Introduction
Osteosarcoma, the most common primary bone
malignancy, accounts for ~20% of all bone tumors and ~5% of all
pediatric tumors (1). Osteosarcoma
has an overwhelming tendency for invasion and early metastasis
(2). Although treatment with surgery
and neoadjuvant chemotherapy appears to cure 60–70% of cases
(3), the 5-year survival rate for
patients with recurrent and metastatic osteosarcoma is only 20%
(4,5).
Research investigating the mechanism of osteosarcoma development
has primarily focused on chromosomal abnormalities, genetic
alterations of tumor suppressor genes, activation of oncogenes and
dysregulation of major signaling pathways. However, the molecular
events that lead to the development of osteosarcoma are not yet
fully understood.
Inhibitor of growth 4 (ING4) functions as a tumor
suppressor, thus serving an inhibitory role during the generation
and development of various types of tumor, including thyroid
(6), gastric (7), lung (8)
and breast cancer (9). ING4
physically interacts with and phosphorylates p65, a subunit of
nuclear factor-κB (NF-κB), therefore suppressing the activity of
NF-κB (10). The inhibition of NF-κB
negatively regulates various target genes, including matrix
metalloproteinase (MMP)-2, MMP-9, interleukin (IL)-6, IL-8,
cyclooxygenase-2 and colony stimulating factor-3. The
downregulation of these cytokines inhibits angiogenesis and tumor
cell growth (11–15). In RKO colorectal cancer cells, ING4
expression was able to decrease the cell population in the S-phase
of the cell cycle in a p53-dependent manner, as well as upregulate
p21 expression (16). In addition,
ING4 may induce G2/M cell cycle arrest, and enhance
chemosensitivity to etoposide and doxorubicin in HepG2
hepatocellular carcinoma cells (17).
In general, there are two regulatory mechanisms of the cell cycle:
p21 and Bcl-2-associated X protein (Bax). ING4 upregulates p21 and
Bax in a p53-independent manner. The upregulation of p21 enhances
its binding to cyclin B1, while the compound of cyclin
B1/cyclin-dependent kinase 1 is crucial for progression in the cell
cycle (18). Conversely, in the
p53-defective SaoS-2 cell line, ING4 cannot upregulate p21 or Bax,
and is therefore suspected to regulate the cell cycle by directly
interacting with p300 or p65 (19).
Furthermore, ING4 is able to upregulate Bax while downregulating B
cell lymphoma-2 (Bcl-2), thus, decreasing the ratio of Bcl-2/Bax,
resulting in cytochrome c release from the mitochondrion and
activation of caspase-3. Activated caspase-3 disrupts the function
of poly(ADP-ribose) polymerase and, as a result, DNA cannot be
repaired. Therefore, ING4 may also induce apoptosis through
activation of the mitochondrial apoptotic pathway in a
p53-independent manner (20–22). Evidence suggests that ING4 may
suppress the expression of the target gene, hypoxia-inducible
factor-1α, by interacting with its plant homeodomain finger motif
under hypoxic conditions (23–25). In
addition, ING4 serves a role in the suppression of loss of contact
inhibition elicited by the overexpression of the proto-oncogene Myc
or MYCN; however, ING4 cannot suppress the transcriptional activity
of the proto-oncogene (26).
Furthermore, ING4 is able to interact with liprin α-1, a novel
ING4-associated protein, to prevent invasion and metastasis
(27).
ING4 expression is decreased in a number of tumor
tissues and the degree of low expression corresponds with tumor
grade (11,18,20,28–31).
Allelic loss and mutation of ING4 have also been observed in
various cancer cell lines (26,32,33), thus
suggesting that ING4 is a promising tumor suppressor. Despite this,
there are currently no previously published studies that have
investigated ING4 expression in osteosarcoma tissues. Therefore,
the exact role of ING4 in the tumorigenesis and progression of
osteosarcoma has yet to be established. In the present study, the
expression of ING4 was analyzed in osteosarcoma tissue, and
associations between the expression of the ING4 protein and several
clinical characteristics were evaluated.
