Introduction
Bladder cancer is the ninth most commonly occurring
cancer globally. In total, 70–80% of all bladder cancer patients
initially present with superficial disease (i.e., non-muscle
invasive) (1). Non-muscle invasive
bladder cancers [Ta, T1 or carcinoma in situ (CIS)] are a
heterogeneous group of tumors that vary in terms of oncological
outcome (2,3).
Generally, the initial approach to managing
non-muscle invasive bladder cancer is cystoscopic observation
followed by transurethral resection. The recurrence rate in
non-muscle invasive bladder cancer is high following resection and
the disease can progress to muscle invasive cancer, which has a
poor prognosis (4).
Since there is considerable risk for the recurrence
and/or progression of tumors following transurethral resection,
adjuvant intravesical therapies are recommended for all patients at
an intermediate to high risk of recurrence (5). Current treatment guidelines recommend
that patients at risk of recurrence should be treated with adjuvant
intravesical immunotherapy with bacillus Calmette-Guerin (BCG) or
adjuvant intravesical chemotherapy with mitomycin C, epirubicin or
doxorubicin (5). The time to the
initial cancer recurrence, but not disease progression, is reduced
by intravesical chemotherapy, and the treatment is associated with
minor side-effects (6). BCG appears
to be superior to intravesical chemotherapy with regard to the rate
of recurrence, the response rate and the percentage of patients
remaining tumor-free; however BCG is more toxic than chemotherapy
(5–11).
Although evidence suggests that BCG is superior to
the majority of chemotherapies, its superiority with respect to
mitomycin C is less clear. Two prior meta-analyses found that tumor
recurrence was reduced with maintenance BCG compared with mitomycin
C (9,12). Another meta-analysis found that the
superiority of BCG over mitomycin C for tumor recurrence was only
apparent in a subgroup of patients at high risk for tumor
recurrence (13). A different
meta-analysis found that BCG was superior to chemotherapy
(mitomycin C, adriamycin, epirubicin and gemcitabine) with regard
to achieving a complete response or disease-free survival; however,
the evidence did not indicate that one agent was superior to the
others with regard to overall survival (14). The study did, however, find that the
immediate post-operative use of mitomycin or epirubicin was
effective in reducing tumor recurrence (14).
The present meta-analysis further investigated the
benefits of BCG and mitomycin C in the treatment of patients with
superficial bladder cancer by comparing progression-free survival
(PFS) rates in patients treated with either mitomycin C or BCH
following transurethral resection.
Materials and methods
Search strategy
This meta-analysis was conducted in accordance with
the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses guidelines (15). The
Medline (www.ncbi.nlm.nih.gov/pubmed/), Cochrane (www.cochranelibrary.com/) and EMBASE (www.embase.com/)databases were searched between
January 1966 and August 31, 2014 for studies that investigated the
efficacy of the intravesical instillation of chemotherapy in
patients with non-muscle invasive bladder cancer who had been
treated with transurethral resection. Search terms included:
‘Urinary bladder neoplasms’, ‘superficial bladder cancer’ and
‘non-muscle invasive bladder cancer’; ‘bacillus Calmette-Guerin’ or
‘BCG’; ‘mitomycin C’; and ‘intravesical administration’. A list of
potentially relevant studies was identified by two independent
reviewers. A third reviewer was consulted for any
discrepancies.
Included studies had to be randomized controlled
trials that investigated subjects who were ≥18 years of age and who
had been diagnosed with non-muscle invasive bladder cancer [stage
Ta, T1 or carcinoma in situ (CIS)]. Included studies also
had to have reported the numerical data of interest (i.e., PFS
rate) for intravesical BCG and intravesical mitomycin C
administration. Non-English publications, letters, comments,
editorials and case reports were excluded.
Data extraction
Data were extracted by two independent reviewers and
a third reviewer was consulted in the case of any uncertainties or
disagreements. The following information was extracted from studies
that met the inclusion criteria: The name of the first author, year
of publication, study design, demographic data of subjects, regimen
of intervention, PFS rate, recurrence-free survival rate and
adverse events.
Quality assessment
The quality of the studies and included data was
evaluated using the Cochrane Risk of Bias Tool (16). Similar to the method for study
inclusion and data extraction, two independent reviewers performed
the quality assessment and a reviewer was consulted for any
disagreements.
