Introduction
Prostatic stromal sarcoma (PSS) is an extremely rare
type of adult non-epithelial malignant tumor affecting the
prostate. In 1998, Gaudin et al (1) classified PSS into two categories:
Prostatic stromal proliferation of uncertain malignant potential
and PSS. The etiology and pathogenesis of PSS is currently unknown,
and no confirmed risk factors have been identified (1,2). As the
majority of patients present with obstructive urinary symptoms, the
diagnosis of prostatic stromal sarcoma is frequently made following
open prostatectomy or transurethral resection of the prostate
(3). Owing to the rarity of the
tumor, with <30 cases reported in the literature, the optimal
treatment for this disease has not yet been determined (3). The robot-assisted laparoscopic radical
prostatectomy (RLRP) has grown increasingly popular and rapidly
equated itself as the most frequently used modality to treat
organ-confined prostate cancer (4). A
limited number of PSS cases have been treated with RLRP due to the
low prevalence and poor prognosis of such malignancies. The current
study presents the case of a patient with PSS who was treated with
RLRP.
Case report
On April 8, 2014, a 32-year-old man was referred to
the Department of Urology, The First Affiliated Hospital, School of
Medicine, Zhejiang University (Hangzhou, China) with progressive
obstructive voiding symptoms that had persisted for 2 years. The
patient had also developed acute urinary retention that had been
apparent for 3 days. A digital rectal examination revealed a huge
solid mass. Serum levels of prostate-specific antigen (PSA) were
not elevated (0.44 ng/ml; normal range, 0–4 ng/ml). A computed
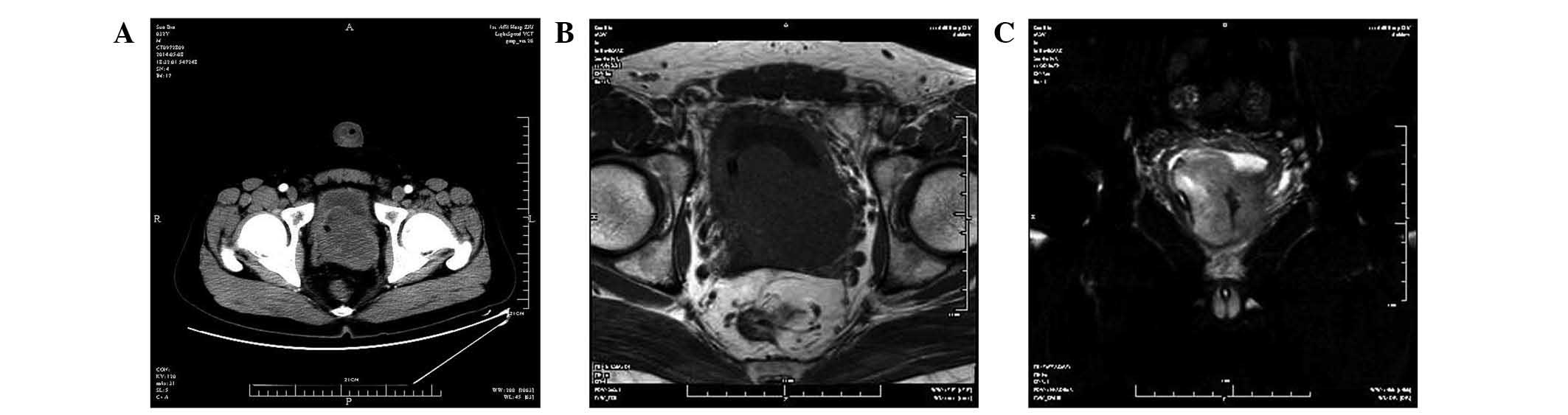
tomography scan (SOMATOM Definition AS20; Siemens AG, Munich,
Germany) of the pelvis showed an 8×6-cm prostatic mass protruding
into the bladder (Fig. 1A). A
magnetic resonance imaging scan (Signa HDxt 3.0T; GE Healthcare
Life Sciences, Chalfont, UK) revealed a multinodular mass, with
homogeneous low signal intensity on T1-weighted imaging (Fig. 1B) and heterogeneous high signal
intensity on T2-weighted imaging (Fig.
