Introduction
Bevacizumab is a recombinant humanized monoclonal
antibody that prevents tumor angiogenesis by inhibiting vascular
endothelial growth factor (VEGF) (1).
Bevacizumab is widely used in the treatment of a variety of
advanced solid tumors, including colorectal cancer (CRC), non-small
cell lung cancer (NSCLC), breast cancer and renal cell cancer
(RCC).
The adverse-effect profile of bevacizumab is
different compared with traditional cytotoxic chemotherapy agents
(2). Bone marrow suppression and
gastrointestinal (GI) toxicity are commonly associated with
chemotherapy; by contrast, frequently reported adverse effects of
bevacizumab include hypertension, proteinuria and epistaxis
(2). Bevacizumab is also associated
with other serious side effects, such as hemorrhage, GI tract
perforation, wound dehiscence, arterial and venous thromboembolism,
hypertensive crisis, reversible posterior leukoencephalopathy,
neutropenia, infections, nephritic syndrome and congestive heart
failure (3). The importance of
bevacizumab-associated hemorrhage is highlighted by the Food and
Drug Administration (FDA)-issued black box warning, which
recognizes that in patients with NSCLC treated with
bevacizumab-containing chemotherapy, fatal pulmonary hemorrhage may
occur. However, little is known regarding spontaneous perirenal
hematoma associated with bevacizumab treatment. To date, only one
case has been reported in the literature (4). The patient was treated with bevacizumab
(5 mg/kg, intravenous, 90 min biweekly), but developed perirenal
hematoma after 11 cycles of chemotherapy (4). The patient received a conservative
treatment approach and experienced symptomatic improvement
(4).
The present study reports the case of a 44-year-old
woman that developed a perirenal hematoma during treatment with
bevacizumab-containing chemotherapy for colon cancer.
Case report
In April 2012, a 44-year-old woman presented at
Soonchunhyang University Bucheon Hospital (Bucheon, Korea)
emergency room suffering with hematochezia and abdominal pain for
10 days. Contrast-enhanced computed tomography (CT) and colonoscopy
revealed a diffuse wall thickening on the proximal sigmoid colon
with metastasis to the lungs, peritoneum, T2 vertebrae,
retroperitoneum and hilar, and subcarinal lymph nodes. The patient
was diagnosed with sigmoid colon cancer [poorly-differentiated
adenocarcinoma; stage IV, pT3N2M1 (5); epidermal growth factor receptor