Statistical analysis
The primary outcome for this meta-analysis was the
5-year PFS rate. Odds ratios (ORs) and 95% confidence intervals
(CIs) were calculated for binary outcomes for each individual study
and for those studies combined. An OR of <1 indicated that the
BCG group was favored. A χ2-based test of homogeneity
was performed and the inconsistency index (I2) and Q
statistics were determined. If the I2 statistic was
>50%, a random-effects model (DerSimonian-Laird method) was
used. Otherwise, a fixed-effects model (Mantel-Haenszel method) was
employed. Combined effects were calculated and a two-sided P-value
of <0.05 was considered to indicate statistical significance.
Sensitivity analysis was performed using the leave one-out
approach. Publication bias was not assessed as >5 studies are
required to detect funnel plot asymmetry (17). All analyses were performed using
Comprehensive Meta-Analysis statistical software, version 2.0
(Biostat, Englewood, NJ, USA).
Results
Literature search
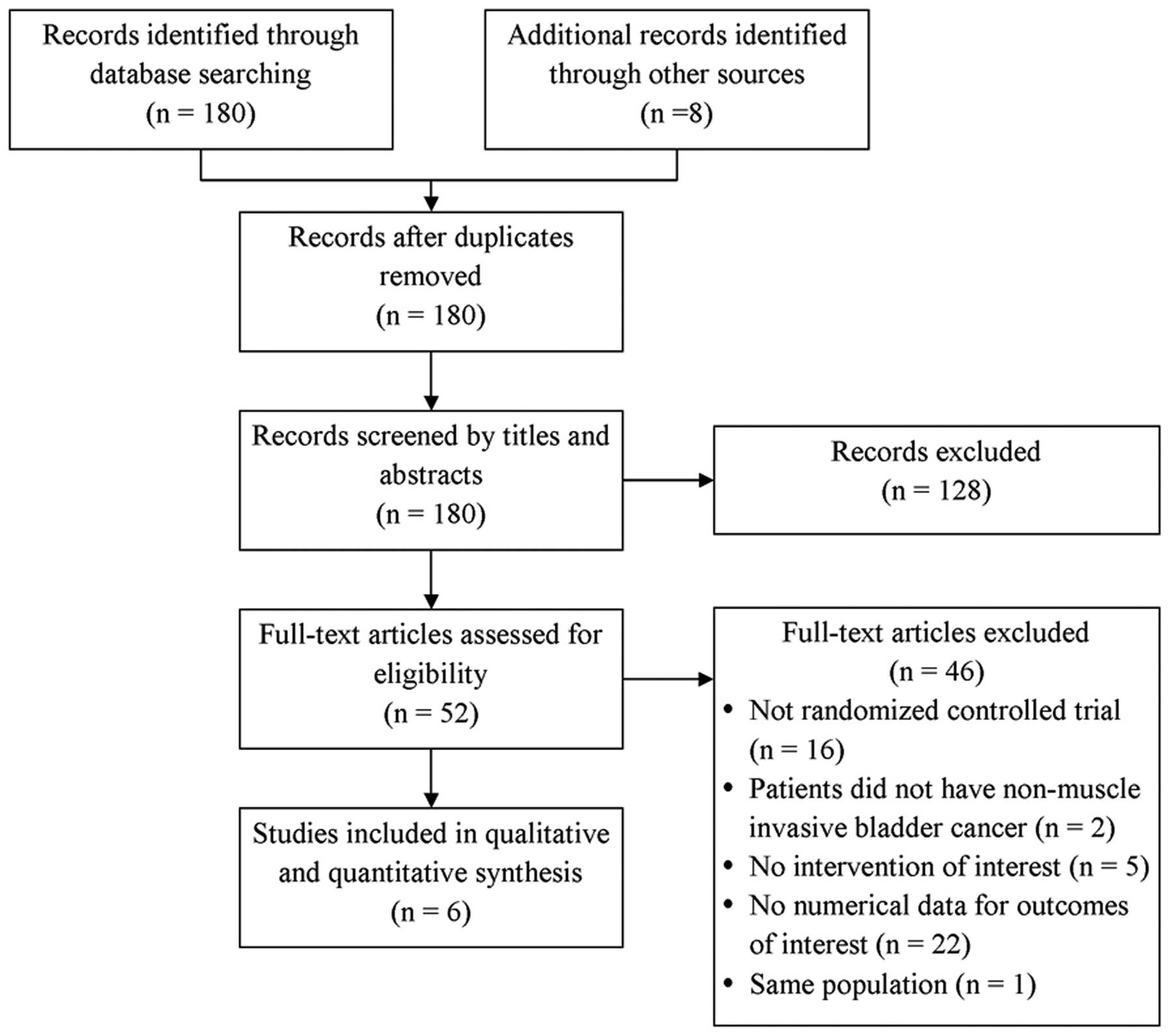
The database search identified 180 studies and a
hand-search identified an additional 8 studies (Fig. 1). Of the 188 studies, 8 studies were
excluded as they were duplicates and 128 were excluded as they were
clearly irrelevant. Another 46 studies were excluded as they were
not randomized control trials (n=16), the patients did not have
non-muscle invasive bladder cancer (n=2), the studies did not
report interventions of interest (n=5), the studies did not
quantitatively report data of interest (n=22) or the studies
included the same population of patients as another included study
(n=1). A total of 6 randomized controlled studies were included in
the final analysis (18–23).
