Introduction
Gastric cancer is a worldwide health issue that is
commonly associated with gastrointestinal symptoms. Chylothorax
refers to the accumulation of lymphatic fluid in the pleura, due to
the obstruction or disruption of the thoracic duct (1,2). Gastric
carcinoma with chylothorax and lymphedema as the initial
manifestations has rarely been reported. To date, only 14 case
reports of gastric carcinoma associated with chylothorax and
lymphedema are available (3–16).
When a patient presents with chylothorax, the
gastric cancer is typically at an advanced stage and, thus, the
prognosis is considerably poorer compared to the survival time of
patients with gastric cancer without chylothorax; however, the
mechanisms of gastric carcinoma-induced chylothorax have yet to be
elucidated. Limited information has been provided by the existing
literature; of note, in the 14 previously reported cases reviewed
in the present study, chylothorax has been demonstrated to be
closely associated with the presence of lymphedema. However, it is
unclear how lymphedema affects the outcome of patients with
chylothorax.
The current study presents the rare case of a
63-year-old woman diagnosed with gastric carcinoma, presenting with
the initial manifestations of chylothorax and lymphedema of the
lower extremities, and reviews the literature. The aim of the
present case report is to obtain an improved understanding of the
mechanisms of gastric carcinoma-induced chylothorax and lymphedema
in order to achieve a earlier diagnosis of gastric cancer.
Case report
A 63-year-old woman with no relevant medical history
was admitted to The Second Xiangya Hospital of Central South
University (Changsha, China) in March 2014, presenting with gradual
bilateral lower extremity swelling for 8 months and dyspnea for 4
months. No obvious weight loss or fever was observed, and no
complaints of gastrointestinal symptoms were noted during the last
5 months. The patient only complained of decreased appetite.
Physical examination showed dullness to percussion with decreased
breath sounds at the right thorax, as well as mild bilateral
non-pitting edema of the lower extremities. Two enlarged lymph
nodes were identified upon palpation: A left supraclavicular lymph
node measuring 2×2.5 cm and a right neck lymph node measuring 1×1.5
cm.
The laboratory examinations performed revealed the
following: Routine blood and urine tests, renal function tests and
tuberculosis-spot tests were all normal; however, the fecal occult
blood test was weakly positive. Tumor markers identified in the
blood were as follows: Carcinoembryonic antigen (CEA), 6.830 ng/ml
(normal value in blood, <5.000 ng/ml); and cancer antigen 125,
205.82 kU/l (normal value, <35.00 kU/l).
A chest radiograph (Brivo™ DR-F X-ray system; GE
Healthcare, Chicago, IL, USA) revealed a large pleural effusion on
the right side and a small pleural effusion on the left (Fig. 1). Ultrasound confirmed the enlargement
of the left supraclavicular and right neck lymph nodes with the
addition of minimal ascites. No other enlarged superficial lymph
nodes were detected by the ultrasound. Furthermore, a computed
tomography (CT) scan revealed a clearly thickened gastric antrum
wall and an enlarged retroperitoneal lymph node measuring 1.0×1.2
cm with a small pericardial effusion, but no obvious enlarged
mediastinal lymph nodes.
