Introduction
Lung adenocarcinoma (LAC) is a major histological
subtype of lung cancer (1) and a
heterogeneous tumor that includes various histological subtypes,
molecular alterations and responses to chemotherapy and targeted
therapy (2). Patients with LAC have
benefited less from traditional chemotherapy (3). With the increasing administration of
targeted therapy, the driver genes harbored in LAC may receive
increased consideration. Targeted therapy has been associated with
great achievements towards improving the clinical outcomes of LAC
patients (4). For example, targeted
therapy has been shown to increase the median progression-free
survival time of patients when compared with standard chemotherapy
(5). The epidermal growth factor
receptor (EGFR) and Kristen rat sarcoma viral oncogene homolog
(KRAS) mutations have been reported as the major driver genes in
LAC; the mutations are expressed exclusively and are characterized
by distinct histological subtypes (6,7). EGFR
mutations have been verified as good independent prognostic factors
for patients who are treated with gefitinib and erlotinib therapy
(8). LAC with KRAS mutations
demonstrates resistance to EGFR-targeted therapies. Therefore, KRAS
and EGFR mutations have been screened together for the routine
molecular testing of LAC (9). The
anaplastic lymphoma receptor tyrosine kinase (ALK) rearrangement
was first detected in LAC by Soda et al (10) in 2007. The detection of this
rearrangement was a revolutionary point in the history of molecular
therapy for LAC, and crizotinib as a specific targeted drug has
obtained encouraging outcomes with regard to improving the survival
of patients who harbor ALK rearrangements (11).
Increasing numbers of studies have focused on the
histological subtypes of LAC following the novel classification
proposed by the International Association for the Study of Lung
Cancer/American Thoracic Society/European Respiratory Society
(IASLC/ATS/ERS) (12,13). Finding the association between the
histological subtype and mutation status of LAC patients is
crucial. Numerous previous studies have evaluated the association
between EGFR/KRAS mutation status, ALK rearrangement and
histological subtype (14–17). However, the results of these studies
have been controversial. EGFR mutations have most often been
examined in papillary and micropapillary subtypes, and KRAS
mutations in solid subtypes (6). Yet,
Yoshizawa et al (14) reported
that EGFR mutations were significantly associated with lepidic and
papillary subtypes, and KRAS mutations with adenocarcinomas with
mucin production. In addition, a previous study confirmed that a
solid signet-ring cell pattern and a mucinous cribriform pattern
were the most common histological features in ALK-positive tumors
(16), whereas another study revealed
a significant association between intra- and/or extra-cytoplasmic
mucin and cribriform patterns in tumors with ALK rearrangements
(17).
Following the established IASLC/ATS/ERS
classification of LAC, the purpose of the present study was to
identify the associations between EGFR/KRAS mutation alterations
and ALK rearrangements and the predominant subtypes in 200 LAC
patients.
Patients and methods
Patients
Cases of invasive LAC in which the patients had
undergone surgery at the Beijing Chest Hospital (Beijing, China)
between March 2005 and June 2014 were randomly selected from the
hospital medical archives. Clinical data were collected, including
gender, age, smoking status, tumor size, lymph node metastases and
stage. The use of archived samples and the protocols in the present
study were approved by the Medical Ethics Committee of Beijing
Chest Hospital, Capital Medical University (approval no.,
2014-No.10).
‘Never smokers’ were defined as patients who had
smoked <100 cigarettes in their lifetime. The patients were not
provided any treatment prior to surgery. Tumor staging was decided
according to the 7th edition of the American Joint Committee for
Cancer staging system (18). The
morphology of all samples was reviewed by at least two
pathologists, according to the 2011 IASLC/ATS/ERS international
multidisciplinary classification of LAC (19).
Tumor samples
Formalin-fixed, paraffin-embedded (FFPE) samples,
stained with hematoxylin and eosin, were reviewed by two
pathologists. FFPE blocks were divided into 5–10 sections of 5-µm
thickness. EGFR and KRAS gene mutations were screened using the
direct DNA sequencing method (20).
Mutations in exons 18, 19, 20 and 21 of EGFR and codons 12 and 13
of KRAS mutations were detected in the present study.