Materials and methods
Materials
The present study was approved by the Ethics
Commitee of The First Affiliated Hospital of Harbin Medical
University (Harbin, China; approval no., HYDEC-D-2016-051) and was
conducted according to the Declaration of Helsinki Principles
(34). Written informed consent was
obtained from the patients or their families for the publication of
this study. The tissue microarrays (TMAs) used for this study were
purchased from Ailina Xi'an Biological Technology Co., Ltd. (Xi'an,
China). The OS804 osteosarcoma tissue microarray, containing 40
cases of osteosarcoma, and the BO244b tissue microarray, containing
1 case of osteosarcoma and 11 cases of normal bone tissue (in the
absence of any other bony disease), were obtained during trauma
surgery and each contained duplicate cores. All primary
osteosarcoma biopsy specimens were obtained at the time of
diagnosis, prior to any chemotherapy treatment, and were assessed
according to the Enneking classification for malignant bone tumors
(35,36). The tissues were formalin-fixed
(Sigma-Aldrich, St. Louis, MO, USA) and paraffin-embedded
(Sigma-Aldrich), and cut into 5-µm thick sections with 1.5-mm
individual cores. The array sections were mounted on
positively-charged, super plus glass slides (Sigma-Aldrich). The
primary antibody, mouse anti-human ING4 polyclonal antibody (3
µg/ml; catalog no., ab90551), was purchased from Abcam (Cambridge,
UK). The EliVision™ Plus kit (catalog no., KIT-9902) and
3,3′-diaminobenzidine (DAB; catalog no., DAB-0031/1031), which was
used as substrate chromogen, were purchased from Fuzhou Maixin
Biotech Co., Ltd. (Fuzhou, China).
Immunohistochemistry
Normal bone tissues (n=11) were selected as the
positive control and the primary antibody was replaced with
phosphate-buffered saline (PBS; Sigma-Aldrich) to function as the
negative control. The expression of ING4 protein was analyzed by
immunohistochemistry. Sections were baked for 30 min at 60°C, and
were subsequently deparaffinized by washing with xylene
(Sigma-Aldrich) and rehydrated in a graded alcohol series. Antigen
retrieval was performed using 1X antigen retrieval solution
(catalog no., 03690; Sigma-Aldrich) for 2 min in a pressure cooker
at boiling point, as observed by steaming. The sections were cooled
to room temperature, washed three times in PBS for 5 min, incubated
for 10 min in 3% hydrogen peroxide (Sigma-Aldrich) at room
temperature and washed again three times in PBS for 5 min.
Subsequently, the sections were incubated at 4°C overnight with the
primary antibody, diluted to 1:100 in antibody diluent
(Sigma-Aldrich) solution. The slides were washed three times in
0.01% Tween-20 (Sigma-Aldrich) PBS for 5 min, incubated for 20 min
with polymer enhancer (Sigma-Aldrich) at room temperature, washed
again three times with 0.1% Tween-20 PBS for 5 min and incubated
for 30 min at room temperature with polymerized horseradish
peroxidase anti-mouse immunoglobulin G (catalog no., ZB-2305;
dilution, 1:500; ZSGB-Bio, Beijing, China). The tissues were
subsequently incubated in peroxidase substrate DAB solution until
the desired stain intensity had developed and rinsed in tap water.
Finally, the slides were counterstained with Hematoxylin QS
(catalog no., H-3404; Vector Laboratories, Inc., Burlingame, CA,
USA), and cleared and mounted with permanent mounting medium
(Vector Laboratories, Inc.).
Evaluation and scoring of the
TMAs
A semiquantitative scoring system was used to
evaluate the immunohistochemistry results of ING4 protein localized
to the nucleus. Immunoreactivity was assessed by the percentage of
positive cells and the strength of staining. The percentage of
positive cells was scored as follows: 1, <11%; 2, 11–50%; 3,
51–75%; and 4, >75%. The strength of staining was scored as
follows: No staining, 0; light brown, 1; brown, 2; and dark brown,
3. The final score was determined by multiplying the proportion of
positive cells score with the strength of staining score. Final
scores were classified as follows: <3, (−); 3–5, (+); 6–9, (++);
and >9, (+++) (21). A final score
of (−) and (+) represented low expression, whilst (++) and (+++)
represented high expression. All cases, including 41 osteosarcoma
cases and 11 normal bone cases, were evaluated by three
independent, blinded observers simultaneously, and a consensus
score was established for each core. All sections were observed
under an optical microscope (TCS NT; Leica Microsystems, Wetzlar,
Germany).
