Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer in males worldwide and the second most common in
females, with an estimated 1.4 million cases occurring in 2012
(1). There are no typical symptoms
until the late stages of the disease, which may then include
abdominal pain, hematochezia and change of stool customs. Two
thirds of CRC cases are colon cancer (2), for which the main treatment is radical
surgery. Approximately 40% of patients experience recurrence, and
this is consequently the main cause of mortality among affected
individuals (3). It has been reported
that the average survival time for patients with tumor recurrence
is 7 months, which can be increased to ~29.9 months when a second
surgery is performed (4). For this
reason, when treating patients with recurrent colon cancer, the
main aim is to achieve a radical resection or to at least perform
palliative surgery.
Neoadjuvant chemoradiotherapy is a feasible option
in order to realize an R0 resection for local recurrent colon
cancer. Perioperative radiation therapy and chemotherapy can also
reduce the recurrence rate and prolong the survival time of
patients (5–7). Irinotecan (CPT-11) has shown anti-tumor
effects in multiple types of tumor is also used in the neoadjuvant
chemotherapy for colon cancer (8).
Intraperitoneal (IP) chemotherapy with CPT-11 is also applied to
treat various intra-abdominal tumors, including colon cancer
(9,10). Intensity-modulated radiotherapy (IMRT)
has been widely applied in the treatment of cancer due to its
potential to provide sharp dose gradients at the junction of the
tumor and the adjacent critical organs. It is considered to be safe
and effective, and may be applied in the treatment of various types
of cancer (11).
The present study reports a case of locally
recurrent colon cancer, in which a radical reoperation was
performed following neoadjuvant IMRT and IP chemotherapy. Written