DNA extraction, polymerase chain
reaction (PCR) amplification and direct sequencing for EGFR/KRAS
mutations
FFPE blocks were divided into 10–15 4-µm thick
sections for DNA extraction according to the previously described
protocol (21). Briefly, PCR was
performed with 100 ng template DNA in a 50-µl volume that contained
0.75 units of HotStarTaq DNA polymerase (Fermentas International
Inc.; Thermo Fisher Scientific, Waltham, MA, USA), 5 µl PCR buffer,
0.8 µM deoxyribonucleotide triphosphate, 0.5 µM of each primer and
various concentrations of MgCl2, depending on the
various markers. PCR was performed on a Genepro Thermal Cycler
(TC-E-96G; Hangzhou Bioer Technology Co., Ltd., Hangzhou, China)
and the cycling conditions were as follows: 40 cycles of a
denaturation step at 94°C for 45 sec, a primer annealing step at
56°C for 30 sec and an elongation step at 72°C for 30 sec; and a
final extension step at 72°C for 10 min. The nucleic acid used for
mutations was based on the reference sequence NM_005228.3
(www.ncbi.nlm.nih.gov/gene/?
term=NM_005228.3). The primers (Sangon Biotech Co., Ltd., Shanghai,
China) were designed for EGFR/KRAS mutations using Primer 3
software (primer3.ut.ee/) as follows: EGFR mutation: Exon 18
forward, 5′-CAACCAAGCTCTCTTGAGGATC-3′ and reverse,
5′-CCCAGCCCAGAGGCCTGT-3′; exon 19 forward,
5′-GCAGCATGTGGCACCATCTC-3′ and reverse, 5′-AGAGCCATGGACCCCCACAC-3′;
exon 20 forward, 5′-CACACTGACGTGCCTCTCC-3′ and reverse, 5′-AGC AGG
TAC TGG GAG CCA AT-3′; and exon 21 forward,
5′-TCTGTCCCTCACAGCAGGGTCT-3′ and reverse,
5′-GCTGGCTGACCTAAAGCCACC-3′. β-actin was used as positive control:
forward, 5′-AGAGATGGCCACGGCTGCTT-3′ and reverse,
5′-ATTTGCGGTGGACGATGGAG-3′. The primer sequence for the
amplification of KRAS 12 and 13 codons mutation test referred to
the study by Franklin et al (22) as follows: Forward,
5′-AAGGCCTGCTGAAAATGAC-3′ and reverse, 5′-TGGTCCTGCACCAGTAATATG-3′.
The analysis was performed according to the manufacturer's
protocols using an ABI PRISM 377 DNA Sequencer (Applied Biosystems,