Clinical outcome measures of the
osteosarcoma specimens
The Enneking classification system for malignant
bone tumors is based on considerations of grade and metastasis
(35,36). The stages of osteosarcoma are divided
into I, II and III based upon the compartmentalization of the
lesion. Stage I comprises of low grade lesions without metastases;
stage II comprises of high grade lesions without metastases; and
stage III comprises low or high grade lesions with metastases.
Stages I and II are further subdivided as intracompartmental (A) or
extracompartmental (B). Thus, all the specimens used in the present
study were grouped as IA, IB, IIA, IIB or III.
Statistical analysis
The Mann-Whitney U test was used to analyze the
diversity in the expression of ING4 between the osteosarcoma and
bone tissue specimens. In addition, all clinicopathological
variables were analyzed in association with the ING4
immunohistochemical score using univariate logistic regression
analysis modeling. For statistical analysis of the ordinal data,
gender was categorized as male or female; age at diagnosis as
<30 or ≥30 years; anatomical site as distal femur, proximal
tibia or other; histological type as osteoblastic or other; and
Enneking classification as IA, IB, IIA, IIB or III. Data was
presented as the mean ± standard error of the mean. SPSS version
19.0 software (SPSS, Inc., Chicago, IL, USA) was used to perform
the statistical analyses, and P<0.05 was considered to indicate
a statistically significant difference.
Results
The mean age of the 41 patients with osteosarcoma
(28 men and 13 women) was 31 years (range, 11–64 years). The
anatomical sites of the primary tumor were the distal femur (n=27),
proximal tibia (n=6), humerus (n=3), scapula (n=2), rib (n=2) and
fibula (n=1). The histological type of the 41 specimens included
osteoblastic (n=26), chondroblastic (n=7), fibroblastic (n=4),
periosteal (n=3) and telangiectatic (n=1). Metastatic lesions were
identified in only 1 patient. Patient survival information was not
obtained. The mean age of the 11 control cases, including 9 men and
2 women, was 59 years at the time when normal bone tissue was
obtained (range, 41–75 years). The trauma sites of the normal bone
tissues consisted of the femur (n=5), the femoral head (n=3), tibia
(n=2) and humerus (n=1).
Each duplicated core of the 41 osteosarcoma
specimens and 11 normal bone tissues had the same
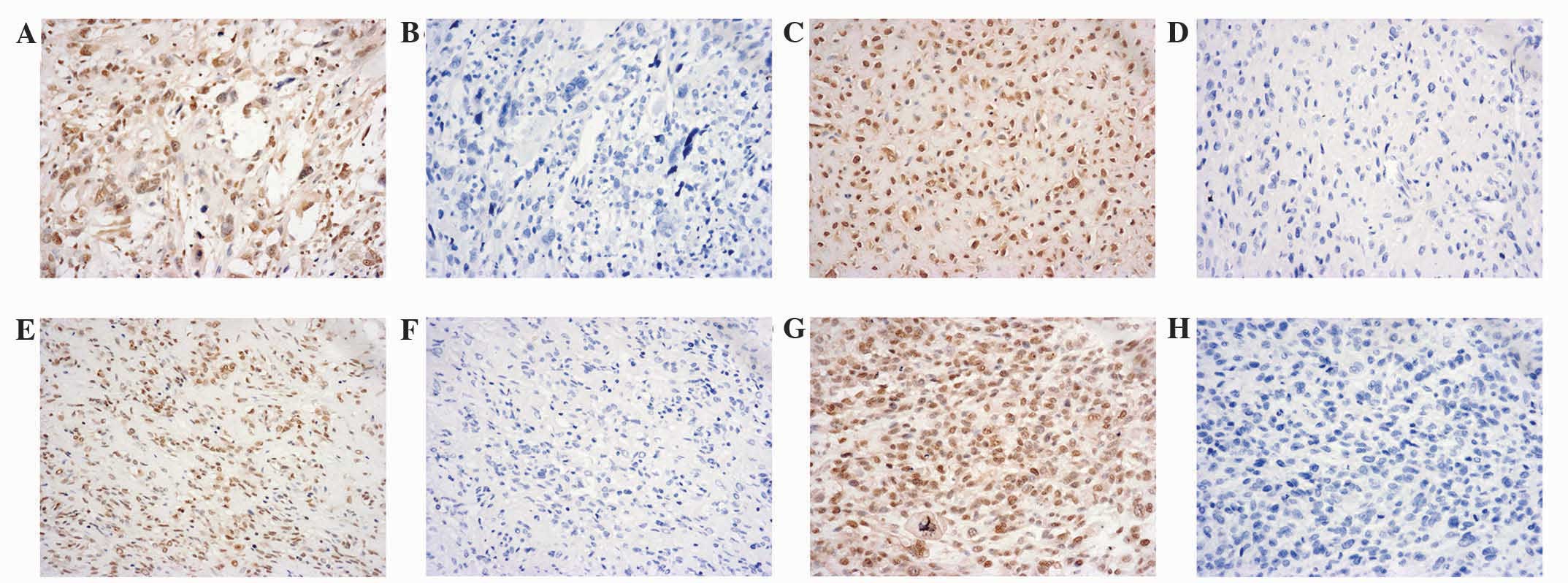
immunohistochemical staining score. Representative
immunohistochemical staining results for the osteosarcoma tissues
are presented in Fig. 1. A final
score of (−) was observed in 7 (17.1%) osteosarcoma specimens
(Fig. 1A), a score of (+) in 19
(46.3%) specimens (Fig. 1C), a score
of (++) in 11 (26.8%) specimens (Fig.