informed consent was provided by the patient for publication of
this study. The study was approved by the Ethics Committee of
Nanjing Drum Tower Hospital (Nanjing, China).
Case report
A 28-year-old man experienced hematochezia for 6
months and was first diagnosed with sigmoid colon carcinoma by
colonoscopy at the People's Hospital of Suqian City (Suqian, China)
on June 1, 2012. The patient underwent a partial sigmoidectomy and
surgical bladder repair. The pathological diagnosis was of a grade
I–II adenocarcinoma of the sigmoid colon, according to Broders
(12) carcinoma grading system, of
which 50% was mucoid carcinoma. The carcinoma invaded the
full-thickness of the colon wall and the muscle layer of the
bladder. No lymph node metastasis was evident (0/29 nodes). The
patient then underwent 6 cycles of a docetaxel combined with
oxaliplatin, 5-fluorouracil and calcium folinate regimen (detailed
treatment dosage data were not available). The serum levels of
carcinoembryonic antigen (CEA), cancer antigen (CA)19-9, CA-125 and
CA242 during the whole course were normal.
At 8 months post-adjuvant chemotherapy, the patient
was presented to Nanjing Drum Tower Hospital on October 23, 2013,
due to hematochezia that had persisted for several days. Laboratory
tests revealed normal levels of tumor markers, including CEA,
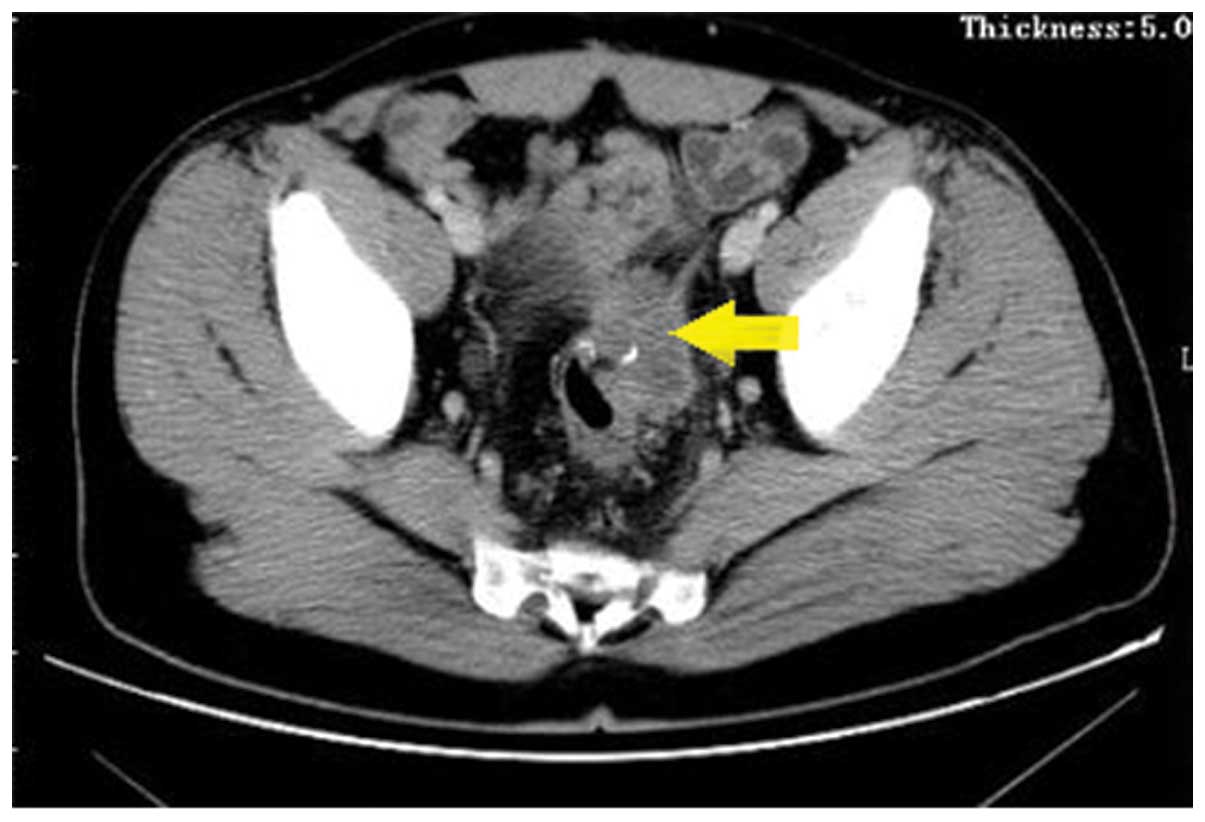
CA19-9, CA125 and CA242. Computed tomography (CT) scans revealed a
mass, ~4.2 cm in diameter, at the anastomotic site, invading the
bladder and the left ureter (Fig. 1).
No other metastases in the abdominal organs or lymph nodes were
detected by CT. Colonoscopy biopsy revealed grade II
adenocarcinoma, which was conjectured to be the result of the
recurrence of the primary tumor.
The patient was treated by a multidisciplinary team,
including surgeons, radiation oncologists and physicians. As the
carcinoma encroached on the surrounding organs, the surgeons