Study characteristics
A total of 1,289 patients were encompassed by all 6
studies (Table I). Overall, the
studies were similar with respect to the distributions of age and
gender between patients who received intravesical mitomycin C or
BCG (Table I); ages ranged from
63.5–68 years and the majority of patients were male (range,
71–88%). Dosing regimens across the included studies varied and all
the intravesical regimens were applied subsequent to surgery.
 | Table I.Study characteristics of the included
studies. |
Table I.
Study characteristics of the included
studies.
| First author,
year | Study type | Type of patients | Comparison | Dosage of
intervention | Cases, n | Age, years | Male,% | (Ref.) |
|---|
| Friedrich et
al, 2007 | RCT | Intermediate-risk
superficial bladder cancer | MMC | 20 mg weekly for 6
weeks followed by monthly instillations for 3 years | 153 | 67 | 87 | (18) |
|
|
|
| BCG | RIVM 2×108
CFU weekly for 6 weeks | 163 | 67 | 80 |
|
| Ojea et al,
2007 | RCT | Intermediate-risk
superficial bladder cancer | MMC | 30 mg weekly for 6
weeks followed by instillations every 2 weeks for 12 weeks | 149 | 64 | 87 | (19) |
|
|
|
| BCG | 27 mg weekly for 6
weeks followed by instillations every 2 weeks for 12 weeks | 142 | 65 | 88 |
| Di Stasi et
al, 2003 | RCT | High-risk superficial
bladder cancer | MMC | 40 mg electromotive
MMC instillation with 20-mA electric current for 30 min/40 mg
passive MMC with a dwell time of 60 min | 72 | 67 | 72 | (20) |
|
|
|
| BCG | 81 mg BCG with a
dwell time of 120 min | 36 | 67 | 75 |
|
| Malmström et
al, 1999 | RCT | Superficial bladder
cancer | MMC | 40 mg MMC for 2
years | 125 | NA | NA | (21) |
|
|
|
| BCG | 20 mg BCG for 2
years | 125 | NA | NA |
|
| Krege et al,
1996 | RCT | Superficial bladder
cancer | MMC | 20 mg in 150 ml
sodium chloride every 2 weeks during year 1 followed by monthly
instillations during year 2 | 113 | 68 | 84 | (22) |
|
|
|
| BCG | 120 mg in 50 ml
sodium chloride weekly for 6 weeks and once a month for 4
months | 102 | 64 | 80 |
|
| Rintala et
al, 1991 | RCT | Frequently
recurrent superficial bladder cancer | MMC | 20–40 mg weekly for
1st month flowed by monthly instillations for 2 years | 58 | 67 | 71 | (23) |
|
|
|
| BCG | 6×108
CFU weekly during the first month, and once a month for a 2-year
period | 51 | 68 | 76 |
|
Across the studies, PFS rate ranged from 34–75% for
mitomycin C and from 47–81% for BCG (Table II). Recurrence-free survival rate was
higher for mitomycin C compared with BCG (range, 37–88.3% for
mitomycin C and 21–68.5% for BCG). Common adverse events reported
across studies for the two treatments were hematuria, fever and
cystitis.
 | Table II.Summary of outcome measurements and
adverse events/toxicity. |
Table II.