1E) and a score of (+++) in 4 (9.8%) specimens (Fig. 1G). Negative controls were used for all
osteosarcoma specimens (Fig. 1B, D, F and
H). Representative immunohistochemical staining results for the
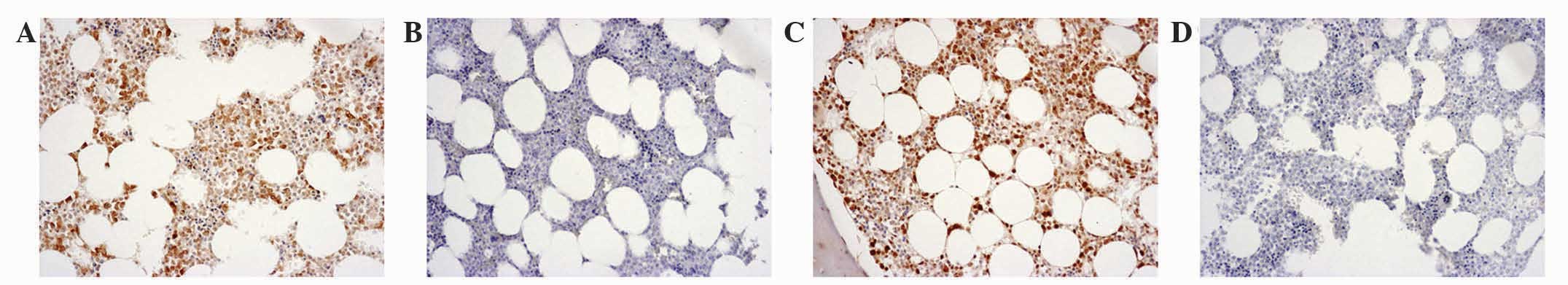
normal bone tissues are presented in Fig.
2. A score of (++) was observed in 2 (18.2%) normal bone
specimens (Fig. 2A) and a score of
(+++) in 9 (81.8%) specimens (Fig.
2C). Negative controls were used for all normal bone specimens
(Fig. 2B and D). Immunohistochemical
staining scores of (−) and (+) were not observed in the normal bone
specimens. A significant difference was observed between ING4
immunohistochemical staining scores in the osteosarcoma and normal
bone tissues (P<0.001) (Table
I).
 | Table I.Comparison of ING4 expression between
osteosarcoma and normal bone tissue specimens. |
Table I.
Comparison of ING4 expression between
osteosarcoma and normal bone tissue specimens.
|
| Immunohistochemical
score |
|
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Group | − | + | ++ | +++ | Mean rank | U-value | P-value |
|---|
| Osteosarcoma | 7 | 19 | 11 | 4 | 21.90 |
|
|
| Normal bone | 0 | 0 | 2 | 9 | 43.64 |
|
|
| Total | 7 | 19 | 13 | 13 |
| 37.00 | <0.001 |
According to the Enneking classification system for
malignant bone tumors (35,36), 2 osteosarcoma specimens with a score
of (+++) were classified as IA; 3 osteosarcoma specimens, including
a score of (++) in 2 and (+++) in 1, were classified as IB; 9
osteosarcoma specimens, including a score of (+) in 3 and (++) in
6, were classified as IIA; 26 osteosarcoma specimens, including a
score of (−) in 6, (+) in 16, (++) in 3 and (+++) in 1, were
classified as IIB; and only 1 osteosarcoma specimen with a score of
(−) was classified as III. No statistical significance was observed
between gender, age at diagnosis, anatomical site or histological
type and immunohistochemical staining of ING4. However, a
significant negative correlation was identified between the
immunohistochemical staining scores and Enneking classification
results of the 41 osteosarcoma specimens (P=0.002) (Table II).