indicated that it was not possible to perform a radical resection
and that pre-operative neoadjuvant therapy was necessary. The
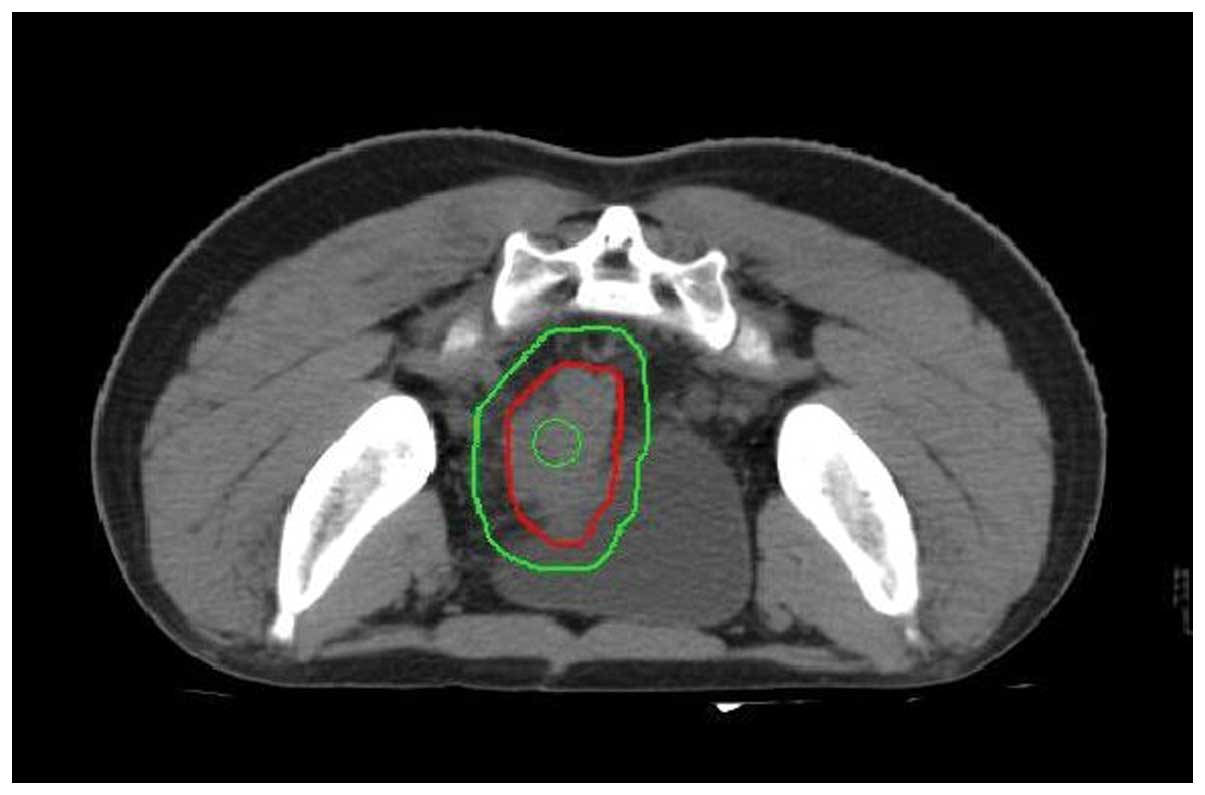
patient was then administered intensity modulation radiation
therapy (IMRT). The planning target volume (PTV) consisted of the
clinical target volume plus a 0.8 to 1-cm margin, and the PTV1 (a
region of the PTV with a higher dose) consisted of the gross tumor
volume plus a 0.3-cm margin. The total dose prescription for the
PTV was 50 Gy and the total dose prescription for the PTV1 was 60
Gy, which were each delivered at 2 Gy per fraction (Fig. 2). The radiotherapy was delivered over
6 weeks. Concurrent IP perfusion chemotherapy using CPT-11 (100
mg/m2) was administered weekly, five times during the
radiotherapy course (Fig. 2).
Following the concurrent radiation therapy and
chemotherapy, CT scans revealed no evident tumor at the anastomotic
site or any other metastases, indicating a clinical complete
response (Fig. 3). Subsequently, the
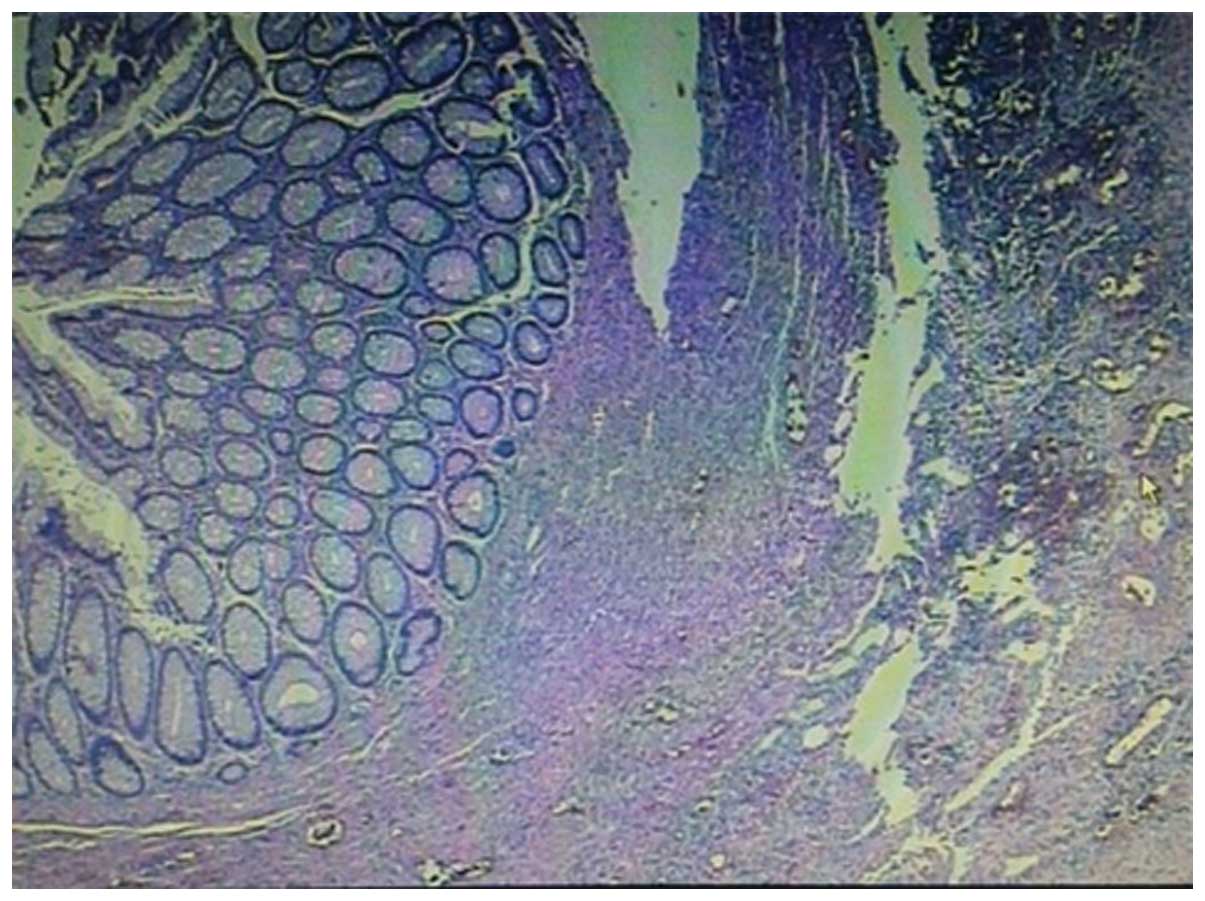
patient underwent a second surgery to resect the remaining sigmoid
colon. The pathological examination revealed no definite invasive
adenocarcinoma or lymph node metastasis, thus, the patient achieved
a pathological complete response (pCR) (Fig. 4). Four additional 4-week cycles of
intravenous (IV) systemic CPT-11 chemotherapy (150
mg/m2, days 1 and 15) combined with oral Xeloda (1,500
mg/m2, days 1–14) were administered. At the time of
writing this study, the patient remains alive, with a disease-free
survival time of ~10 months.
Discussion
The majority of colon cancer local recurrences occur
at 6 months to 2 years after the first surgery (13). While 30–40% of patients with stage
II/III colon cancer experience recurrence, only 10–20% of them can
undergo radical surgery (14,15). It has also been reported that the
5-year survival rate may be 19–35% for patients who undergo a