Summary of outcome measurements and
adverse events/toxicity.
| First author,
year | Comparison | Progression-free
survival rate, % | Recurrence-free
survival rate, % | Adverse
events/toxicity, % cases | (Ref.) |
|---|
| Friedrich et
al, 2007 | MMC vs. BCG | NA | (2-year) 88.3 vs.
68.5 (3-year) 86.1 vs. 65.5 | Dysuria: 20.5 vs.
17.3; hematuria: 9.4 vs. 17.6; fever: 2.4 vs. 9.3 | (18) |
| Ojea et al,
2007 | MMC vs. BCG | (5-year) 58 vs.
75 | NA | Local toxicity:
30.2 vs. 65.4; systemictoxicity: 4.6 vs. 11.2 | (19) |
| Di Stasi et
al, 2003 | MMC vs. BCG | (5-year) 75 vs.
81 | (5-year) 37 vs.
21 | Hematuria: 19.4 vs.
72.2; fever: 0.0 vs. 19.4; cystitis: 30.6 vs. 66.7; urinary
frequency: 18.1 vs. 58.3; general malaise: 1.4 vs. 30.5; allergic
reactions: 6.9 vs. 0.0 | (20) |
| Malmström et
al, 1999 | MMC vs. BCG | (5-year) 34 vs.
47 | NA | In 6 patients
(2.4%) a contracted bladder developed (<100 ml); after MMC in 3
patients and BCG in 1 patient, and after crossover to BCG in 2
patients | (21) |
| Krege et al,
1996 | MMC vs. BCG | NA | (3-year) 46.7 vs.
59.6 | Haematuria: 3 vs.
6; fever: 0 vs. 18; cystitis: 16 vs. 34; skin alteration: 6 vs. 0;
epididymitis: 3 vs. 10 | (22) |
| Rintala et
al, 1991 | MMC vs. BCG | (1-year) 39 vs.
72 | NA | Cystitis: 2 vs. 9;
eczema: 5.2 vs. 0.0 | (23) |
5-year PFS rate
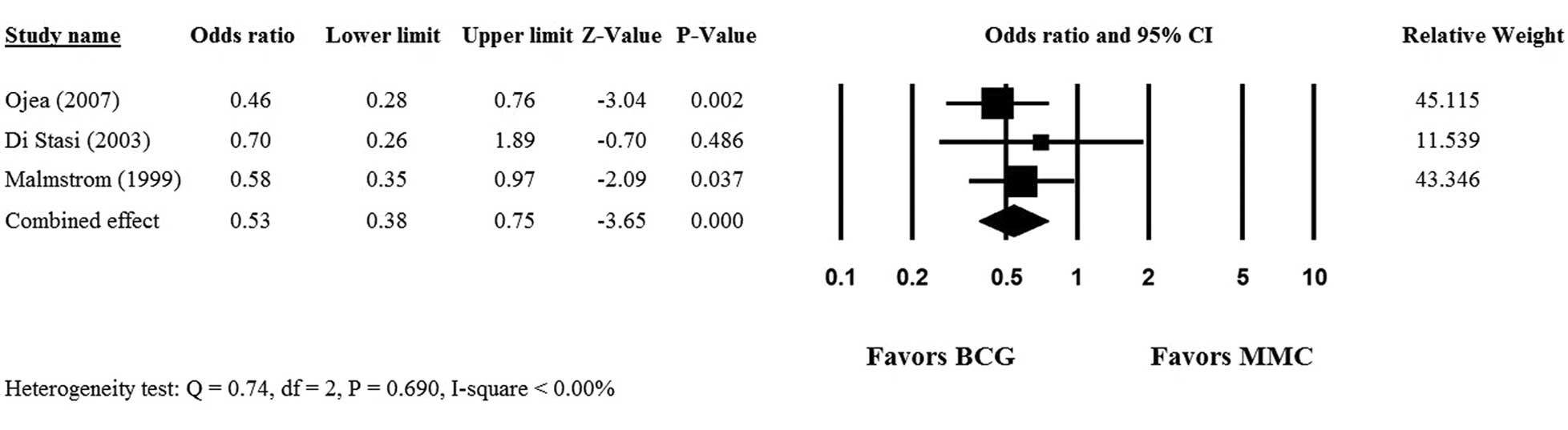
Only 3 (19–21) of the 6 studies provided complete
5-year PFS data for patients who received intravesical mitomycin C
or BCG, and hence, were included in the meta-analysis. No
heterogeneity was observed among the 3 studies, therefore, a
fixed-effects model was used (Q statistic, 0.74;
I2<0.00%; P=0.690). The overall analysis revealed a
significant difference in 5-year PFS rate between the mitomycin C
and BCG groups (OR, 0.53; 95% CI, 0.38–0.75; P<0.001) and
indicated that BCG was superior to mitomycin C therapy in patients
with non-muscle invasive bladder cancer following transurethral
resection (Fig. 2).