1C). No enlarged lymph nodes were detected. A transperineal,
ultrasound-guided prostate biopsy was performed on April 16, 2014.
The histopathological examination revealed spindle tumor cells with
cytologic atypia and mitoses, thus resulting in a diagnosis of PSS.
On May 14, 2014, the patient underwent RLRP without pelvic
lymphadenectomy.
For the RLRP, the patient was placed in the dorsal
lithotomy position with his arms secured to his side. A
pneumoperitoneum was created using a VERESS pneumoperitoneum needle
(Karl Storz GmbH & Co. KG, Tuttlingen, Germany). The trocars
were positioned with similar placement to a standard robotic
prostatectomy: A 12-mm camera port was placed 2 cm cephalad to the
umbilicus; two 8-mm metal robotic ports were placed bilaterally
along the midclavicular line at the level of the umbilicus; an
accessory 5-mm port for suction was placed lateral and superior to
the camera port; a second 12-mm assistant port was placed 8–10 cm
lateral to the right robotic port; and a third 8-mm robotic port
was placed 8 cm lateral to the left robotic port, 2 cm above and
anteriorly to the anterior superior iliac spine. The table was
moved to a deep Trendelenburg position. The da Vinci robotic system
(Intuitive Surgical Inc., Sunnyvale, CA, USA) was brought between
the patient's legs, and the four arms were connected to the
corresponding ports.
From a technical standpoint, the surgery was
extremely challenging. The anterior prostatic surface and the
endopelvic fascia were difficult to expose due to the lack of
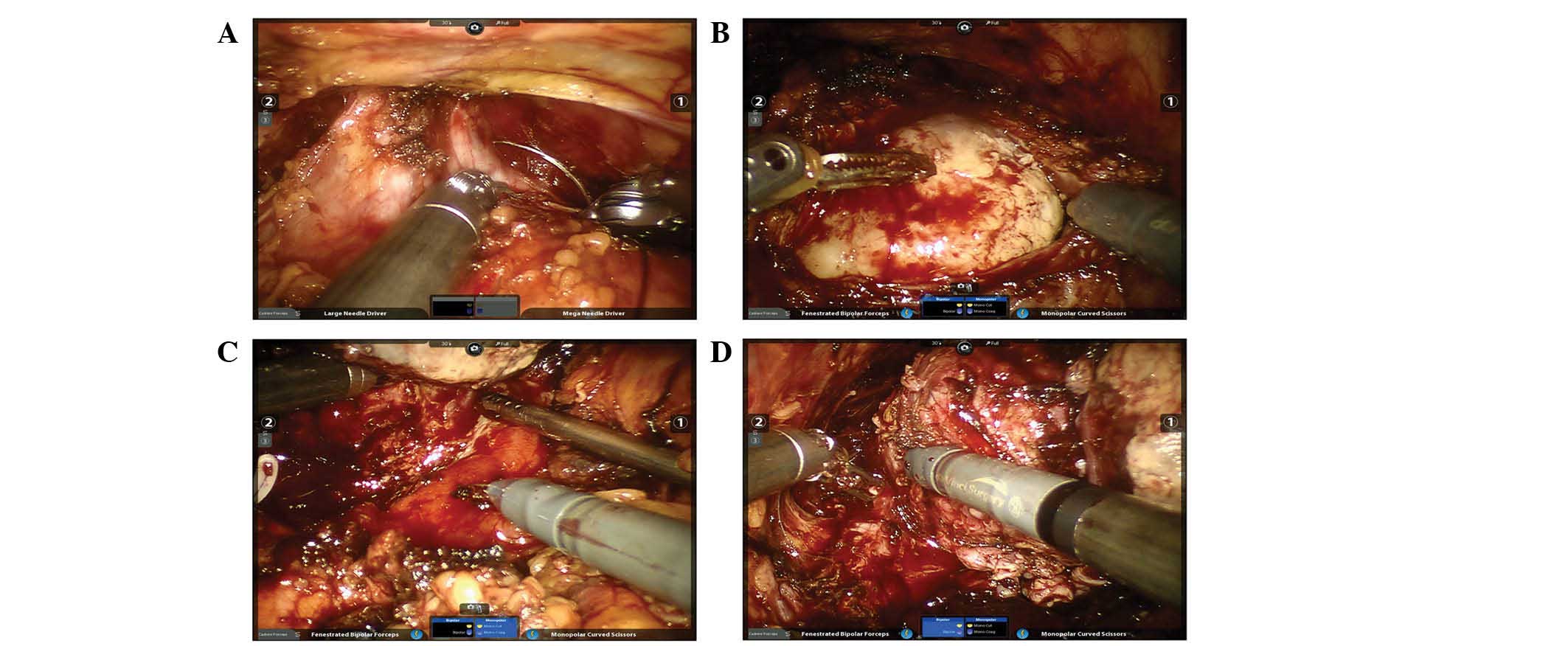
space. Control of the dorsal vein plexus was achieved by a
figure-of-eight ligation, using 2–0 Vicryl sutures (Ethicon;
Johnson & Johnson, New Brunswick, NJ, USA), with the advantage
of flexibility (Fig. 2A). Considering
that the preservation of the bladder neck is impossible and that
the plane between the prostate and bladder neck could not be
identified, the bladder was directly and widely incised to fully
expose the mass protruding into the bladder (Fig. 2B). The bladder neck dissection was
initiated bilaterally along the tumor. However, the dissection of
the posterior bladder neck was challenging due to the poor
elevation of the prostate. The third arm was used to lift the mass
and gain increased elevation (Fig.
2C). This challenge became more significant during the
posterior dissection for the marked adhesion between the rectum and
the prostate. In order to minimize the risk of rectal injury, the
dissection of the apical and lateral pedicles was performed first
and progressed toward the midline (Fig.
2D). Following the removal of the tumor, the large bladder neck
was reconstructed using the tennis racket technique with a 2–0
Vicryl suture in running fashion. The wide gap between the bladder
and urethra that was left following the removal of the huge mass
resulted in tension on the anastomosis, which was reduced by
additional mobilization of the bladder, decreased Trendelenberg