second radical surgery, but <5% for inoperable patients. Radical
surgery can prolong survival markedly, with the 5-year survival
rate increasing up to 54%; even in patients who exhibit positive
surgical margins, the 5-year survival rate can increase to 25%
(14,16,17).
In the present case, the patient experienced
recurrence after surgery and 6 cycles of adjuvant chemotherapy.
However, according to the surgeons, the tumor could not be removed
via radical surgery and so neoadjuvant medical treatment was a
requisite. CPT-11, a widely used second-line anticancer agent,
which has been shown to have cytotoxic activity in patients with
colorectal, gastric, pancreatic, lung, ovarian, breast and cervical
cancers, was chosen for application. In the liver and other
tissues, carboxyl esterase converts CPT-11 to its active
metabolite, SN-38, which is potently inhibits the nuclear enzyme,
topoisomerase I. This enzyme exhibits cytotoxic activity that is
100–1,000-fold greater than that of CPT-11 (18). CPT-11 has been demonstrated to extend
the overall survival of metastatic colorectal cancer patients when
an IV administration (19). In a
mouse model, IP administration of CPT-11 was significantly more
effective than IV administration with regard to antitumor activity
against peritoneal seeding and liver metastases (20). The survival benefit of IP chemotherapy
has been documented in ovarian and gastric cancer, for which
randomized trials showed significant a survival advantage for
patients receiving IP chemotherapy (21–23). An IP
CPT-11 pharmacokinetic study performed in a pig model confirmed the
ability of the drug to achieve a peritoneal exposure level at least
30 times higher than that of systemic exposure. The peak
concentration of peritoneal SN-38 was also achieved earlier than
that of plasma SN-38, suggesting that IP infusion of CPT-11 could
be an efficient route of administration in patients with abdominal
carcinomas (24).
