Introduction
Teratomas may be categorized as either mature or
immature, and originate from the three pluripotent germ cell
layers: The ectoderm (skin and neural tissue), the endoderm
(gastrointestinal and bronchial epithelium, and thyroid tissue),
and the mesoderm (fat, bone, muscle and cartilage) (1). Immature teratomas are rare tumors that
develop in the ovaries, accounting for 0.25–3% of tumors that
develop in the ovaries, with individuals at a greater risk during
their first two decades of life (1).
By contrast, mature teratomas occur most commonly in women aged
between 20–40 years (2). Teratomas
have high recurrence and metastasis rates, and immature tumor
tissues may be converted into mature tissues following
post-surgical recurrence. The conversion of immature teratoma is
characterized by slow growth, so symptoms are not typical. Clinical
doctors often neglect the diagnosis of teratomas. If the size of
the tumor is >6 cm, it is common for surgeons to utilize a
surgical treatment. Recurrence typically develops subsequent to
surgical excision of the tumor, often within the first year of
primary therapy (3). The present
study describes the case of a patient who underwent excision of an
immature teratoma, which later developed into mature teratomas
found as extensive abdominal metastases to the liver, spleen and
pelvic cavity, identified after 10 years.
Case report
A 24-year-old female was referred to the Department
of General Surgery, The Second Hospital of Dalian Medical
University (Dalian, China), on 24 April 2013, due to intermittent
upper abdominal pain. Upon physical examination of the right
abdomen, a hard, large mass that moved under palpation was
identified. Abdominal tenderness, rebound pain and muscular tension
were impalpable, with no other positive signs. When examining the
medical history of the patient in detail, an immature right ovarian
teratoma that had been diagnosed 10 years previously was noted, for
which the patient had undergone a laparotomy and right ovarian
oophorectomy. The post-surgical pathological analysis had
identified an immature teratoma, with an official diagnosis of
grade 2 immature teratoma [Norris grading system (4)]. Adjuvant therapy had been administered,
consisting of 3 cycles of bleomycin (20 U/m2), etoposide
(1,675 mg/m2) and cisplatin (20 mg/m2),
following surgery. At the last chemotherapy session, no residual
tumor had been observed. The patient had subsequently been lost to
follow-up.
Based on the medical history of the patient,
recurrence of the teratoma was now suspected. Notably, laboratory
tests exhibited elevated levels of the serum markers cancer antigen
(CA)-125 and CA19-9 (Table I). In
order to assess the giant tumor in the abdomen, the patient
underwent a sonographic examination and computed tomography (CT)
scan. The contrast-enhanced total abdominal CT scan revealed a
complex cystic mass, measuring 18.6×15.7 cm2, on the
right lobe of the liver (Fig. 1A).
Additionally, a further tumor that exhibited the same properties as
the giant mass was detected on the spleen (Fig. 1B).
 | Table I.Dynamic change of tumor markers. |
Table I.
Dynamic change of tumor markers.