 | Table II.Correlation between
clinicopathological variables and immunohistochemical staining
score of ING4 in the osteosarcoma tissue specimens. |
Table II.
Correlation between
clinicopathological variables and immunohistochemical staining
score of ING4 in the osteosarcoma tissue specimens.
|
| Immunohistochemical
score |
|
|---|
|
|
|
|
|---|
| Characteristic | Low (n=26) | High (n=15) | P-value |
|---|
| Gender |
|
| 0.389 |
|
Male | 19 | 9 |
|
|
Female | 7 | 6 |
|
| Age at diagnosis,
years |
|
| 0.923 |
|
<30 | 10 | 6 |
|
|
≥30 | 16 | 9 |
|
| Anatomical
site |
|
| 0.671 |
| Distal
femur | 16 | 11 |
|
|
Proximal tibia | 5 | 1 |
|
|
Other | 5 | 3 |
|
| Histological
type |
|
| 0.743 |
|
Osteoblastic | 16 | 10 |
|
|
Other | 10 | 5 |
|
| Enneking
classification |
|
| 0.002 |
| IA | 0 | 2 |
|
| IB | 0 | 3 |
|
|
IIA | 3 | 6 |
|
|
IIB | 22 | 4 |
|
|
III | 1 | 0 |
|
Discussion
Previous studies have demonstrated that ING4
expression is suppressed or reduced in a number of malignancies,
and the degree of expression is associated with the tumor grade
(11,18,20,28–31).
To the best of our knowledge, no study has investigated the level
of expression of ING4 in human osteosarcoma tissue as compared with
normal bone tissue, or the prognostic value of ING4 expression in
patients diagnosed with osteosarcoma.
Similar to the results of previous studies regarding
other malignant tumors (11,18,20,28–31),
the results of the present study demonstrated that the osteosarcoma
tissues had significantly decreased expression of ING4 when
compared with the normal bone tissues (P<0.001). The majority of
the osteosarcoma specimens had a score of (−) or (+), whilst no
normal bone specimens had a score of (−) or (+). However, the mean
age of the 11 patients from whom the normal bone specimens were
obtained (59 years old) was markedly higher than that of the 41
patients with osteosarcoma (31 years old). This may be because the
11 normal bone tissues were obtained from patients who underwent
surgery following trauma. Data as to whether the patients had
implicit or asymptomatic osteoporosis, which are highly prevalent
in older individuals, could not obtained. However, to the best of
our knowledge, there are currently no available studies that
demonstrate a correlation between ING4 expression level in bone and
potential osteoporosis. Consequently, the results of the present
study are considered to be reliable and objective.
In the present study, no significance was observed
between gender, age at diagnosis, anatomical site or histological
type and immunohistochemical staining of ING4. Instead, it was
noted that the degree of reduction in ING4 expression correlated
with the progression from low to high grades of osteosarcoma,
according to the Enneking classification system for malignant bone
tumors (P=0.002). ING4, a novel tumor suppressor of the ING family,
has potential tumor-suppressing effects that are exerted through
various signaling pathways, including tumorigenesis, cell cycle
regulation, angiogenesis, cell apoptosis, DNA repair, migration and
transcriptional regulation (10–27,37–43).
These functions of ING4, which acts as an oncogene suppressor in
numerous tumor types, have been identified repeatedly in
vitro and in vivo (10–27,37–43).
To the best of our knowledge, the present study is the first to
investigate the immunoreactivity of ING4 in osteosarcoma tissue.