Inc.; Thermo Fisher Scientific).
The detection of the ALK rearrangement was performed
using Ventana immunohistochemistry on a Benchmark XT autostainer
(Ventana Medical Systems, Inc., Tucson, AZ, USA), and with
monoclonal rabbit anti-human ALK antibody (ready to use; clone,
D5F3; catalog no., 790–4794; Ventana Medical Systems, Inc.). The
Optiview DAB IHC detection kit (catalog no., 860–099; Ventana
Medical Systems, Inc.) was used according to the manufacturer's
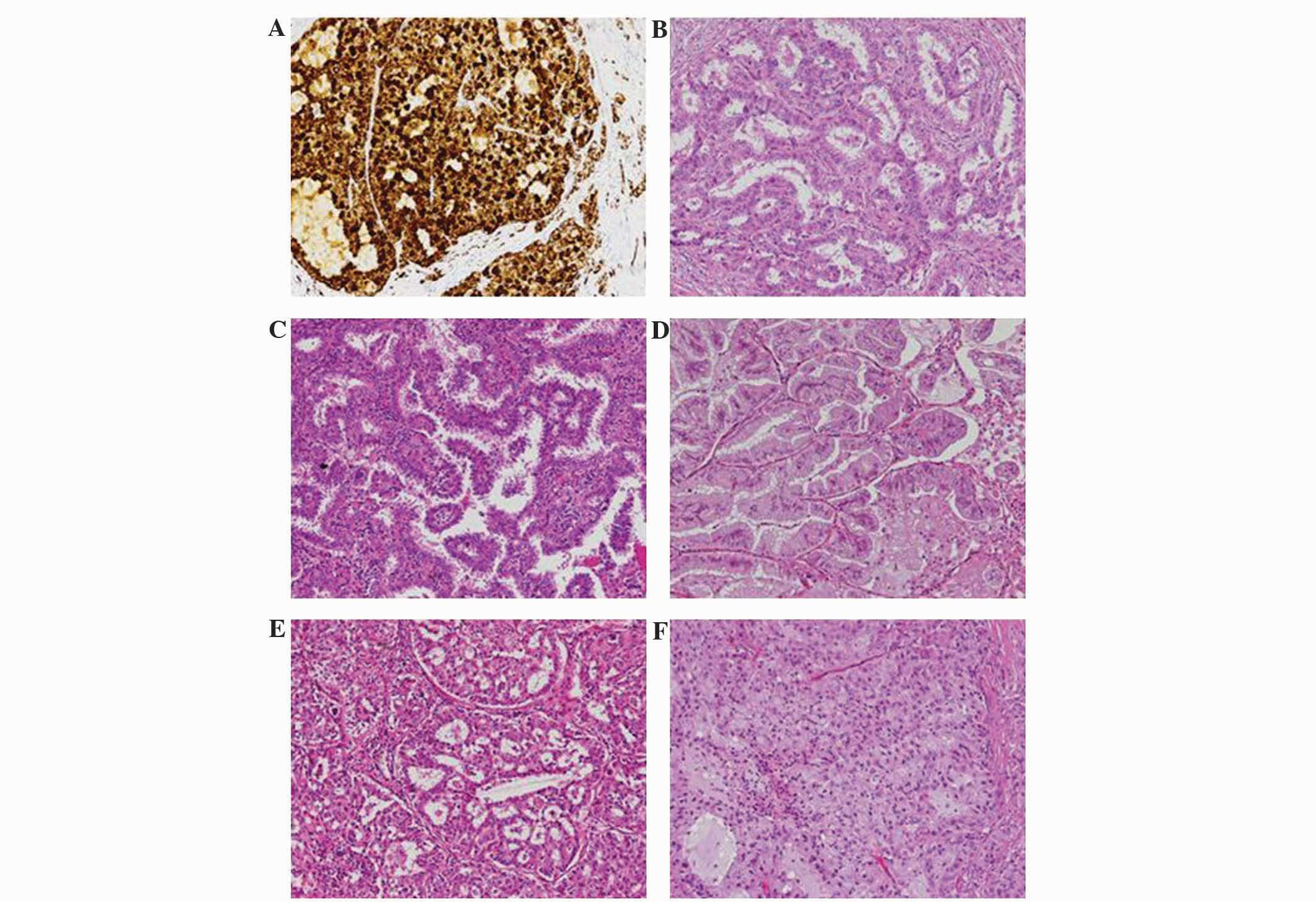
protocols. Positive staining was indicated by strong granular
staining in the tumor cell cytoplasm (Fig. 1A), in any percentage of positive tumor
cells; otherwise, the expression of ALK was considered to be
negative (23).
Statistical analysis
The associations between EGFR/KRAS mutation status
and ALK rearrangement, clinical factors and the predominant subtype
were compared by Fisher's exact test. All statistical tests were
performed on SPSS version 21.0 (IBM SPSS, Armonk, NY, USA), and
values of P<0.05 for the two-tailed test were considered to
indicate a statistically significant difference.
Results
Patient characteristics
Out of 200 patients, 95 (47.5%) were male. The
median age was 59 years (range, 23–79 years) and 61 patients had a
history of smoking. All patients were diagnosed at various clinical
stages: 55 patients at stage IA, 23 patients at stage IB, 17
patients at stage IIA, 3 patients at stage IIB, 80 patients at
stage IIIA, 13 patients at stage IIIB and 9 patients at stage IV.
The tissue sections were confirmed to possess enough tumor cells
for EGFR and KRAS mutation and ALK fusion gene assays. The clinical
data, including gender, age, smoking status, tumor size, lymph node
metastasis and clinical stage, were reviewed. The
clinicopathological features are summarized in Table I.
 | Table I.Clinicopathological characteristics of
EGFR/KRAS mutation status and ALK rearrangement in 200
patients. |
Table I.
Clinicopathological characteristics of
EGFR/KRAS mutation status and ALK rearrangement in 200
patients.