|
|
| Level post-surgery,
kU/l |
|
|
|---|
|
|
|
|
|
|
|---|
| Tumor marker | Level prior to
surgery, kU/l | 7 days | 30 days | 180 days | Normal range,
kU/l |
|---|
| CA19–9 | >7000.00 | 1927.51 | 250.50 | 23.00 | 0.00–35.00 |
| CA12–5 | 440.63 |
201.70 |
40.00 | 5.20 | 0.00–35.00 |
With consideration of the tumor size, the
compression symptoms (the right ventricle, right kidney and
pancreas had shifted to the left; Fig. 1C
and D) and the elevated serum markers, a diagnosis of an
immature teratoma was the most probable. The patient subsequently
underwent a laparotomy. Possible invasion of the tumor into the
liver could not be excluded, therefore, a cardiopulmonary blood
bypass was prepared. Following entry into the peritoneal cavity,
the mass was removed from the right lobe of the liver, the first
porta hepatis was blocked and the right hepatic vein was resected
where the tumor had infiltrated. Subsequently, complete removal of
the mass from the spleen surface was successful. The giant tumor
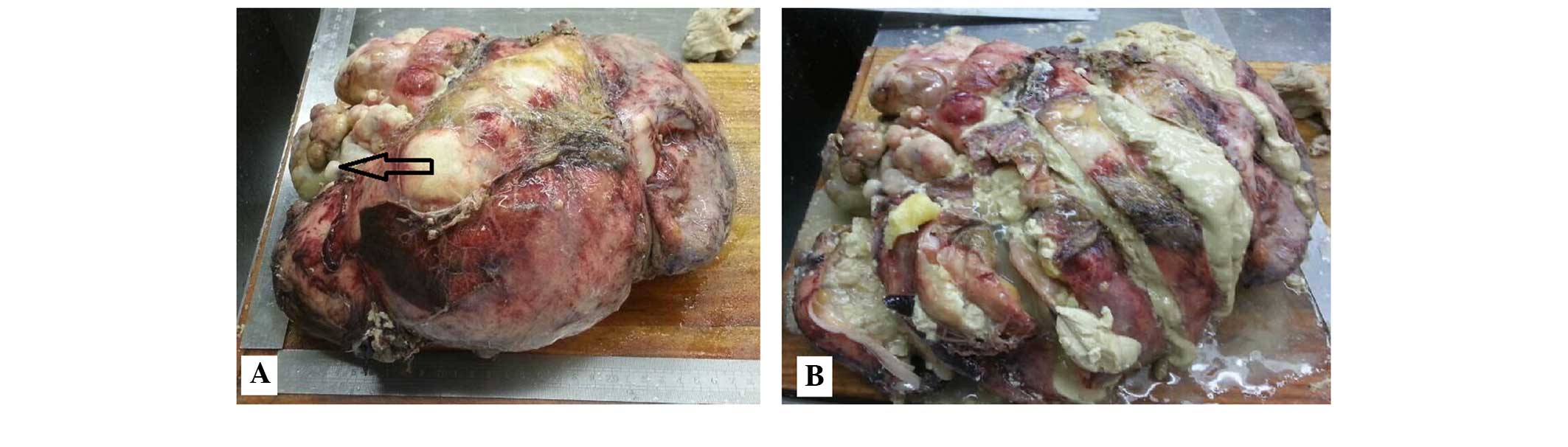
was rich in sebaceous materials, with hair shaft tissues and teeth
identified inside the mass (Fig. 2A and