Sensitivity analysis
Sensitivity analyses were performed using the
leave-one-out approach in which the meta-analysis of the 5-year PFS
rate was performed with each study removed in turn. The direction
and magnitude of combined estimates did not vary markedly with the
removal of the studies (Fig. 3),
indicating that the meta-analysis had good reliability and that the
data was not overly affected by any one study.
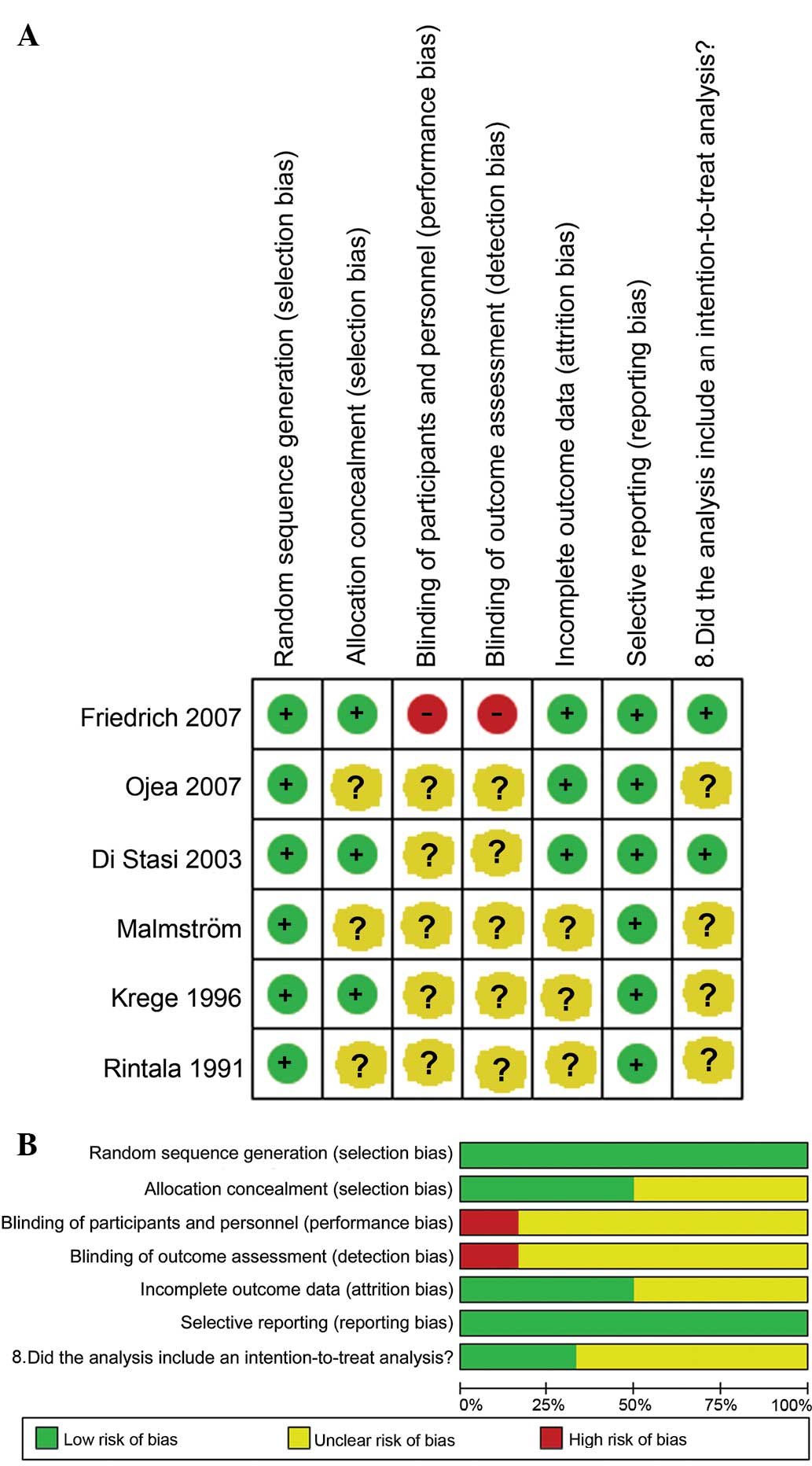
Quality assessment
As shown in Fig. 4A and
B, the data from the 6 studies were of good quality, although
performance and detection biases were also present. The studies
indicated either that the patients and personnel were blinded to
randomization, or that randomization was not reported within the
study.