Following admission, turbid-yellow fluid (~500 ml)
was obtained from the space between the lungs and the chest wall by
right-sided thoracentesis. The effusion contained
1,250×106/l leukocytes, with 29.7 g/l albumin (32.4 g/l
serum albumin), 136.5 U/l lactate dehydrogenase (LDH), 7.2 U/l
adenosine deaminase (ADA), 5.80 mmol/l glucose and 19.04 ng/ml CEA
(normal value in pleural effusion, <6.500 ng/ml). Thoracentesis
was performed again 7 days later, yielding ~400 ml milky fluid
containing 4.43 mmol/l triglycerides (1.56 mmol/l serum
triglycerides), 2.92 mmol/l cholesterol (5.13 mmol/l serum
cholesterol), 25.1 g/l albumin, 117.2 U/l LDH, 5.8 U/l ADA, 6.98
mmol/l glucose, 1,200×106/l leukocytes and 17.33 ng/ml
CEA. The examination for chylomicrons in the pleural effusion was
positive. The difference in the factors identified in the first and
second effusions was due to the alteration in the diet of the
patient; between the first and second effusion the patient
increased her intake of dietary fat leading to an increase in
triglycerides and chylomicrons and the milky appearance of the
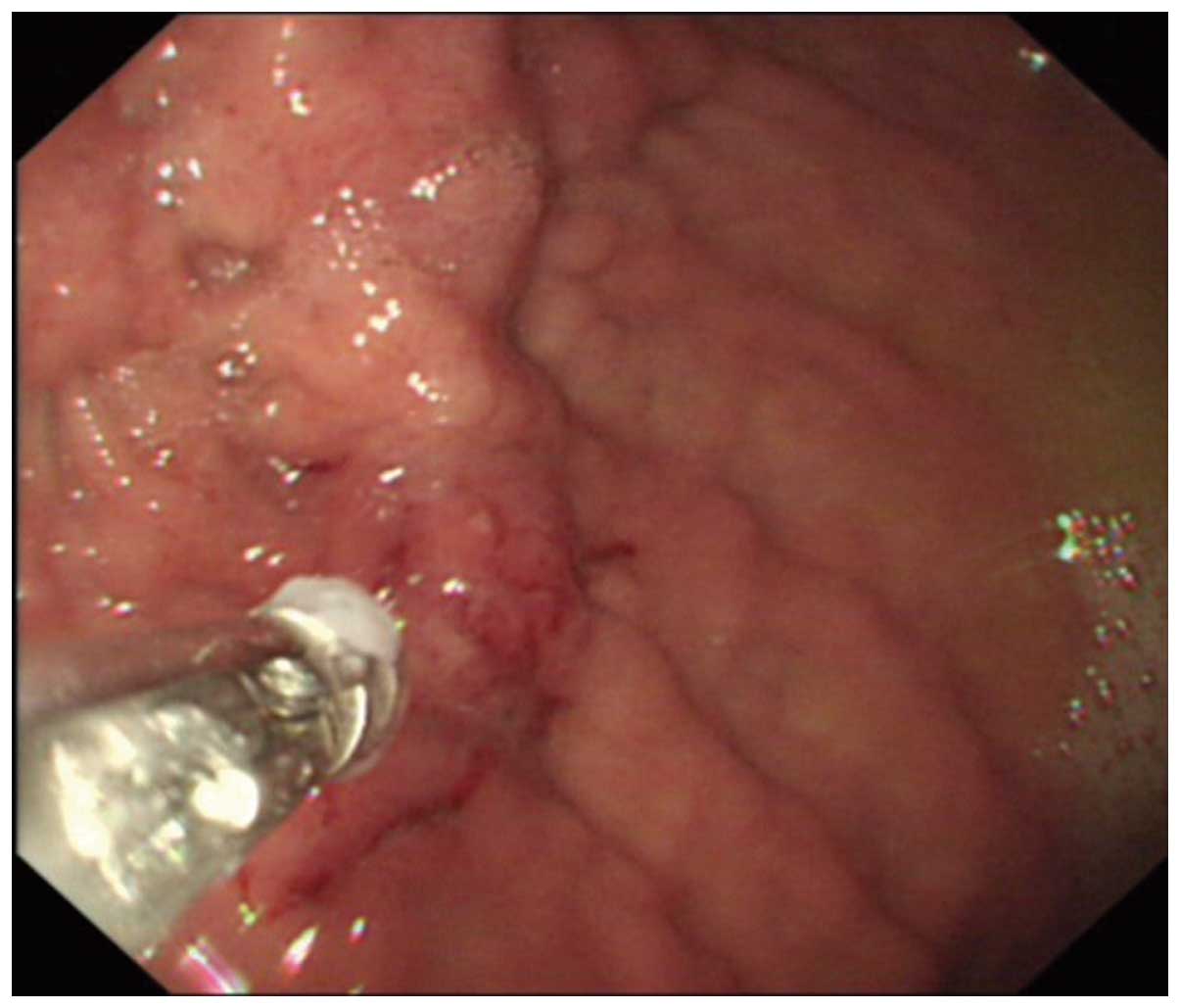
effusion fluid. An irregular apophysis lesion measuring 3.0×3.5 cm
was observed at the gastric fundus by upper gastrointestinal
endoscopy (Fig. 2) (GIF-PQ260 Video
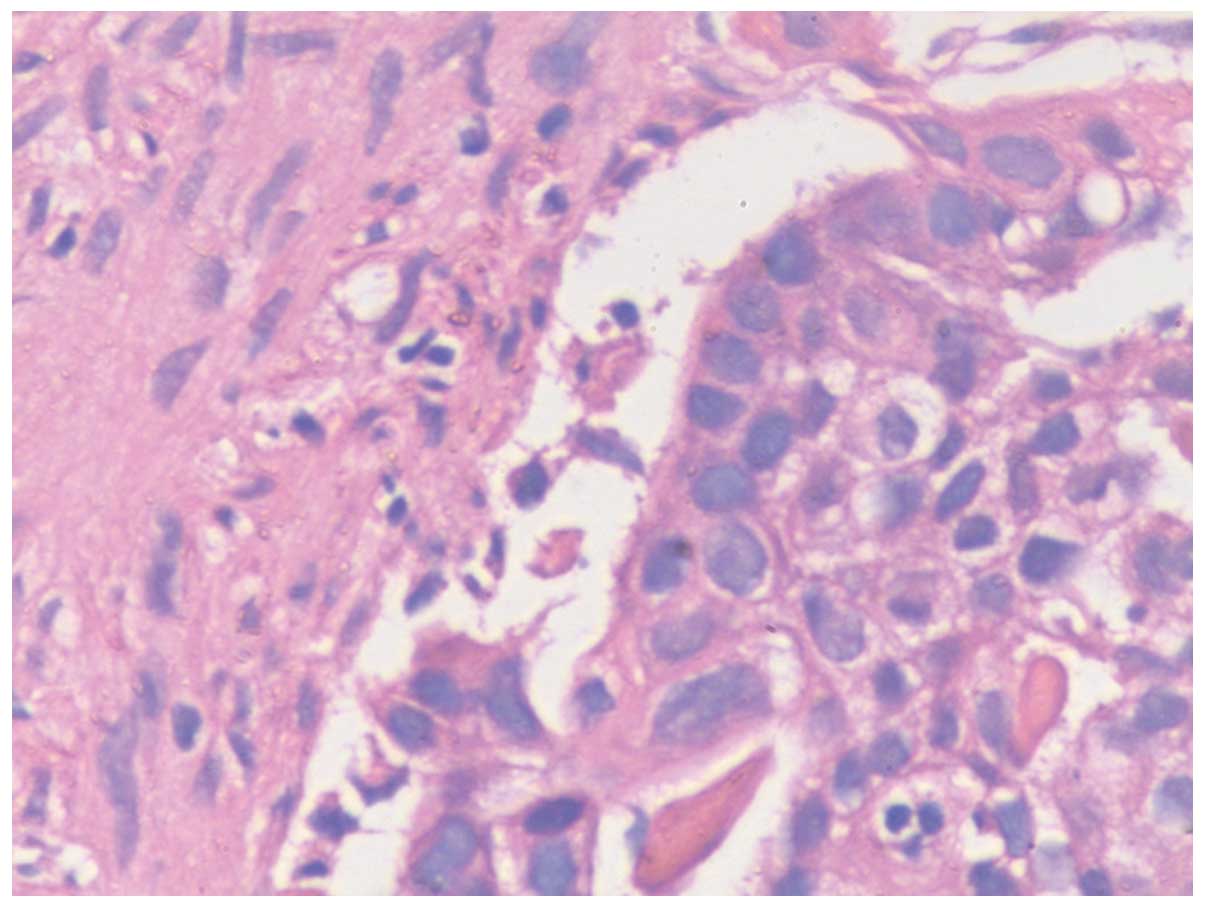
Gastroscope; Olympus Corporation, Tokyo, Japan). Hematoxylin and
eosin staining of the biopsy was undertaken at the Department of
Pathology, The Second Xiangya Hospital of Central South University,
and confirmed a poorly differentiated gastric adenocarcinoma
(Fig. 3). The patient refused further
treatment and follow-ups, and was discharged in April 2014 at her
own request. The patient succumbed 4.5 months following the
diagnosis of chylothorax in August 2014.