|
| EGFR | KRAS | ALK
rearrangement |
|---|
|
|
|
|
|
|---|
| Clinical
features | Mutation | WT | P-value | Mutation | WT | P-value | Positive | Negative | P-value |
|---|
| Gender |
|
| 0.016 |
|
| 0.323 |
|
| 0.663 |
| Male | 35 | 60 |
| 11 | 84 |
| 12 | 83 |
|
|
Female | 57 | 48 |
| 7 | 98 |
| 11 | 94 |
|
| Age, years |
|
| 0.478 |
|
| 0.089 |
|
| 0.001 |
|
Range | 28–76 | 23–79 |
| 50–78 | 23–79 |
| 23–69 | 28–79 |
|
|
Median | 59 | 57 |
| 63 | 58 |
| 47 | 61 |
|
|
<60 | 46 | 60 |
| 6 | 100 |
| 20 | 86 |
|
|
≥60 | 46 | 48 |
| 12 | 82 |
| 3 | 91 |
|
| Smoking status |
|
| 0.542 |
|
| 0.429 |
|
| 0.810 |
|
Yes | 26 | 35 |
| 7 | 54 |
| 6 | 55 |
|
| No | 66 | 73 |
| 11 | 128 |
| 17 | 122 |
|
| Tumor size, cm |
|
| 0.139 |
|
| 0.001 |
|
| 0.564 |
| ≤5 | 80 | 85 |
| 9 | 156 |
| 18 | 147 |
|
|
>5 | 12 | 23 |
| 9 | 26 |
| 5 | 30 |
|
| LN metastasis |
|
| 0.257 |
|
| 0.622 |
|
| 0.046 |
|
Yes | 45 | 62 |
| 11 | 96 |
| 17 | 90 |
|
| No | 47 | 46 |
| 7 | 86 |
| 6 | 87 |
|
| Clinical stage |
|
| 1.000 |
|
| 1.000 |
|
| 0.076 |
|
I+II | 45 | 53 |
| 9 | 89 |
| 7 | 91 |
|
|
III+IV | 47 | 55 |
| 9 | 93 |
| 16 | 86 |
|
Histological features
According to the IASLC/ATS/ERS classification, the
patients were grouped into the following subtypes: Lepidic
predominant, 5 patients (2.5%); acinar predominant, 77 patients
(38.5%); solid predominant with mucin production, 52 patients
(26.0%); papillary predominant, 49 patients (24.5%); invasive
mucinous adenocarcinoma, 10 patients (5.0%); micropapillary
predominant, 7 patients (3.5%); lepidic component, 51 patients
(25.5%); intra-/extra-cytoplasmic mucus, 47 cases (23.5%);
cribriform pattern, 12 patients (6.0%); and signet-ring cell, 9
patients (4.5%) (Fig. 1B–F).
Associations between EGFR/KRAS
mutations and ALK rearrangements and the predominant morphological
structure EGFR mutations
EGFR mutations were examined in 92 patients (46%),
including 6 cases with mutations in exon 18, 42 cases in exon 19, 6
cases in exon 20 and 32 cases in exon 21. The remaining 6 patients
exhibited multiple mutations, of which 3 patients possessed
sensitive mutations in exons 18, 19 or 21; and 3 patients possessed
sensitive and primarily resistant mutations in the exon 20
insertion. The frequency of EGFR mutation was increased in females
compared with males (P=0.016).
The predominant subtype in 92 EGFR-mutant tumors was
as follows: 3 (60.0%) lepidic predominant, 43 (55.8%) acinar
predominant, 26 (53.1%) papillary predominant, 49 (57.1%) acinar
predominant, 15 (28.8%) solid predominant with mucin production and
1 (10%) invasive mucinous adenocarcinoma.
EGFR mutations were more common in patients with the
acinar predominant subtype (43/77; 55.8%; P=0.030) and the
papillary predominant subtype (26/49; 53.1%; P=0.006). Compared
with the solid predominant subtype with mucin production (15/52;
28.8%; P=0.004) and invasive mucinous adenocarcinoma (1/10; 10.0%;
P=0.007), EGFR mutations were more common in patients with the
acinar predominant subtype (43/77, 55.8%). The papillary
predominant subtype (26/49; 53.1%) was more frequently detected in
EGFR-mutant patients compared with patients with the solid
predominant subtype with mucin production (15/52; 28.8%; P=0.016)
and invasive mucinous adenocarcinoma (1/10; 10.0%; P=0.016). EGFR
mutations were less commonly identified in adenocarcinoma with
mucin production (11/47; 23.4%; P=0.001) (Table II).
 | Table II.Correlation between EGFR/KRAS
mutation status and ALK rearrangement with the histological
subtype. |
Table II.
Correlation between EGFR/KRAS
mutation status and ALK rearrangement with the histological
subtype.