Discussion
The present meta-analysis evaluated the benefit of
BCG compared with mitomycin C in the treatment of patients with
superficial bladder cancer by analyzing PFS rate in patients
treated with either of these drugs following transurethral
resection. It was found that BCG was superior to mitomycin C with
regard to 5-year PFS rate (P<0.001).
The present meta-analysis differed from several
prior meta-analyses (4,14,24) in
that the present study quantitatively evaluated a head-to-head
comparison of BCG and mitomycin C using only randomized controlled
studies. The present analysis did not include studies that
evaluated combination (i.e., BCG plus mitomycin C) therapies.
Two previous meta-analyses assessed the benefit of
BCG compared with mitomycin C in patients with non-muscle invasive
bladder cancer (10,25). The study by Sylvester et al
(2002) included 9 randomized trials with 700 CIS patients and
compared BCG to either mitomycin C, epirubicin, adriamycin, or
sequential dosing of mytomycin C and Adriamycin. Although the
difference in the long-term benefit of BCG compared with mitomycin
C was smaller compared with other chemotherapies, BCG was superior
to mitomycin C in trials with maintenance BCG with regard to
disease recurrence (OR, 0.57; P<0.04) (10). The study concluded that compared with
chemotherapy, BCG significantly lowered the risk of short- and
long-term treatment failure in patients with superficial bladder
cancer.
Malmström et al (2009) performed a
meta-analysis that compared the efficacy of BCG and mitomycin C
using individual patient data from randomized trials (25). The analysis included 9 studies with
2,820 patients. At 4.4 years post-initiation of treatment, 43% of
the patients exhibited tumor recurrence. In general, there was no
difference in the time to first recurrence between BCG and
mitomycin C therapy (P=0.09). However, with BCG maintenance
therapy, there was a 32% reduction in the risk of recurrence with
BCG compared with mitomycin C (P<0.0001). No significant
difference was found between BCG and mitomycin C with regard to
bladder cancer-related mortality, overall survival or disease
progression. The difference between the findings of Malmström et
al (2009) and the present analysis may reflect the differences
in the studies included and analyses performed (25).
The present study did not evaluate the type of
regimen (i.e., co-administration, dose and maintenance therapy vs.
induction therapy). Prior studies found BCG to be more effective at
reducing recurrence when used as maintenance therapy, but that a
sole induction course was not superior to mitomycin C (10,11,14,24).
It was also found that maintenance BCG therapy was associated with
greater adverse events, but a lower recurrence rate, compared with
other therapies. It has been proposed that the benefit of the
reduced rate of recurrence outweighs the risk of complications,
particularly in patients that are at a high risk of recurrence
(24). Prior studies have not
clarified if the dose of BCG affects the outcome (24,26–28). Also,
one prior meta-analysis did not find a significant difference in
terms of recurrence-free survival and PFS between patients who
received BCG plus mitomycin C compared with those who received BCG
or mitomycin C alone (24). The
present analysis did not assess the addition of mitomycin C to BCG
therapy, or the effect of the timing or dose of either therapy on
reducing the rate of recurrence or PFS. Future studies are required
to further evaluate the effect of dose and combination therapy on
recurrence rate and PFS in patients with superficial bladder cancer
following transurethral resection.