Written informed consent was obtained from the
patient's family for the publication of the study.
Discussion
Chylothorax is diagnosed based on the presence of
chylomicrons in the pleural effusion. In the majority of cases, it
occurs following thoracic surgery and it is associated with
malignant tumors, particularly lymphoma (1,2).
To date, 20 cases of chylothorax and lymphedema
associated with gastric carcinoma can be found in the literature,
however, the full text or abstract could be accessed in only 14 of
them (3–16). Despite the publication of these cases,
the mechanism of gastric carcinoma-induced chylothorax has yet to
be determined. Data collected from the present case and the 14
cases found in the literature are presented in Table I.
 | Table I.Characteristics of patients diagnosed
with gastric carcinoma with chylothorax and lymphedema as the
initial manifestations. |
Table I.
Characteristics of patients diagnosed
with gastric carcinoma with chylothorax and lymphedema as the
initial manifestations.
| Author | Age,
years/gender | RE symptoms | GI symptoms |
Chylothorax/location | Lymphedema | Mediastinal/cervical
lymph nodes | Endoscopic
findings | Pathological
diagnosis | Survival time,
months | Refs |
|---|
| Segal et al
(1986) | 69/M | NA | NA | Y/B | NA | NA | NA | NA | NA | (3) |
| Bautz et al
(1991) | 38/F | Y | N | Y/B | N | N | Antral gastritis | Sig | 2.0 | (4) |
| Shibata et al
(1998) | 58/F | Y | Y | Y/B | Y | N | Irregular
erosion | Sig | 4.0 | (5) |
| Mogulkoc et al
(1999) | 19/F | Y | N | Y/B | Y | N | Negative | Sig | 3.5 | (6) |
| Majoor et al
(2007) | 64/M | Y | N | Y/R | Y | N | Gastric ulcer | Por | 6.0 | (7) |
| Lanznaster et
al (2007) | NA | NA | NA | NA/L | Y | Y | Gastric ulcer | Sig | NA | (8) |
| Kayacan et al
(2008) | 28/F | Y | N | Y/B | Y | NA | Not performed | Sig | 7.0 | (9) |
| Devaraj et al
(2014) | 23/M | Y | N | Y/B | Y | Y | Gastric ulcer | Sig | 1.5 | (10) |
| Yamada et al
(2001) | 58/F | Y | N | Y/B | Y | N | Irregular ulcer | Por | 4.0 | (11) |
| Watanabe et al
(2004) | 66/F | Y | N | Y/B | N | NA | Gastric ulcer | Sig | NA | (12) |
| Miyazaki et al
(2007) | 64/M | Y | N | Y/B | Y | Y | Irregular ulcer | Por | 3.5 | (13) |
| Miwa et al
(2009) | 77/F | Y | N | Y/B | Y | Y | NA | Sig | 1.0 | (14) |
| Yoshizawa et
al (2013) | 61/F | Y | N | Y/B | Y | Y | Gastric ulcer | Sig | 10.0 | (15) |
| Martín-Joven et
al (1996) | NA | NA | NA | Y/NA | NA | NA | NA | NA | NA | (16) |
| Present case | 63/F | Y | N | Y/B | Y | Y | Irregular
apophysis | Por | 4.5 |
The patients from the reviewed case reports
typically presented with respiratory rather than gastrointestinal
symptoms. Of the 14 cases, 9 exhibited signet ring cell carcinoma
(4–6,8–10,12,14,15)
and 3 had poorly differentiated gastric adenocarcinoma (7,11,13). Eleven of these patients presented with
bilateral chylothorax with a right effusion predominance. By the
time chylothorax was diagnosed, the majority of patients had missed
the opportunity for surgery and succumbed a few months later (mean
time, 4.3 months).
Limited information on the mechanism of gastric
carcinoma-induced chylothorax has been provided in the literature.