|
| EGFR | KRAS | ALK
rearrangement |
|---|
|
|
|
|
|
|---|
| Predominant
subtypea | Mutation | WT | P-value | Mutation | WT | P-value | Positive | Negative | P-value |
|---|
| Lepidic
predominant |
|
|
Yes | 3 | 0 |
| 0 | 0 |
| 0 | 0 |
|
| No | 89 | 108 |
| 18 | 182 |
| 23 | 177 |
|
| Acinar
predominant |
|
|
Yes | 43 | 34 | 0.030 | 1 | 76 | 0.002 | 6 | 71 | 0.256 |
| No | 49 | 74 |
| 17 | 106 |
| 17 | 106 |
|
| Papillary
predominant |
|
|
Yes | 26 | 23 | 0.038 | 3 | 46 | 0.120 | 1 | 48 | 0.004 |
| No | 66 | 85 |
| 15 | 136 |
| 22 | 129 |
|
| Micropapillary
predominant |
|
|
Yes | 4 | 3 |
| 0 | 7 |
| 2 | 5 |
|
| No | 88 | 105 |
| 18 | 175 |
| 21 | 172 |
|
| Solid
predominant |
|
|
Yes | 15 | 37 | 0.006 | 9 | 43 | 0.023 | 13 | 39 | 0.002 |
| No | 77 | 71 |
| 9 | 139 |
| 10 | 138 |
|
| Invasive mucinous
adenocarcinoma |
|
|
Yes | 1 | 9 | 0.040 | 5 | 5 | 0.004 | 1 | 9 | 1.000 |
| No | 91 | 99 |
| 13 | 177 |
| 22 | 168 |
|
KRAS mutations
In total, 18 patients (9.0%) harbored KRAS
mutations, and 17 tumors were confirmed to contain KRAS mutations
within codon 12 and 1 tumor contained KRAS mutations in codon 13.
KRAS mutations were significantly associated with tumor size
(P=0.001).
KRAS mutations were distributed in 1 (1.3%) patient
with the acinar predominant subtype, 3 (6.1%) with the papillary
predominant, 9 (17.3%) with the solid predominant subtype with
mucin production, and 5 (50%) with invasive mucinous
adenocarcinomas. No KRAS mutation was identified in the lepidic and
micropapillary predominant subtypes.
KRAS mutations occurred most frequently in the solid
predominant subtype (9/52; 17.3%; P=0.023) and the invasive
mucinous adenocarcinoma subtype (5/10; 50.0%; P=0.004), and less
frequently occurred in the acinar predominant subtype (1/77; 1.3%;
P=0.002) and lepidic component (1/50; 2.0%; P=0.047). KRAS
mutations were more common in the invasive mucinous subtype (5/10;
50.0%) compared with acinar predominant tumors (1/77; 1.3%;
P<0.001) and the papillary predominant subtype (3/49; 6.1%;
P=0.002). KRAS mutations were also characterized by adenocarcinoma
with mucin production (9/47; 19.1%; P=0.008) (Table II).
ALK rearrangements
Among the 200 patients, ALK rearrangements were
screened in 23 patients (11.5%). The predominant subtypes in tumors
harboring ALK rearrangements were as follows: Acinar predominant, 6
patients (7.8%); lepidic predominant, 0 patients (0.0%); papillary
predominant, 1 patient (2.0%); micropapillary predominant, 2
patients (28.6%); solid predominant subtype with mucin production,
13 patients (25.0%); and invasive mucinous adenocarcinoma, 1
patient (10.0%). The ALK fusion gene was significantly associated
with young age (P=0.001) and lymph node metastasis (P=0.046).
ALK rearrangements occurred most often in patients
with the solid predominant subtype with mucin production (13/52;
25.0%; P=0.002) and were less common in the papillary predominant
subtype (1/49; 2.0%; P=0.004). ALK-positive tumors occurred more
frequently in the solid predominant subtype with mucin production
(13/52; 25%) compared with the acinar predominant (6/77; 7.8%;
P=0.010) and papillary predominant (1/49; 2.0%; P=0.001) subtypes.
ALK-positive tumors had a tendency to express a characteristic
morphological pattern, including solid predominant subtype with
mucin production (13/52; 25.0%), cribriform structure (7/12; 58.3%;
P<0.001), intra-/extra-cytoplasmic mucin (14/47; 29.8%;
P<0.001) and signet-ring cell presence (7/9; 77.8%; P<0.0001)
(Table II).
Coexistence of EGFR/KRAS mutation
status and ALK rearrangement
Only 1 sample possessed the EGFR mutation (L858R)
and ALK rearrangement, and another possessed an insertion mutation
in exon 20 of the EGFR gene and a missense mutation in codon 12 of
the KRAS gene.
Discussion
Prior to the establishment of the novel
IASLC/ATS/ERS LAC classification, certain studies demonstrated the
association between EGFR/KRAS mutation status or ALK rearrangement
and adenocarcinoma histology according to the 2004 World Health
Organization classification (6,16). Motoi
et al (6) reported a
significant association between the papillary and micropapillary
LAC subtypes and the EGFR mutation.