tilt and perineal pressure.
The patient recovered uneventfully post-operatively,
and was discharged 2 weeks after the surgery. The resected specimen
was a yellow-white tumor of 8 cm in diameter, with occasional
hemorrhagic foci. A pathological examination of the specimen
revealed a PSS with negative surgical margins. Formalin-fixed,
paraffin-embedded, 5-µm thick sections were immunohistochemically
diffusely positive for cluster of differentiation (CD34; anti-mouse
antibody; catalog no., 14-0341-85; dilution, 1:250; eBioscience,
Inc., San Diego, CA, USA), and focally positive for B-cell lymphoma
2, but not for CD117, vimentin, epithelial membrane antigen and
progesterone receptor. No recurrence or distant metastasis of the
tumor has occurred following the surgical intervention, and the
patient continues to be followed up.
Discussion
Prostate sarcoma is a rare malignancy that is
associated with a poorer prognosis compared with prostate cancer.
PSS is an extremely rare subtype of prostate sarcoma with <30
documented cases (2,3,5,6). The majority of these lesions present in
the sixth and seventh decades of life, and the majority of patients
present with symptoms of urethral obstruction, such as in the
present case (2,3,5,6). Patients with PSS usually have a PSA
level within the normal range. Histologically, the tumor cells have
an ovoid, spindle shape, with pleomorphic nuclei. CD34, CD117,
vimentin and progesterone receptor may assist in distinguishing
between these tumors and other prostatic mesenchymal neoplasms, for
example, rhabdomyosarcoma and leiomyosarcoma are negative for CD34
and positive for vimentin (7).
PSS presents a significant therapeutic challenge.
Due to the rare occurrence of PSS and the paucity of published
literature, the optimal treatment for the disease is unknown. Osaki
et al (2) presented a case of
PSS in which a suprapubital radical prostatectomy was performed
without adjuvant therapy, with no recurrence reported at 8 years
post-surgery. Reese et al (3)
applied an aggressive multimodality approach in the management of a
PSS, including neoadjuvant chemotherapy and radiation, which
resulted in a complete response in the primary lesion, followed by
radical cystoprostatectomy. Chang et al (8) reported one case of PSS treated with
radical cystoprostatectomy followed by radiotherapy, and the
patient was alive and well 5 months after treatment. The outcomes
of these studies are challenging to interpret due to the
heterogeneity of treatment modalities. However, a complete radical
surgical resection (radical prostatectomy or cystoprostatectomy)
remains the preferred treatment that is most likely to result in
long-term survival (1). In the
present study, with consideration to the particularly young age of
the patient, and due to no apparent bladder invasion on the
pre-operative images, the decision was made to perform a radical
prostatectomy with the expectation of sparing the bladder.
