et al: Class III beta-tubulin isotype
predicts response in advanced breast cancer patients randomly
treated either with single-agent doxorubicin or docetaxel. Clin
Cancer Res. 14:4511–4516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan SF, Zhu LJ, Zheng WE, Chen H, Wu LL,
Zhang W, Sun HY and Chen WJ: Expression of β-tubulin III and
survivin in advance stage breast cancer correlates with
chemotherapeutic effects of docetaxel. Asian Pac J Cancer Prev.
13:361–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jung M, Koo JS, Moon YW, Park BW, Kim SI,
Park S, Lee SH, Hong S, Rha SY, Chung HC, et al: Overexpression of
class III beta tubulin and amplified HER2 gene predict good
response to paclitaxel and trastuzumab therapy. PLoS One.
7:e451272012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang YI, Sparano JA, Fineberg S, Stead L,
Sunkara J, Horwitz SB and McDaid HM: High expression of class III
β-tubulin predicts good response to neoadjuvant taxane and
doxorubicin/cyclophosphamide-based chemotherapy in estrogen
receptor-negative breast cancer. Clin Breast Cancer. 13:103–108.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pentheroudakis G, Batistatou A, Kalogeras
KT, Kronenwett R, Wirtz RM, Bournakis E, Eleftheraki AG, Pectasides
D, Bobos M, Papaspirou I, et al: Prognostic utility of β-tubulin
isotype III and correlations with other molecular and
clinicopathological variables in patients with early breast cancer:
A translational Hellenic Cooperative Oncology Group (HeCOG) study.
Breast Cancer Res Treat. 127:179–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Horak CE, Pusztai L, Xing G, Trifan OC,
Saura C, Tseng LM, Chan S, Welcher R and Liu D: Biomarker analysis
of neoadjuvant doxorubicin/cyclophosphamide followed by ixabepilone
or Paclitaxel in early-stage breast cancer. Clin Cancer Res.
19:1587–1595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dumontet C, Krajewska M, Treilleux I,
Mackey JR, Martin M, Rupin M, Lafanechère L and Reed JC: BCIRG 001
molecular analysis: Prognostic factors in node-positive breast
cancer patients receiving adjuvant chemotherapy. Clin Cancer Res.
16:3988–3997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruiz C, Seibt S, Al Kuraya K, Siraj AK,
Mirlacher M, Schraml P, Maurer R, Spichtin H, Torhorst J, Popovska
S, et al: Tissue microarrays for comparing molecular features with
proliferation activity in breast cancer. Int J Cancer.
118:2190–2194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Al-Kuraya K, Schraml P, Torhorst J, Tapia
C, Zaharieva B, Novotny H, Spichtin H, Maurer R, Mirlacher M,
Köchli O, et al: Prognostic relevance of gene amplifications and
coamplifications in breast cancer. Cancer Res. 64:8534–8540. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ploussard G, Terry S, Maillé P, Allory Y,
Sirab N, Kheuang L, Soyeux P, Nicolaiew N, Coppolani E, Paule B, et
al: Class III beta-tubulin expression predicts prostate tumor
aggressiveness and patient response to docetaxel-based
chemotherapy. Cancer Res. 70:9253–9264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Minner S, Wittmer C, Graefen M, Salomon G,
Steuber T, Haese A, Huland H, Bokemeyer C, Yekebas E, Dierlamm J,
et al: High level PSMA expression is associated with early PSA
recurrence in surgically treated prostate cancer. Prostate.
71:281–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choschzick M, Lebeau A, Marx AH, Tharun L,
Terracciano L, Heilenkötter U, Jaenicke F, Bokemeyer C, Simon R,
Sauter G and Schwarz J: Overexpression of cell division cycle 7
homolog is associated with gene amplification frequency in breast
cancer. Hum Pathol. 41:358–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choschzick M, Lassen P, Lebeau A, Marx AH,
Terracciano L, Heilenkötter U, Jaenicke F, Bokemeyer C, Izbicki J,
Sauter G and Simon R: Amplification of 8q21 in breast cancer is
independent of MYC and associated with poor patient outcome. Mod
Pathol. 23:603–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burandt E, Jens G, Holst F, Jänicke F,
Müller V, Quaas A, Choschzick M, Wilczak W, Terracciano L, Simon R,
et al: Prognostic relevance of AIB1 (NCoA3) amplification and
overexpression in breast cancer. Breast Cancer Res Treat.
137:745–753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stockinger A, Eger A, Wolf J, Beug H and
Foisner R: E-cadherin regulates cell growth by modulating
proliferation-dependent beta-catenin transcriptional activity. J
Cell Biol. 154:1185–1196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Roy F and Berx G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kitase Y and Shuler CF: Microtubule
disassembly prevents palatal fusion and alters regulation of the
E-cadherin/catenin complex. Int J Dev Biol. 57:55–60. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Levallet G, Bergot E, Antoine M, Creveuil
C, Santos AO, Beau-Faller M, de Fraipont F, Brambilla E, Levallet
J, Morin F, et al: High TUBB3 expression, an independent prognostic
marker in patients with early non-small cell lung cancer treated by
preoperative chemotherapy, is regulated by K-Ras signaling pathway.
Mol Cancer Ther. 11:1203–1213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao S, Zhao X, Lin B, Hu Z, Yan L and Gao
J: Clinical implications of REST and TUBB3 in ovarian cancer and
its relationship to paclitaxel resistance. Tumour Biol.
33:1759–1765. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carrara L, Guzzo F, Roque DM, Bellone S,
Emiliano C, Sartori E, Pecorelli S, Schwartz PE, Rutherford TJ and
Santin AD: Differential in vitro sensitivity to patupilone
versus paclitaxel in uterine and ovarian carcinosarcoma cell lines
is linked to tubulin-beta-III expression. Gynecol Oncol.
125:231–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Katsetos CD, Legido A, Perentes E and Mörk
SJ: Class III beta-tubulin isotype: A key cytoskeletal protein at
the crossroads of developmental neurobiology and tumor
neuropathology. J Child Neurol. 18:851–867. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Panda D, Miller HP, Banerjee A, Ludueña RF
and Wilson L: Microtubule dynamics in vitro are regulated by
the tubulin isotype composition. Proc Natl Acad Sci USA.
91:11358–11362. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Falconer MM, Echeverri CJ and Brown DL:
Differential sorting of beta tubulin isotypes into
colchicine-stable microtubules during neuronal and muscle
differentiation of embryonal carcinoma cells. Cell Motil
Cytoskeleton. 21:313–325. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sève P and Dumontet C: Is class III
beta-tubulin a predictive factor in patients receiving
tubulin-binding agents? Lancet Oncol. 9:168–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jordan MA, Toso RJ, Thrower D and Wilson
L: Mechanism of mitotic block and inhibition of cell proliferation
by taxol at low concentrations. Proc Natl Acad Sci USA.
90:9552–9556. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dumontet C and Sikic BI: Mechanisms of
action of and resistance to antitubulin agents: Microtubule
dynamics, drug transport, and cell death. J Clin Oncol.
17:1061–1070. 1999.PubMed/NCBI
|
|
39
|
Narvi E, Jaakkola K, Winsel S,
Oetken-Lindholm C, Halonen P, Kallio L and Kallio MJ: Altered TUBB3
expression contributes to the epothilone response of mitotic cells.
Br J Cancer. 108:82–90. 2013. View Article : Google Scholar : PubMed/NCBI
|


