Although several previous studies have identified
the significant associations of the driver gene mutations and the
predominant subtypes according to the proposed IASLC/ATS/ERS LAC
classification (24,25), the association between the genotype
and subtype of LAC remains unclear. Numerous studies have
identified the associations between EGFR mutations and the
predominant subtypes subsequent to the publication of the novel
IASLC/ATS/ERS classification. Wang et al (26) examined 332 patients with primary LAC,
and 149 patients (44.9%) were found to harbor EGFR mutations; the
EGFR mutation rate was significantly increased in the papillary
predominant subtype compared with the solid predominant subtype
with mucin production (P=0.008). Song et al (24) reviewed 161 patients who underwent
surgical resections, and EGFR mutation was examined in 67 cases
(41.6%). The EGFR mutation was identified more frequently in the
micropapillary predominant (P=0.0068) and lepidic component
(P=0.005) subtypes. The study by Yoshizawa et al (14), which retrospectively examined 167
Japanese patients, indicated that the EGFR mutation was observed in
90 patients (53.9%), and demonstrated a significant association
with the adenocarcinomas with non-mucinous lepidic (P<0.0001)
and papillary components (P<0.0001). Zhang et al
(27) enrolled 349 female patients
who were never-smokers and identified EGFR mutations in 266
patients (76.2%); the predominant subtype was the acinar
predominant subtype (P=0.005).
The 4 aforementioned previous studies from Asia
revealed that EGFR mutations were characterized by the acinar,
papillary and micropapillary subtypes, and non-mucinous lepidic
growth. In the present study, EGFR mutations were more common in
the acinar predominant subtype (P=0.030) and papillary predominant
subtype (P=0.006), and were rarely examined in adenocarcinomas with
mucin production (P=0.001). The present findings were consistent
with the studies by Yoshizawa et al (14) and Zhang et al (27). However, the present study did not find
any significant association between EGFR mutations and the
micropapillary and lepidic predominant subtypes, due to the small
sample size for each (n=7 and n=5, respectively).
The significant association noted between KRAS
mutations and histological subtypes is controversial. Wang et
al (26) did not identify any
significant differences in histological subtypes between the KRAS
mutations and the wild-types; whereas, Yoshizawa et al
(14) and Kakegawa et al
(28) indicated that KRAS mutations
were significantly associated with adenocarcinoma of mucinous tumor
subtypes (P<0.001). Rekhtman et al (29) showed that KRAS mutations occurred more
often in adenocarcinomas with a solid growth pattern (P=0.022).
The present findings indicated that KRAS mutations
were characterized by the solid predominant subtype with mucin
production (P=0.023) and invasive mucinous adenocarcinoma
(P=0.004), and were also more frequently identified in LAC with
mucin production (P=0.008). However, KRAS mutations were less
frequently indicated in the acinar predominant subtype (P=0.002)
and adenocarcinomas with lepidic component (P=0.047), which was in
consistent with the report by Rekhtman et al (29).
Based on the IASLC/ATS/ERS classification of LAC,
several studies (15,16) have confirmed that the acinar
predominant subtype, solid growth pattern, cribriform structure,
mucin production and lack of lepidic growth were more common in
ALK-positive patients. In the study by Yoshida et al
(16), a solid signet-ring cell
pattern and a mucinous cribriform pattern were examined in 43 and
56% of ALK-positive cases, respectively. Jokoji et al
(17) indicated that ALK-positive
tumors showed significant associations with
intra-/extra-cytoplasmic mucin (P=0.0001), and the cribriform
pattern with excessive extracytoplasmic mucin (P<0.0001).
Cribriform structure, as a unique pattern of the
acinar subtype, was identified for being associated with the
distinct histopathological features of ALK rearrangement in a
previous study (30). A significant
association between ALK rearrangement and cribriform structure
(P<0.0001) was also indicated in the present study. ALK
rearrangements occurred most often in the solid predominant subtype
with mucin production (P=0.002) and less frequently in the
papillary predominant subtype (P=0.004). ALK-positive tumors were
also characterized by signet-ring cell presence (P<0.001) and
adenocarcinoma with mucin production (P=0.0001).
The EGFR/KRAS mutation status and ALK rearrangement
were mutually exclusive with each other (31). However, several studies (26,32) have
reported the coexistence of two mutations of the three driver
genes. In the present study, 1 tumor possessed the EGFR mutation
(L858R at exon 21) and ALK rearrangement, and 1 tumor possessed the
EGFR mutation (at exon 20) and KRAS mutation (codon 12). The KRAS
mutation and the ALK rearrangement did not occur together in the
sample of patients, and no cases exhibited all three driver gene
mutations. The coexistence of the two genes is rarely reported.
Determination of an effective treatment for patients who exhibit
the coexistence of gene mutations requires a larger study sample
size.