Several limitations of the present study should be
considered when interpreting the results. The analysis used a small
sample size and there was heterogeneity in the types of treatment
regimens used across the studies; these factors may have biased the
results. In addition, the patients and the study personal were not
blinded to the treatment, which also could have affected the
analysis.
In summary, the present meta-analysis found that BCG
was superior to mitomycin C with regard to 5-year PFS rate in
patients with non-muscle invasive bladder cancer following
transurethral resection.
References
|
1
|
Lamm D, Herr H, Jakse G, Kuroda M, Mostofi
FK, Okajima E, Sakamoto A, Sesterhenn I and da Silva FC: Updated
concepts and treatment of carcinoma in situ. Urol Oncol. 4:130–138.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Witjes JA, Palou J, Soloway M, Lamm D,
Kamat AM, Brausi M, Persad R, Buckley R, Colombel M and Böhle A:
Current clinical practice gaps in the treatment of intermediate-
and high-risk non-muscle-invasive bladder cancer (NMIBC) with
emphasis on the use of bacillus Calmette-Guérin (BCG): Results of
an international individual patient data survey (IPDS). BJU Int.
112:742–750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shelley MD, Jones G, Cleves A, Wilt TJ,
Mason MD and Kynaston HG: Intravesical gemcitabine therapy for
non-muscle invasive bladder cancer (NMIBC): A systematic review.
BJU Int. 109:496–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Babjuk M, Oosterlinck W, Sylvester R,
Kaasinen E, Böhle A, Palou-Redorta J and Rouprêt M: European
Association of Urology (EAU): EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder, the 2011 update. Eur Urol.
59:997–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pawinski A, Sylvester R, Kurth KH,
Bouffioux C, van der Meijden A, Parmar MK and Bijnens L: A combined
analysis of European organization for research and treatment of
cancer and medical research Council randomized clinical trials for
the prophylactic treatment of stage TaT1 bladder cancer. European
organization for research and treatment of cancer genitourinary
tract cancer cooperative group and the medical research council
working party on superficial bladder cancer. J Urol. 156:1934–1940;
discussion 1940–1941. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shelley MD, Kynaston H, Court J, Wilt TJ,
Coles B, Burgon K and Mason MD: A systematic review of intravesical
bacillus Calmette-Guérin plus transurethral resection vs.
transurethral resection alone in Ta and T1 bladder cancer. BJU Int.
88:209–216. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han RF and Pan JG: Can intravesical
bacillus Calmette-Guérin reduce recurrence in patients with
superficial bladder cancer? A meta-analysis of randomized trials.
Urology. 67:1216–1223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Böhle A, Jocham D and Bock PR:
Intravesical bacillus Calmette-Guerin versus mitomycin C for
superficial bladder cancer: A formal meta-analysis of comparative
studies on recurrence and toxicity. J Urol. 169:90–95. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sylvester RJ, van der Meijden AP and Lamm
DL: Intravesical bacillus Calmette-Guerin reduces the risk of
progression in patients with superficial bladder cancer: A
meta-analysis of the published results of randomized clinical
trials. J Urol. 168:1964–1970. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Böhle A and Bock PR: Intravesical bacille
Calmette-Guérin versus mitomycin C in superficial bladder cancer:
Formal meta-analysis of comparative studies on tumor progression.
Urology. 63:682–686; discussion 686–687. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malmström PU: Management of superficial
bladder cancer: What is new? Curr Opin Urol. 10:447–451. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shelley MD, Wilt TJ, Court J, Coles B,
Kynaston H and Mason MD: Intravesical bacillus Calmette-Guérin is
superior to mitomycin C in reducing tumour recurrence in high-risk
superficial bladder cancer: A meta-analysis of randomized trials.
BJU Int. 93:485–490. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shelley MD, Mason MD and Kynaston H:
Intravesical therapy for superficial bladder cancer: A systematic
review of randomised trials and meta-analyses. Cancer Treat Rev.
36:195–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate health care
interventions: explanation and elaboration. Ann Intern Med.