The thoracic duct is primarily located at the right side of the
pleura; this may help explain the development of bilateral
chylothorax, particularly the right effusion predominance (17). Dervaraj et al (10) reported the case of an enlarged left
supraclavicular lymph node measuring 2.2×1.3 cm, which led to the
compression of the left internal jugular vein by 80% compared with
the right internal jugular vein. Dervaraj et al considered
that the increase in thoracic duct pressure caused by the
compressing enlarged lymph node had an important role in the
formation of chylothorax. However, as a large number of collateral
and lymphovenous networks exist in the backflow of the thoracic
duct (17,18), Shibata et al (5) stated that merely the compression of the
thoracic duct was insufficient to cause chylothorax. Yoshizawa
et al (15) instead proposed
that the invasion of the metastatic mediastinal lymph nodes into
the thoracic duct was the cause of chylothorax. However,
mediastinal and enlarged supraclavicular lymph nodes were absent in
5/14 cases (4–7,11).
Metastatic tumor cells infiltrating the lymph vessels close to the
surface of the skin of patients were detected by biopsies in the
studies by Bautz et al (4),
Shibata et al (5) and Mogulkoc
et al (6). Majoor et al
(7) suggested that the invasion of
metastatic tumor cells into the thoracic duct was the direct cause
of chylothorax. In the present case, no obvious mediastinal
enlarged lymph nodes were detected by the CT scan; however, an
enlarged supraclavicular lymph node was observed. Based on the data
collected from the present study and the existing literature, the
conclusion was drawn that the invasion of metastatic tumor cells
into the thoracic duct was the primary cause of chylothorax, and
that the compression of enlarged, metastatic external lymph nodes
may also contribute to the occurrence of chylothorax.
Of note, chylothorax was found to be accompanied by
lymphedema in 10/14 cases (5–11,13–15). There
are two types of lymphedema: Primary lymphedema, which is caused by
lymphatic malformation, and secondary lymphedema, which is a result
of obstruction or disruption of the lymphatic system, for example,
as a consequence of tumors, surgery, trauma, infection and
inflammation (19). As no other
underlying cause could be identified, lymphedema was considered to
be caused by the micrometastasis of gastric carcinoma cells
obstructing or infiltrating into the lymphatic networks located
close to the skin. The association between chylothorax/lymphedema
and gastric carcinoma has not been previously explained. The
presentation of lymphedema preceded that of chylothorax 4 and 1.5
years in the reports of Shibata et al (5) and Mogulkoc et al (6), respectively. This phenomenon was also
observed in the present case, in which the lymphedema presented 4
months prior to the chylothorax. It has been shown that lymphatic
vessels closely intercommunicate through collateral circulation,
resulting in lymph reflux into the thoracic duct (17). We propose that the close association
between lymphedema/chylothorax and gastric carcinoma in the cases
reviewed herein, including the present case, may be a consequence
of the infiltration of gastric carcinoma cells into the lymphatic
circulatory system. First, the micrometastatic cells arrive at the
lymphatic networks located close to the surface of the skin causing
lymphedema. Subsequently, the tumor cells spread throughout the
lymphatic circulatory system, resulting in chylothorax, or, at an
advanced stage, developing into metastatic retroperitoneal lymph
nodes.
In conclusion, differential diagnosis of chylothorax
of unknown cause should consider gastric carcinoma regardless of
gastrointestinal symptoms. Additionly, early detection of malignant
tumors should be performed when patients present with lymphedema of
uncertain causes. The findings of the present and previous studies
suggest that chylothorax and lymphedema in gastric carcinoma may be
a result of the infiltration of gastric carcinoma cells into the
lymphatic circulatory system.
Glossary
Abbreviations
Abbreviations:
|
CEA
|
carcinoembryonic antigen
|
|
ADA
|
adenosine deaminase
|
|
LDH
|
dehydrogenase
|
References
|
1
|
Romero S: Nontraumatic chylothorax: Curr
Opin Pulm Med. 6:287–291. 2000.
|
|
2
|
Schild HH, Strassburg CP, Welz A and Kalff
J: Treatment options in patients with chylothorax. Dtsch Arztebl
Int. 110:819–826. 2013.PubMed/NCBI
|
|
3
|
Segal R, Waron M, Reif R and Zecler E:
Chylous ascites and chylothorax as presenting manifestations of
stomach carcinoma. Isr J Med Sci. 22:897–899. 1986.PubMed/NCBI
|
|
4
|
Bautz JB, Delaney MD, Mostaghim R and
Lodato RF: Chylothorax as presenting manifestation of
adenocarcinoma with probable gastric primary. Chest. 99:1044–1045.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shibata K, Kitagawa S, Fujimura M and
Matsuda T: Chylothorax associated with inflammatory carcinoma.