Considering that Enneking classification system has prognostic
implications for tumor invasion and metastasis, the findings of the
current study indicate that ING4 may also serve a suppressive role
in the tumorigenesis, invasion and soft tissue extension of
osteosarcoma. A limited number of studies have investigated the
tumor suppressor effect of ING4 in osteosarcoma. It was previously
reported that adenovirus (Ad)-mediated ING4 gene transfer
significantly induced growth inhibition and apoptosis in MG-63
human osteosarcoma cells, and intratumoral injections of Ad-ING4 in
athymic nude mice bearing osteosarcoma tumors significantly
inhibited osteosarcoma xenograft tumor growth (44). A different study indicated that ING4
could suppress osteosarcoma progression through mitochondrial and
NF-κB signaling pathways (45).
However, further research is required to determine the exact tumor
suppressive mechanism of ING4 in osteosarcoma.
One limitation of the present study was that the
overall survival data of the 41 patients could not be obtained.
However, the Enneking grade of the osteosarcoma specimens at the
time of diagnosis was obtained during TMA analysis. A significant
negative correlation was observed between the ING4
immunohistochemical staining scores and Enneking grade. Therefore,
this suggests that ING4 may be also negatively correlated with
survival of patients with osteosarcoma.
In conclusion, the results of the present study
indicate that ING4 expression may be a promising prognostic marker
for patients with osteosarcoma. The significant negative
correlation observed between ING4 expression and Enneking grade may
provide insight into the mechanism of tumor progression in
osteosarcoma, and additionally provide a potential target for novel
therapeutic strategies, with the aim of improving the length and
quality of life in patients with osteosarcoma.
Acknowledgements
The authors would like to thank Professor Xiangning
Meng (Laboratory of Medical Genetics, Harbin Medical University,
Harbin, China) for providing technical support.
Glossary
Abbreviations
Abbreviations:
|
ING4
|
inhibitor of growth 4
|
|
TMAs
|
tissue microarrays
|
|
NF-κB
|
nuclear factor-κB
|
References
|
1
|
Hansen MF: Genetic and molecular aspects
of osteosarcoma. J Musculoskelet Neuronal Interact. 2:554–560.
2002.PubMed/NCBI
|
|
2
|
Hayden JB and Hoang BH: Osteosarcoma:
Basic science and clinical implications. Orthop Clin North Am.
37:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Link MP, Goorin AM, Miser AW, Green AA,
Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR, Kirkpatrick
JA, et al: The effect of adjuvant chemotherapy on relapse-free
survival in patients with osteosarcoma of the extremity. N Engl J
Med. 314:1600–1606. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eccles SA and Welch DR: Metastasis: Recent
discoveries and novel treatment strategies. Lancet. 369:1742–1757.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rodriguez NI, Hoots WK, Koshkina NV,
Morales-Arias JA, Arndt CA, Inwards CY, Hawkins DS, Munsell MF and
Kleinerman ES: COX-2 expression correlates with survival in
patients with osteosarcoma lung metastases. J Pediatr Hematol
Oncol. 30:507–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang CJ, Yang D and Luo YW: Recombinant
ING4 suppresses the migration of SW579 thyroid cancer cells via
epithelial to mesenchymal transition. Exp Ther Med. 10:603–607.
2015.PubMed/NCBI
|
|
7
|
Zhang H, Zhou X, Xu C, Yang J, Xiang J,
Tao M and Xie Y: Synergistic tumor suppression by
adenovirus-mediated ING4/PTEN double gene therapy for gastric
cancer. Cancer Gene Ther. 23:13–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan A, Yang C, Chen Z, Li C and Cai L:
miR-761 promotes progression and metastasis of non-small cell lung
cancer by targeting ING4 and TIMP2. Cell Physiol Biochem. 37:55–66.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu J, Zhu Y, Xu C, Xu H, Zhou X, Yang J,
Xie Y and Tao M: Adenovirus-mediated p53 and ING4 gene co-transfer
elicits synergistic antitumor effects through enhancement of p53
acetylation in breast cancer. Oncol Rep. 35:243–522.