151:W65–W94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higgins JPT: Cochrane Collaboration
Handbook for Systematic Reviews of Interventions Version 5.1.0
[updated March 2011]. The Cochrane Collaboration. 2011.simplewww.cochrane-handbook.orgAccessed. November
12–2014
|
|
17
|
Sutton AJ, Duval SJ, Tweedie RL, Abrams KR
and Jones DR: Empirical assessment of effect of publication bias on
meta-analyses. BMJ. 320:1574–1577. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Friedrich MG, Pichlmeier U, Schwaibold H,
Conrad S and Huland H: Long-term intravesical adjuvant chemotherapy
further reduces recurrence rate compared with short-term
intravesical chemotherapy and short-term therapy with Bacillus
Calmette-Guérin (BCG) in patients with non-muscle-invasive bladder
carcinoma. Eur Urol. 52:1123–1129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ojea A, Nogueira JL, Solsona E, Flores N,
Gómez JM, Molina JR, Chantada V, Camacho JE, Piñeiro LM, Rodríguez
RH, et al: A multicentre, randomised prospective trial comparing
three intravesical adjuvant therapies for intermediate-risk
superficial bladder cancer: Low-dose bacillus Calmette-Guerin (27
mg) versus very low-dose bacillus Calmette-Guerin (13.5 mg) versus
mitomycin C. Eur Urol. 52:1398–1406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Stasi SM, Giannantoni A, Stephen RL,
Capelli G, Navarra P, Massoud R and Vespasiani G: Intravesical
electromotive mitomycin C versus passive transport mitomycin C for
high risk superficial bladder cancer: A prospective randomized
study. J Urol. 170:777–782. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Malmström PU, Wijkström H, Lundholm C,
Wester K, Busch C and Norlén BJ: 5-year followup of a randomized
prospective study comparing mitomycin C and bacillus
Calmette-Guerin in patients with superficial bladder carcinoma.
Swedish-Norwegian bladder cancer study group. J Urol.
161:1124–1127. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krege S, Giani G, Meyer R, Otto T and
Rübben H: A randomized multicenter trial of adjuvant therapy in
superficial bladder cancer: Transurethral resection only versus
transurethral resection plus mitomycin C versus transurethral
resection plus bacillus Calmette-Guerin. Participating Clinics. J
Urol. 156:962–966. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rintala E, Jauhiainen K, Alfthan O,
Hansson E, Juusela H, Kanerva K, Korhonen H, Permi J, Sotarauta M,
Vaalasti T, et al: Intravesical chemotherapy (mitomycin C) versus
immunotherapy (bacillus Calmette-Guérin) in superficial bladder
cancer. Eur Urol. 20:19–25. 1991.PubMed/NCBI
|
|
24
|
Zhu S, Tang Y, Li K, Shang Z, Jiang N,
Nian X, Sun L and Niu Y: Optimal schedule of bacillus
calmette-guerin for non-muscle-invasive bladder cancer: A
meta-analysis of comparative studies. BMC Cancer. 13:3322013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malmström PU, Sylvester RJ, Crawford DE,
Friedrich M, Krege S, Rintala E, Solsona E, Di Stasi SM and Witjes
JA: An individual patient data meta-analysis of the long-term
outcome of randomised studies comparing intravesical mitomycin C
versus bacillus Calmette-Guerin for non-muscle-invasive bladder
cancer. Eur Urol. 56:247–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoneyama T, Ohyama C, Imai A, Ishimura H,
Hagisawa S, Iwabuchi I, Mori K, Kamimura N, Koie T, Yamato T and
Suzuki T: Low-dose instillation therapy with bacille
Calmette-Guérin Tokyo 172 strain after transurethral resection:
Historical cohort study. Urology. 71:1161–1165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar A, Dubey D, Bansal P, Mandhani A and
Naik S: Urinary interleukin-8 predicts the response of standard and
low dose intravesical bacillus Calmette-Guerin (modified Danish
1331 strain) for superficial bladder cancer. J Urol. 168:2232–2235.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Irie A, Uchida T, Yamashita H, Matsumoto
K, Satoh T, Koh H, Shimura S, Iwamura M and Baba S: Sufficient
prophylactic efficacy with minor adverse effects by intravesical
instillation of low-dose bacillus Calmette-Guérin for superficial
bladder cancer recurrence. Int J Urol. 10:183–189. 2003. View Article : Google Scholar : PubMed/NCBI
|