In conclusion, the present data revealed a distinct
association between EGFR/KRAS mutation status and the ALK
rearrangement and predominant subtype, according to the novel
IASLC/ATS/ERS LAC scheme. EGFR mutations were significantly
associated with the acinar and papillary predominant subtypes,
whereas KRAS mutations were significantly associated with the solid
predominant subtype and invasive mucinous adenocarcinoma. ALK
rearrangements were significantly characterized by the solid
predominant subtype with mucin production, the cribriform pattern
and a signet-ring cell appearance. The histological subtype
proposed by the novel IASLC/ATS/ERS LAC classification may aid the
identification of patients with specific genotypes and
individualized treatment.
References
|
1
|
Devesa SS, Bray F, Vizcaino AP and Parkin
DM: International lung cancer trends by histologic type: Male:
Female differences diminishing and adenocarcinoma rates rising. Int
J Cancer. 117:294–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reck M, von Pawel J, Zatloukal P, Ramlau
R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N and
Manegold C: Phase III trial of cisplatin plus gemcitabine with
either placebo or bevacizumab as first-line therapy for nonsquamous
non-small-cell lung cancer: AVAil. J Clin Oncol. 27:1227–1234.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jorge SEDC, Kobayashi SS and Costa DB:
Epidermal growth factor receptor (EGFR) mutations in lung cancer:
Preclinical and clinical data. Braz J Med BiolRes. 47:929–939.
2014. View Article : Google Scholar
|
|
5
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: West Japan Oncology Group: Gefitinib versus cisplatin plus
docetaxel in patients with non-small-cell lung cancer harbouring
mutations of the epidermal growth factor receptor (WJTOG3405): An
open label, randomised phase 3 trial. Lancet Oncol. 11:121–128.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Motoi N, Szoke J, Riely GJ, Seshan VE,
Kris MG, Rusch VW, Gerald WL and Travis WD: Lung adenocarcinoma:
Modification of the 2004 WHO mixed subtype to include the major
histologic subtype suggests correlations between papillary and
micropapillary adenocarcinoma subtypes, EGFR mutations and gene
expression analysis. Am J Surg Pathol. 32:810–827. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yatabe Y, Koga T, Mitsudomi T and
Takahashi T: CK20 expression, CDX2 expression, K-ras mutation and
goblet cell morphology in a subset of lung adenocarcinomas. J
Pathol. 203:645–652. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al:
EGF receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Riely GJ, Marks J and Pao W: KRAS
mutations in non-small cell lung cancer. Proc Am Thorac Soc.
6:201–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berge EM, Lu X, Maxson D, Barón AE,
Gadgeel SM, Solomon BJ, Doebele RC, Varella-Garcia M and Camidge
DR: Clinical benefit from pemetrexed before and after crizotinib
exposure and from crizotinib before and after pemetrexed exposure
in patients with anaplastic lymphoma kinase-positive non-small-cell
lung cancer. Clinical Lung Cancer. 14:636–643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Warth A, Muley T, Meister M, Stenzinger A,
Thomas M, Schirmacher P, Schnabel PA, Budczies J, Hoffmann H and
Weichert W: The novel histologic international association for the
study of lung cancer/American thoracic society/European respiratory
society classification system of lung adenocarcinoma is a
stage-independent predictor of survival. J Clin Oncol.
30:1438–1446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Russell PA, Barnett SA, Walkiewicz M,
Wainer Z, Conron M, Wright GM, Gooi J, Knight S, Wynne R, Liew D
and John T: Correlation of mutation status and survival with
predominant histologic subtype according to the new IASLC/ATS/ERS
lung adenocarcinoma classification in stage III (N2) patients. J
Thorac Oncol. 8:461–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshizawa A, Sumiyoshi S, Sonobe M,
Kobayashi M, Fujimoto M, Kawakami F, Tsuruyama T, Travis WD, Date H
and Haga H: Validation of the IASLC/ATS/ERS lung adenocarcinoma
classification for prognosis and association with EGFR and KRAS
gene mutations: Analysis of 440 Japanese patients. J Thorac
Oncology. 8:52–61. 2013. View Article : Google Scholar
|
|
15
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida A, Tsuta K, Nakamura H, Kohno T,
Takahashi F, Asamura H, Sekine I, Fukayama M, Shibata T, Furuta K
and Tsuda H: Comprehensive histologic analysis of ALK-rearranged
lung carcinomas. Am J Surg Pathol. 35:1226–1234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jokoji R, Yamasaki T, Minami S, Komuta K,
Sakamaki Y, Takeuchi K and Tsujimoto M: Combination of
morphological feature analysis and immunohistochemistry is useful
for screening of EML4-ALK-positive lung adenocarcinoma. J Clin
Pathol. 63:1066–1070. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC lung cancer staging project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimada Y, Saji H, Nomura M, Matsubayashi
J, Yoshida K, Kakihana M, Kajiwara N, Ohira T and Ikeda N: Cancer
stem cell-related marker expression in lung adenocarcinoma and
relevance of histologic subtypes based on IASLC/ATS/ERS
classification. Onco Targets Ther. 6:1597–1604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kosaka T, Yatabe Y, Onozato R, Kuwano H
and Mitsudomi T: Prognostic implication of EGFR, KRAS, and TP53
gene mutations in a large cohort of Japanese patients with
surgically treated lung adenocarcinoma. J Thorac Oncol. 4:22–29.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai YR, Zhang HQ, Qu Y, Mu J, Zhao D, Zhou
LJ, Yan H, Ye JW and Liu Y: Expression of MET and SOX2 genes in
non-small cell lung carcinoma with EGFR mutation. Oncol Rep.