2016.PubMed/NCBI
|
|
10
|
Garkavtsev I, Kozin SV, Chernova O, Xu L,
Winkler F, Brown E, Barnett GH and Jain RK: The candidate tumour
suppressor protein ING4 regulates brain tumour growth and
angiogenesis. Nature. 428:328–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klironomos G, Bravou V, Papachristou DJ,
Gatzounis G, Varakis J, Parassi E, Repanti M and Papadaki H: Loss
of inhibitor of growth (ING-4) is implicated in the pathogenesis
and progression of human astrocytomas. Brain Pathol. 20:490–497.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Zhang Q, Cai L, Wang Y, Wang Q,
Huang X, Fu S, Bai J, Liu J, Zhang G and Qi J: Inhibitor of growth
4 induces apoptosis in human lung adenocarcinoma cell line A549 via
Bcl-2 family proteins and mitochondria apoptosis pathway. J Cancer
Res Clin Oncol. 135:829–835. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Yu L, Wang Y, Zhang Y, Wang Y and
Zhang G: Expression of tumor suppressor gene ING4 in ovarian
carcinoma is correlated with microvessel density. J Cancer Res Clin
Oncol. 138:647–655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nozell S, Laver T, Moseley D, Nowoslawski
L, De Vos M, Atkinson GP, Harrison K, Nabors LB and Benveniste EN:
The ING4 tumor suppressor attenuates NF-kappaB activity at the
promoters of target genes. Mol Cell Biol. 28:6632–6645. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie YF, Sheng W, Xiang J, Zhang H, Ye Z
and Yang J: Adenovirus-mediated ING4 expression suppresses
pancreatic carcinoma cell growth via induction of cell-cycle
alteration, apoptosis and inhibition of tumor angiogenesis. Cancer
Biother Radiopharm. 24:261–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shiseki M, Nagashima M, Pedeux RM,
Kitahama-Shiseki M, Miura K, Okamura S, Onogi H, Higashimoto Y,
Appella E, Yokota J and Harris CC: p29ING4 and p28ING5 bind to p53
and p300 and enhance p53 activity. Cancer Res. 63:2373–2378.
2003.PubMed/NCBI
|
|
17
|
Zhang X, Xu LS, Wang ZQ, Wang KS, Li N,
Cheng ZH, Huang SZ, Wei DZ and Han ZG: ING4 induces G2/M cell cycle
arrest and enhances the chemosensitivity to DNA-damage agents in
HepG2 cells. FEBS Lett. 570:7–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Z, Xie Y, Sheng W, Miao J, Xiang J and
Yang J: Tumor-suppressive effect of adenovirus-mediated inhibitor
of growth 4 gene transfer in breast carcinoma cells in vitro and in
vivo. Cancer Biother Radiopharm. 25:427–437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J and Li G: Cell cycle regulator ING4
is a suppressor of melanoma angiogenesis that is regulated by the
metastasis suppressor BRMS1. Cancer Res. 70:10445–10453. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cai L, Li X, Zheng S, Wang Y, Wang Y, Li
H, Yang J and Sun J: Inhibitor of growth 4 is involved in
melanomagenesis and induces growth suppression and apoptosis in
melanoma cell line M14. Melanoma Res. 19:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Cai L, Chen H, Zhang Q, Zhang S,
Wang Y, Dong Y, Cheng H and Qi J: Inhibitor of growth 4 induces
growth suppression and apoptosis in glioma U87MG. Pathobiology.
76:181–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Cai L, Liang M, Wang Y, Yang J and
Zhao Y: ING4 induces cell growth inhibition in human lung
adenocarcinoma A549 cells by means of Wnt-1/beta-catenin signaling
pathway. Anat Rec (Hoboken). 291:593–600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hung T, Binda O, Champagne KS, Kuo AJ,
Johnson K, Chang HY, Simon MD, Kutateladze TG and Gozani O: ING4
mediates crosstalk between histone H3 K4 trimethylation and H3
acetylation to attenuate cellular transformation. Mol Cell.
33:248–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozer A and Bruick RK: Regulation of HIF by
prolyl hydroxylases: Recruitment of the candidate tumor suppressor
protein ING4. Cell Cycle. 4:1153–1156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ozer A, Wu LC and Bruick RK: The candidate
tumor suppressor ING4 represses activation of the hypoxia inducible
factor (HIF). Proc Natl Acad Sci USA. 102:7481–7486. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim S, Chin K, Gray JW and Bishop JM: A
screen for genes that suppress loss of contact inhibition:
Identification of ING4 as a candidate tumor suppressor gene in
human cancer. Proc Natl Acad Sci USA. 101:16251–16256. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen JC, Unoki M, Ythier D, Duperray A,
Varticovski L, Kumamoto K, Pedeux R and Harris CC: Inhibitor of
growth 4 suppresses cell spreading and cell migration by
interacting with a novel binding partner, liprin alpha1. Cancer
Res. 67:2552–2558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang F, Luo LB, Tao YM, Wu F and Yang LY:
Decreased expression of inhibitor of growth 4 correlated with poor
prognosis of hepatocellular carcinoma. Cancer Epidemiol Biomarkers
Prev. 18:409–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li J, Martinka M and Li G: Role of ING4 in
human melanoma cell migration, invasion and patient survival.