Open surgery, the standard laparoscopic technique
and the robot-assisted technique are surgical options for the
management of PSS. Previously, the feasibility of laparoscopic
surgery for PSS has not been reported. To the best of our
knowledge, only the study by Choi et al (9) has previously reported on the experience
of conducting a robot-assisted excision of a PSS, in 2014. RLRP has
become a common, widely accepted and effective surgical choice for
patients with prostate cancer. In the present case, the traditional
laparoscopic instruments were limited by the narrow manipulation
space in the deep pelvis. The da Vinci robot-assisted laparoscopic
surgical system possesses various advantages over standard
laparoscopic surgery (10,11). The da Vinci system generates an
accurate three-dimensional depth of field using a high resolution,
and the third arm maintains a strong retraction for exposure, thus
enabling the surgeon to acquire a visual field (10,11).
Furthermore, the da Vinci system offers a high degree of freedom
for operating the instruments, and achieves the separation and
dissection of tumors with more precision, while minimizing the
possibility of intraoperative complications, including ureteral and
rectal injury (10,11).
PSS is an aggressive disease with a poor prognosis.
Despite the challenges that were experienced due to the large tumor
size and adhesions, the en bloc resection of the tumor with
negative margins and favorable short-term results observed in the
present case demonstrate that robotic extirpation is a feasible and
effective option for the management of PSS.
References
|
1
|
Gaudin PB, Rosai J and Epstein JI:
Sarcomas and related proliferative lesions of specialized prostatic
stroma: A clinicopathologic study of 22 cases. Am J Surg Pathol.
22:148–162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Osaki M, Osaki M, Takahashi C, Miyagawa T,
Adachi H and Ito H: Prostatic stromal sarcoma: Case report and
review of the literature. Pathol Int. 53:407–411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reese AC, Ball MW, Efron JE, Chang A,
Meyer C and Bivalacqua TJ: Favorable response to neoadjuvant
chemotherapy and radiation in a patient with prostatic stromal
sarcoma. J Clin Oncol. 30:e353–e355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wirth MP and Froehner M: Robot-assisted
radical prostatectomy: The new gold standard? Eur Urol. 57:750–751.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tamada T, Sone T, Miyaji Y, Kozuka Y and
Ito K: MRI appearance of prostatic stromal sarcoma in a young
adult. Korean J Radiol. 12:519–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamazaki H, Ohyama T, Tsuboi T, Taoka Y,
Kohguchi D, Iguchi H and Ao T: Prostatic stromal sarcoma with
neuroectodermal differentiation. Diagn Pathol. 7:1732012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Westra WH, Gerald WL and Rosai J: Solitary
fibrous tumor. Consistent CD34 immunoreactivity and occurrence in
the orbit. Am J Surg Pathol. 18:992–998. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang YS, Chuang CK, Ng KF and Liao SK:
Prostatic stromal sarcoma in a young adult: A case report. Arch
Androl. 51:419–424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi SH, Kim TH, Yoon GS, Chung SK, Kim BW
and Kwon TG: Two different surgical approaches for prostatic
stromal sarcoma: Robot-assisted laparoscopic radical prostatectomy
and open radical cysto-prostatectomy with ileal conduit. Korean J
Urol. 55:620–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trabulsi EJ, Zola JC, Colon-Herdman A,
Heckman JE, Gomella LG and Lallas CD: Minimally invasive radical
prostatectomy: Transition from pure laparoscopic to
robotic-assisted radical prostatectomy. Arch Esp Urol. 64:823–829.
2011.(In Spanish). PubMed/NCBI
|
|
11
|
Finkelstein J, Eckersberger E, Sadri H,
Taneja SS, Lepor H and Djavan B: Open versus laparoscopic versus
robot-assisted laparoscopic prostatectomy: The European and US
experience. Rev Urol. 12:35–43. 2010.PubMed/NCBI
|