Unlike recurrent rectal cancer, recurrent colon
cancer is not commonly treated with radiotherapy for the unfixed
lesions and radioactive enteritis of the surrounding small
intestines. However, technological advances in radiation treatment,
particularly planning IMRT, have developed the practice,
particularly for the treatment of multiple tumors, while minimizing
the risk of damage to healthy tissues (25–27). IMRT
has been applied to treat recurrent colon cancer according to the
National Comprehensive Cancer Network treatment guidelines
(28). In the present study, prior to
receiving the treatment, the patient expressed a strong desire to
undergo an anus-preserving procedure, which is difficult to achieve
surgically. The patient later refused to undergo a second surgery
and a radiotherapy plan was formulated with the aim of a radical
cure. However, at the end of radiotherapy, the patient decided to
undergo the required surgery. The post-operative pathological
result revealed a pCR following neoadjuvant therapy, which usually
occurs at a low rate, but allows for improved survival times. It
has been reported that the cumulative 5-year survival rates may
reach 75% for patient with a pCR (29,30).
In conclusion, in the present case, a patient with
recurrent colon cancer was effectively treated by a
multidisciplinary treatment that included IMRT, IP perfusion
chemotherapy and a second surgery. A pCR was achieved, indicating
that IMRT combined with IP perfusion chemotherapy using CPT-11 may
be a feasible neoadjuvant therapy for recurrent colon cancer.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tamas K, Walenkamp A, de Vries E, van Vugt
MA, Beets-Tan RG, van Etten B, de Groot DJ and Hospers GA: Rectal
and colon cancer: Not just a different anatomic site. Cancer
treatment reviews. 41:671–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldberg RM, Fleming TR, Tangen CM,
Moertel CG, Macdonald JS, Haller DG and Laurie JA: Surgery for
recurrent colon cancer: Strategies for identifying resectable
recurrence and success rates after resection. Eastern Cooperative
Oncology Group, the North Central Cancer Treatment Group, and the
Southwest Oncology Group. Ann Intern Med. 129:27–35. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sardi A, Minton JP, Nieroda C,
Sickle-Santanello B, Young D and Martin EW Jr: Multiple
reoperations in recurrent colorectal carcinoma. An analysis of
morbidity, mortality and survival. Cancer. 61:1913–1919. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beart RW: Prevention and management of
recurrent rectal cancer. World J Surg. 15:589–591. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sugarbaker PH: Update on the prevention of
local recurrence and peritoneal metastases in patients with
colorectal cancer. World J Gastroenterol. 20:9286–9291.
2014.PubMed/NCBI
|
|
7
|
Sugarbaker PH: Second-look surgery for
colorectal cancer: Revised selection factors and new treatment
options for greater success. Int J Surg Oncol.
2011:9150782011.PubMed/NCBI
|
|
8
|
André T, Louvet C, Maindrault-Goebel F,
Couteau C, Mabro M, Lotz JP, Gilles-Amar V, Krulik M, Carola E,
Izrael V and de Gramont A: CPT-11 (irinotecan) addition to
bimonthly, high-dose leucovorin and bolus and continuous-infusion
5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal
cancer. GERCOR. Eur J Cancer. 35:1343–1347. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elias D, Goere D, Blot F, Billard V,
Pocard M, Kohneh-Shahri N and Raynard B: Optimization of
hyperthermic intraperitoneal chemotherapy with oxaliplatin plus
irinotecan at 43°C after compete cytoreductive surgery: Mortality
and morbidity in 106 consecutive patients. Ann Surg Oncol.
14:1818–1824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elias D, Matsuhisa T, Sideris L, Liberale
G, Drouard-Troalen L, Raynard B, Pocard M, Puizillou JM, Billard V,
Bourget P and Ducreux M: Heated intra-operative intraperitoneal
oxaliplatin plus irinotecan after complete resection of peritoneal
carcinomatosis: Pharmacokinetics, tissue distribution and
tolerance. Ann Oncol. 15:1558–1565. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kataria T, Rawat S, Sinha S, Garg C,
Bhalla N and Negi P: Dose reduction to normal tissues as compared
to the gross tumor by using intensity modulated radiotherapy in
thoracic malignancies. Radiat Oncol. 1:12006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Broders AC: The grading of carcinoma. Minn
Med. 8:726–730. 1925.
|
|
13
|
Taylor WE, Donohue JH, Gunderson LL,
Nelson H, Nagorney DM, Devine RM, Haddock MG, Larson DR, Rubin J
and O'Connell MJ: The Mayo Clinic experience with multimodality
treatment of locally advanced or recurrent colon cancer. Ann Surg
Oncol. 9:177–185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olson RM, Perencevich NP, Malcolm AW,
Chaffey JT and Wilson RE: Patterns of recurrence following curative
resection of adenocarcinoma of the colon and rectum. Cancer.