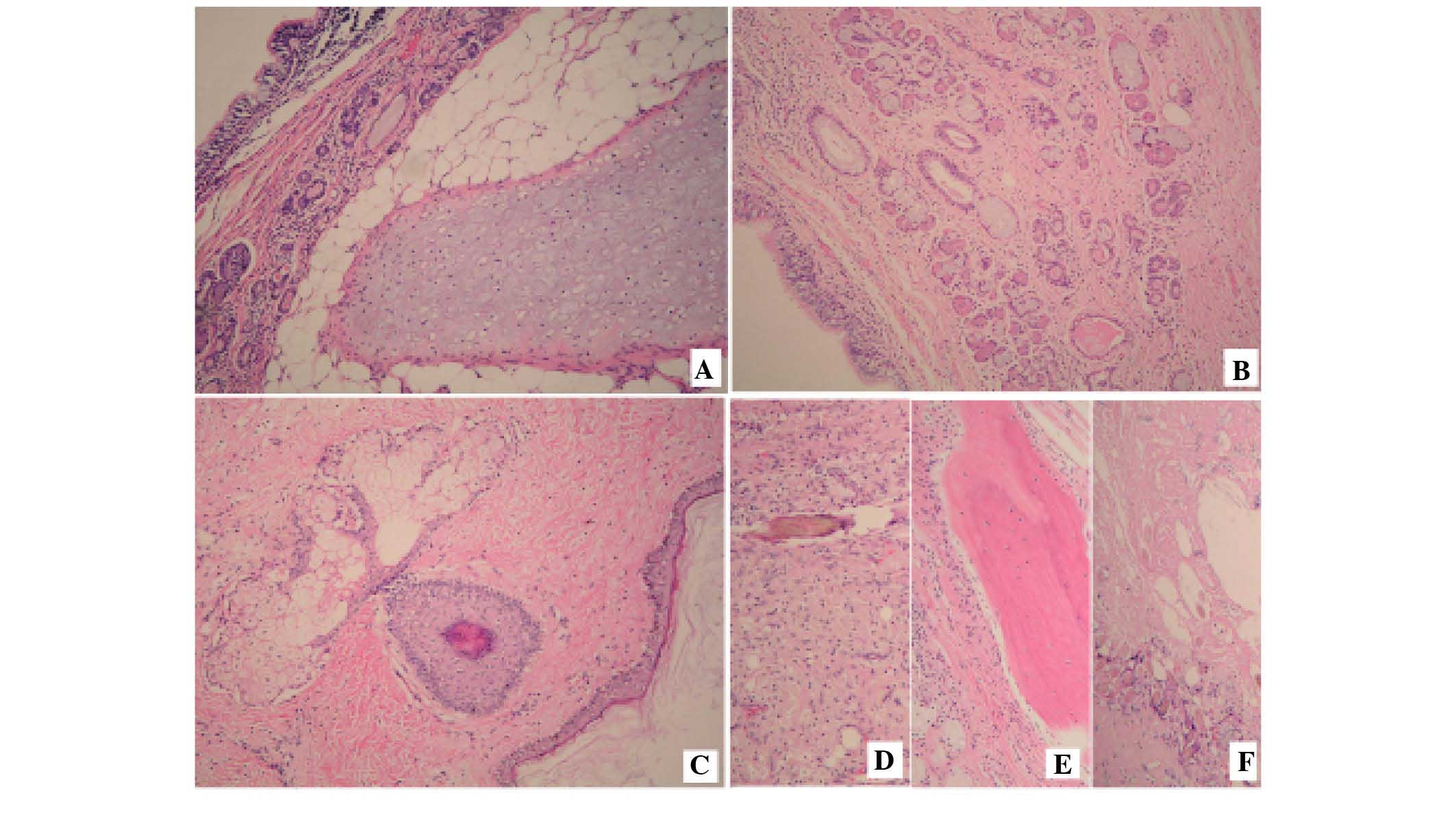
B). The pathology report confirmed that all tumors on the
surface of the liver and the spleen, and in the pelvic cavity were
mature teratomas (Fig. 3). Following
tumor resection, there was no evidence of recurrence or metastasis
during the 1 and 6 month follow-up evaluations and ongoing
follow-up examinations every 3–6 months have been planned.
Discussion
Immature teratomas of the ovary are rare tumors,
accounting for <3% of all teratomas located in the ovaries. The
disease typically occurs during the first two decades of life, and
the tumors are composed of tissue derived two or more germ cell
layers (ectoderm, mesoderm and/or endoderm), containing immature
and embryonal structures (1). The
current case presented with the classic symptoms and histological
appearance associated with immature teratomas. Excision of such
tumors is often followed by local recurrence within the first year
of primary therapy initiation (3).
The present patient was lost to follow-up subsequent to initial
chemotherapy, but developed rare mature teratomas and extensive
abdominal metastases after 10 years.