26:877–885. 2011.PubMed/NCBI
|
|
22
|
Franklin WA, Haney J, Sugita M, Bemis L,
Jimeno A and Messersmith WA: KRAS mutation: Comparison of testing
methods and tissue sampling techniques in colon cancer. J Mol
Diagn. 12:43–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu DP, Dong YJ, Zhang HQ, Wang JH, Qu Y,
Zhou LJ, Su D, Zhang LL, Zhao D and Cai YR: Differential expression
of CRKL and AXL genes in lung adenocarcinoma subtypes according to
the epidermal growth factor receptor and anaplastic lymphoma kinase
gene status. Biomed Rep. 2:481–489. 2014.PubMed/NCBI
|
|
24
|
Song Z, Zhu H, Guo Z, Wu W, Sun W and
Zhang Y: Correlation of EGFR mutation and predominant histologic
subtype according to the new lung adenocarcinoma classification in
Chinese patients. Med Oncol. 30:6452013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsuta K, Kawago M, Inoue E, Yoshida A,
Takahashi F, Sakurai H, Watanabe S, Takeuchi M, Furuta K, Asamura H
and Tsuda H: The utility of the proposed IASLC/ATS/ERS lung
adenocarcinoma subtypes for disease prognosis and correlation of
driver gene alterations. Lung cancer. 81:371–376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Dong Y, Cai Y, Zhou L, Wu S, Liu
G, Su D, Li X, Qin N, Nong J, et al: Clinicopathologic
characteristics of ALK rearrangements in primary lung
adenocarcinoma with identified EGFR and KRAS status. J Cancer Res
Clin Oncol. 140:453–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Sun Y, Pan Y, Li C, Shen L, Li Y,
Luo X, Ye T, Wang R, Hu H, et al: Frequency of driver mutations in
lung adenocarcinoma from female never-smokers varies with
histologic subtypes and age at diagnosis. Clin Cancer Res.
18:1947–1953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kakegawa S, Shimizu K, Sugano M, Miyamae
Y, Kaira K, Araki T, Nakano T, Kamiyoshihara M, Kawashima O and
Takeyoshi I: Clinicopathological features of lung adenocarcinoma
with KRAS mutations. Cancer. 117:4257–4266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rekhtman N, Ang DC, Riely GJ, Ladanyi M
and Moreira AL: KRAS mutations are associated with solid growth
pattern and tumor-infiltrating leukocytes in lung adenocarcinoma.
Mod Pathol. 26:1307–1319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ignatius SH, Ziogas A and Zell JA: Primary
signet-ring carcinoma (SRC) of the lung: A population-based
epidemiologic study of 262 cases with comparison to adenocarcinoma
of the lung. J Thorac Oncol. 5:420–427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gainor JF, Varghese AM, Ou SH, Kabraji S,
Awad MM, Katayama R, Pawlak A, Mino-Kenudson M, Yeap BY, Riely GJ,
et al: ALK rearrangements are mutually exclusive with mutations in
EGFR or KRAS: An analysis of 1,683 patients with non-small cell
lung cancer. Clin Cancer Res. 19:4273–4281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang X, Zhang S, Yang X, Yang J, Zhou Q,
Yin L, An S, Lin J, Chen S, Xie Z, et al: Fusion of EML4 and ALK is
associated with development of lung adenocarcinomas lacking EGFR
and KRAS mutations and is correlated with ALK expression. Mol
Cancer. 9:1882010. View Article : Google Scholar : PubMed/NCBI
|