45:2969–2974. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Willett CG, Tepper JE, Cohen AM, Orlow E
and Welch CE: Failure patterns following curative resection of
colonic carcinoma. Ann Surg. 200:685–690. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Curley SA, Carlson GW, Shumate CR, Wishnow
KI and Ames FC: Extended resection for locally advanced colorectal
carcinoma. Am J Surg. 163:553–559. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Michelassi F, Vannucci L, Ayala JJ,
Chappel R, Goldberg R and Block G: Local recurrence after curative
resection of colorectal adenocarcinoma. Surgery. 108:787–792.
1990.PubMed/NCBI
|
|
18
|
Chabot GG: Clinical pharmacokinetics of
irinotecan. Clin Pharmacokinet. 33:245–259. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saltz LB, Cox JV, Blanke C, Rosen LS,
Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta
N, et al: Irinotecan plus fluorouracil and leucovorin for
metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med.
343:905–914. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cohen MS, Al-kasspooles MF, Williamson SK,
Henry D, Broward M and Roby KF: Combination intraperitoneal
chemotherapy is superior to mitomycin C or oxaliplatin for
colorectal carcinomatosis in vivo. Ann Surg Oncol. 17:296–303.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Armstrong DK, Bundy B, Wenzel L, Huang HQ,
Baergen R, Lele S, Copeland LJ, Walker JL and Burger RA:
Gynecologic Oncology Group: Intraperitoneal cisplatin and
paclitaxel in ovarian cancer. N Engl J Med. 354:34–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alberts DS, Liu P, Hannigan EV, O'Toole R,
Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK
and DuBeshter B: Intraperitoneal cisplatin plus intravenous
cyclophosphamide versus intravenous cisplatin plus intravenous
cyclophosphamide for stage III ovarian cancer. N Engl J Med.
335:1950–1955. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi MK, Ahn BJ, Yim DS, Park YS, Kim S,
Sohn TS, Noh JH, Heo JS, Lee J, Park SH, et al: Phase I study of
intraperitoneal irinotecan in patients with gastric adenocarcinoma
with peritoneal seeding. Cancer Chemother Pharmacol. 67:5–11. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Turcotte S, Sideris L, Younan R, Drolet P
and Dubé P: Pharmacokinetics of intraperitoneal irinotecan in a pig
model. J Surg Oncol. 101:637–642. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mundt AJ, Mell LK and Roeske JC:
Preliminary analysis of chronic gastrointestinal toxicity in
gynecology patients treated with intensity-modulated whole pelvic
radiation therapy. Int J Radiat Oncol Biol Phys. 56:1354–1360.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Uy NW, Woo SY, Teh BS, Mai WY, Carpenter
LS, Chiu JK, Lu HH, Gildenberg P, Trask T, Grant WH and Butler EB:
Intensity-modulated radiation therapy (IMRT) for meningioma. Int J
Radiat Oncol Biol Phys. 53:1265–1270. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vicini FA, Sharpe M, Kestin L, Martinez A,
Mitchell CK, Wallace MF, Matter R and Wong J: Optimizing breast
cancer treatment efficacy with intensity-modulated radiotherapy.
Int J Radiat Oncol Biol Phys. 54:1336–1344. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Engstrom PF, Arnoletti JP, Benson AB III,
Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS,
Enzinger PC, et al: NCCN Clinical Practice Guidelines in Oncology:
Colon cancer. J Natl Compr Canc Netw. 7:778–831. 2009.PubMed/NCBI
|
|
29
|
Passot G, You B, Boschetti G, Fontaine J,
Isaac S, Decullier E, Maurice C, Vaudoyer D, Gilly FN, Cotte E and
Glehen O: Pathological response to neoadjuvant chemotherapy: A new
prognosis tool for the curative management of peritoneal colorectal
carcinomatosis. Ann Surg Oncol. 21:2608–2614. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Benoist S, Brouquet A, Penna C, Julié C,
El Hajjam M, Chagnon S, Mitry E, Rougier P and Nordlinger B:
Complete response of colorectal liver metastases after
chemotherapy: Does it mean cure? J Clin Oncol. 24:3939–3945. 2006.
View Article : Google Scholar : PubMed/NCBI
|