Carcinogenesis. 29:1373–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li M, Jin Y, Sun WJ, Yu Y, Bai J, Tong DD,
Qi JP, Du JR, Geng JS, Huang Q, et al: Reduced expression and novel
splice variants of ING4 in human gastric adenocarcinoma. J Pathol.
219:87–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang QS, Li M, Zhang LY, Jin Y, Tong DD,
Yu Y, Bai J, Huang Q, Liu FL, Liu A, et al: Down-regulation of ING4
is associated with initiation and progression of lung cancer.
Histopathology. 57:271–281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Borkosky SS, Gunduz M, Beder L, Tsujigiwa
H, Tamamura R, Gunduz E, Katase N, Rodriguez AP, Sasaki A, Nagai N
and Nagatsuka H: Allelic loss of the ING gene family loci is a
frequent event in ameloblastoma. Oncol Res. 18:509–518. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim S, Welm AL and Bishop JM: A dominant
mutant allele of the ING4 tumor suppressor found in human cancer
cells exacerbates MYC-initiated mouse mammary tumorigenesis. Cancer
Res. 70:5155–5162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mastroleo I: Post-trial obligations in the
Declaration of Helsinki 2013: Classification, reconstruction and
interpretation. Dev World Bioeth. Oct 19–2015.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Enneking WF, Spanier SS and Goodman MA: A
system for the surgical staging of musculoskeletal sarcoma. Clin
Orthop Relat Res. 153:106–120. 1980.PubMed/NCBI
|
|
36
|
Enneking WF: A system of staging
musculoskeletal neoplasms. Clin Orthop Relat Res. 204:9–24.
1986.PubMed/NCBI
|
|
37
|
Mathema VB and Koh YS: Inhibitor of
growth-4 mediates chromatin modification and has a suppressive
effect on tumorigenesis and innate immunity. Tumour Biol. 33:1–7.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mao ZL, He SB, Sheng WH, Dong XQ and Yang
JC: Adenovirus-mediated ING4 expression reduces multidrug
resistance of human gastric carcinoma cells in vitro and in vivo.
Oncol Rep. 30:2187–2194. 2013.PubMed/NCBI
|
|
39
|
Byron SA, Min E, Thal TS, Hostetter G,
Watanabe AT, Azorsa DO, Little TH, Tapia C and Kim S: Negative
regulation of NF-κB by the ING4 tumor suppressor in breast cancer.
PLoS One. 7:e468232012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han Z, Zhou C, Sun B, Yan Q and Zhang J:
Experimental studies on the inhibition of adenovirus-ING4-OSM
therapy on nasopharyngeal carcinoma proliferation in vitro and in
vivo. Cell Biochem Biophys. 70:1573–1578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lou C, Jiang S, Guo X and Dong XS: ING4 is
negatively correlated with microvessel density in colon cancer.
Tumour Biol. 33:2357–2364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li S, Fan T, Liu H, Chen J, Qin C and Ren
X: Tumor suppressor ING4 overexpression contributes to
proliferation and invasion inhibition in gastric carcinoma by
suppressing the NF-κB signaling pathway. Mol Biol Rep.
40:5723–5732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wei Q, He W, Lu Y, Yao J and Cao X: Effect
of the tumor suppressor gene ING4 on the proliferation of MCF-7
human breast cancer cells. Oncol Lett. 4:438–442. 2012.PubMed/NCBI
|
|
44
|
Xu M, Xie Y, Sheng W, Miao J and Yang J:
Adenovirus-mediated ING4 gene transfer in osteosarcoma suppresses
tumor growth via induction of apoptosis and inhibition of tumor
angiogenesis. Technol Cancer Res Treat. 14:369–378. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li M, Zhu Y, Zhang H, Li L, He P, Xia H,
Zhang Y and Mao C: Delivery of inhibitor of growth 4 (ING4) gene
significantly inhibits proliferation and invasion and promotes
apoptosis of human osteosarcoma cells. Sci Rep. 4:73802014.
View Article : Google Scholar : PubMed/NCBI
|