Mature teratomas are typically benign, however,
malignant transformation may occur, albeit rarely. The peak
incidence of such tumors is reported in women between the ages of
20–40 years. The disease is characterized by slow growth, with a
growth rate of 1.8 mm per year, and when the size of the tumor is
<6 cm, it is common for surgeons to utilize a non-surgical
treatment (1). Growing teratoma
syndrome is an uncommon condition present in men and women, with
appropriately treated germ cell tumors characterized by the
persistence or development of enlarging masses following adjuvant
chemotherapy, normal tumor marker values and the presence of mature
teratomas in the specimen. If a diagnosis of growing teratoma
syndrome is confirmed, the surgeon must determine the operability
of the tumor, whilst also weighing up the risks and benefits of
surgery (5). This is necessary to
allow for the optimal management of the disease and to prevent
unnecessary chemotherapy. In the present case, the serum makers,
CA-125 and CA19-9, exhibited particularly high levels when compared
with levels observed in growing teratoma syndrome. Excision was
performed on tumors that were <2 cm in size. Following the
surgery, pathological sections from different locations in the
tumor showed no immature elements. No further treatment was
administered to the patient. To the best of our knowledge, this
metastasis of a mature teratoma to the liver, the spleen and the
pelvic cavity is particularly rare.
When forming a differential diagnosis for teratomas,
the measurement of the serum levels of CA-125 and CA19-9 is a
common method used for early detection. Ustunyurt et al
(6) reported that serum CA19-9 is the
most reliable tumor marker of mature ovarian cystic teratomas, and
that the levels of serum CA19-9 are often associated with the size
of the teratoma; however, this does not appear to have high
specificity or sensitivity. Bast et al (7) reported that serum CA-125 is a reliable
marker, and that in combination with elevated CA19-9, it may be a
differential character of malignant tumors. Notably, in the present
case, the variation in serum CA-125 and CA19-9 levels was greater
compared with the typical change (Table
I).
Harada et al (8) hypothesized that a young age (<30
years), a large cyst size (>8 cm) and the bilateral occurrence
of mature teratomas are predictive factors for recurrence. In the
present case, the patient was at a high risk of recurrence. In
order to prevent residual disease, frequent post-surgical imaging
analysis and the testing of serum markers is required. In the
literature, to the best of our knowledge, no giant teratoma
patients have been described who have presented with the same
uncommon metastasis as the patient in the current case. Due to the
lack of follow-up appointments in China, the tumor was recognized
too late. To increase the likelihood of detecting recurrent
teratomas, longer follow-up periods are required for patients who
are at a high risk of recurrence.
References
|
1
|
Saba L, Guerriero S, Sulcis R, Virgilio B,
Melis G and Mallarini G: Mature and immature ovarian teratomas: CT,
US and MR imaging characteristics. Eur J Radiol. 72:454–463. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang CF and Lin CK: A case of recurrent,
bilateral ovarian mature teratoma in a young woman. BMC Womens
Health. 14:572014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barwad A, Dey P and Shivalingam J:
Metastatic of mature component in a treated case of immature
teratoma diagnosed on fine-needle aspiration cytology of the liver.
Diagn Cytopathol. 39:711–713. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Norris HJ, Zirkin HJ and Benson WL:
Immature (malignant) teratoma of the ovary: A clinical and
pathologic study of 58 cases. Cancer. 37:2359–2372. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Byrd K, Stany MP, Herbold NC, Leath CA III
and Hamilton CA: Growing teratoma syndrome: Brief communication and
algorithm for management. Aust NZ J Obstet Gynaecol. 53:318–321.
2013. View Article : Google Scholar
|
|
6
|
Ustunyurt E, Gungor T, Iskender C,
Ustunyurt BO, Bilge U and Mollamahmutoglu L: Tumor markers in
mature cystic teratomas of the ovary. Arch Gynecol Obstet.
279:145–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bast RC Jr, Badgwell D, Lu Z, Marquez R,
Rosen D, Liu J, Baggerly KA, Atkinson EN, Skates S, Zhang Z, et al:
New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 15(Suppl
3): 274–281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harada M, Osuga Y, Fujimoto A, Fujimoto A,
Fujii T, Yano T and Kozuma S: Predictive factors for recurrence of
ovarian mature cystic teratomas after surgical excision. Eur J
Obstet Gynecol Reprod Biol. 171:325–328. 2013. View Article : Google Scholar : PubMed/NCBI
|