Intern Med. 37:538–541. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mogulkoc N, Onal B, Okyay N, Günel O and
Bayindir U: Chylothorax, chylopericardium and lymphoedema-the
presenting features of signet-ring cell carcinoma. Eur Respir J.
13:1489–1491. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Majoor CJ, Aliredjo RP, Dekhuijzen PN,
Bulten J and van der Heijden HF: A rare cause of chylothorax and
lymph edema. J Thorac Oncol. 2:247–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lanznaster G, Adami M, Crivellaro C, Kluge
R, Egarter-Vigl E and Wiedermann CJ: Gastric signet-ring cell
carcinoma: Unilateral lower extremity lymphoedema as the presenting
feature. ScientificWorldJournal. 7:1189–1192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kayacan O, Karnak D, Can Ayşe B, Sak
Dizbay S and Beder S: Gastric signet-ring cell adenocarcinoma
presenting with left arm deep-vein thrombosis and bilateral
chylothorax. Clin Appl Thromb Hemost. 14:476–480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Devaraj U, Ramachandran P, Correa M and
Dsouza GA: Chylothorax in gastric adenocarcinoma: A case report and
systematic review of the English literature. Lung India. 31:47–52.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamada M, Kudoh S, Hirata K and Yoshikawa
J: Bilateral chylothorax as initial manifestation of gastric
cancer. Nihon Kokyuki Gakkai Zasshi. 39:343–346. 2001.(In
Japanese). PubMed/NCBI
|
|
12
|
Watanabe K, Yamauchi K, Kobayashi K and
Takeda H: A case of gastric cancer initially presented by bilateral
chylothorax. Nihon Kokyuki Gakkai Zasshi. 42:415–418. 2004.(In
Japanese). PubMed/NCBI
|
|
13
|
Miyazaki S, Noda H, Morita T, Joman M,
Okada M, Moriyama Y, Suzuki K and Takeuchi T: A case of gastric
cancer detected incidentally following to chylothorax, followed by
change in the appearance of pleural effusion with cancer
progression. Nihon Shokakibyo Gakkai Zasshi. 104:1359–1364.
2007.PubMed/NCBI
|
|
14
|
Miwa M, Kasamatsu N, Shibata M, et al: A
case of gastric cancer with bilateral chylothorax. Nihon Kokyuki
Gakkai Zasshi,. 47:1115–1119. 2009.(In Japanese).
|
|
15
|
Yoshizawa K, Sasaki Y, Abe Y, Kanno N,
Mizumoto N, Yagi M, Yaoita T, Iwano D, Nagino K, Sato T, et al:
Chylothorax in a patient with advanced gastric cancer and
mediastinal lymph node metastasis causing thoracic duct
obstruction. Nihon Shokakibyo Gakkai Zasshi. 110:1943–1949.
2013.(In Japanese). PubMed/NCBI
|
|
16
|
Martín-Joven A, Ballesteros Fernández A,
Cervero M, Marco J and Solís J: Chylothorax as presenting form of a
gastric adenocarcinoma. Rev Esp Enferm Dig. 88:880–881. 1996.(In
Spanish). PubMed/NCBI
|
|
17
|
Skandalakis JE, Skandalakis LJ and
Skandalakis PN: Anatomy of the lymphatics. Surg Oncol Clin N Am.
16:1–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schulman A, Fataar S, Dollrymple R and
Tidbury I: The lymphographic anatomy of chylothorax. Br J Radiol.
51:420–427. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murdaca G, Cagnati P, Gulli R, Spanò F,
Puppo F, Campisi C and Boccardo F: Current views on diagnostic
approach and treatment of lymphedema. Am J Med. 125:134–140. 2012.
View Article : Google Scholar : PubMed/NCBI
|