3+/Kirsten ras (KRAS)-wild-type] with metastasis to the
lungs, peritoneum, T2 vertebrae, retroperitoneum and hilar and
subcarinal lymph nodes. The patient underwent a palliative anterior
resection for a partial colon obstruction. Prior to the initiation
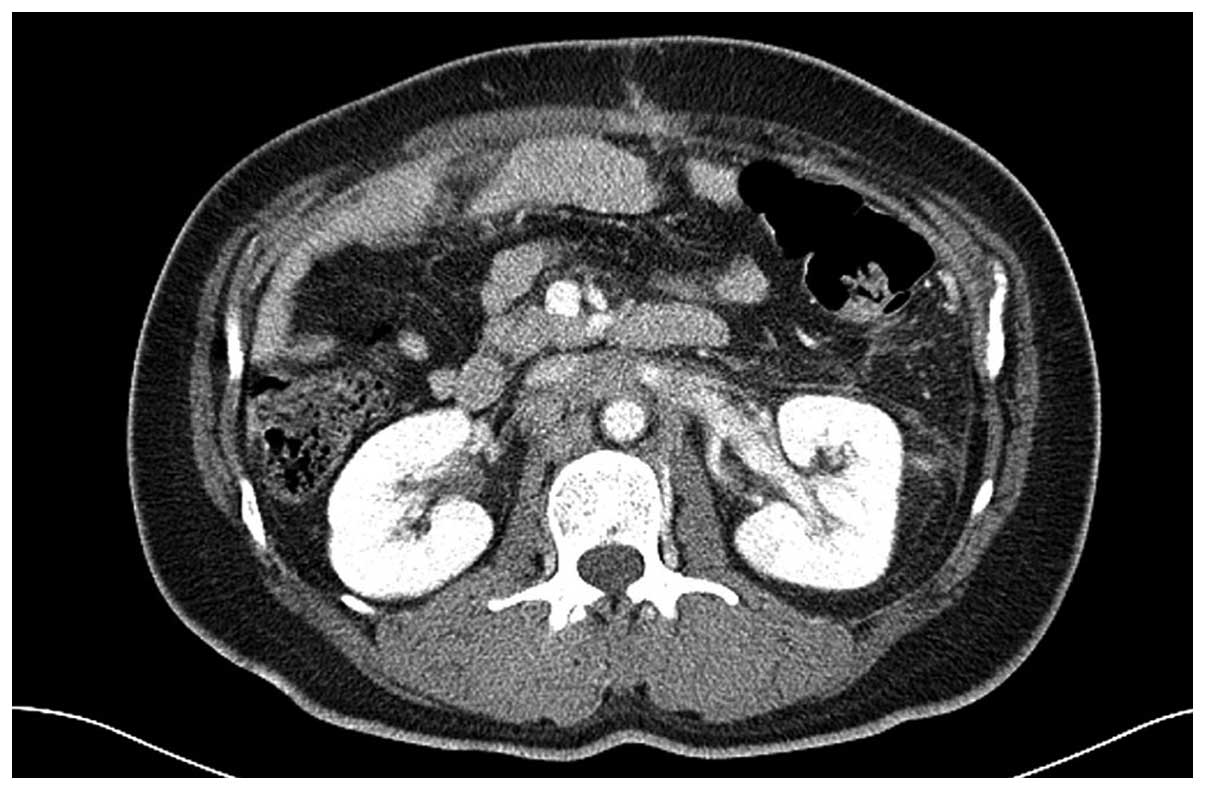
of chemotherapy, baseline CT scans were obtained 4 weeks subsequent
to surgery and no evidence of perirenal hematoma was observed
(Fig. 1). Palliative chemotherapy
consisting of the oxaliplatin (85 mg/m2, intravenous,
120 min biweekly), leucovorin (400 mg/m2, intravenous,
120 min biweekly) and 5-fluorouracil (2,400 mg/m2,
intravenous, 44 h biweekly) (FOLFOX6) regimen was administered, and
bevacizumab (5 mg/kg every 2 weeks) was added to the treatment
regimen following the first cycle of FOLFOX6. At the fourth cycle
of chemotherapy (June 2015), the patient presented with dyspnea and
oliguria. The chemotherapy cycle was delayed, and laboratory tests
were performed to identify the cause of the symptoms. Blood test
results revealed that the patient had an elevated level of white
blood cells (10.86×109 cells/l; normal range,
4–10×109 cells/l) and C-reactive protein (49 mg/l;
normal range, 0–5 mg/l), suggesting an underlying inflammatory
condition. In addition, the patient had a serum urea nitrogen level
of 17.8 mmol/l (normal range, 2.9–7.1 nmol/l), creatinine level of
530.4 µmol/l (normal range, 53–115 µmol/l) and potassium level of
5.9 mmol/l (normal range, 3.5–5.5 nmol/l). Sodium (133 mmol/l;
normal range, 133–142 mmol/l) and chloride (99 mmol/l; normal
range, 98–110 mmol/l) levels were normal, and the patient exhibited
no signs of proteinuria. An arterial blood gas analysis revealed
compensated metabolic acidosis with hypoxia. Chest radiography
revealed that the patient had pulmonary congestion and pleural
effusion. Consequently, emergency hemodialysis was initiated.
Following hemodialysis, the general condition of the
patient improved. A CT scan revealed the presence of a large right
perirenal hematoma, 11.2×9.7 cm in size, with no evidence of active
bleeding (Fig. 2). The hematoma
contained fluid and high-density amorphous materials. The patient
was treated conservatively, including bed rest, a blood transfusion
with 6 units of packed red blood cells, hemodialysis and
prophylactic antibiotic therapy with close monitoring. A follow-up
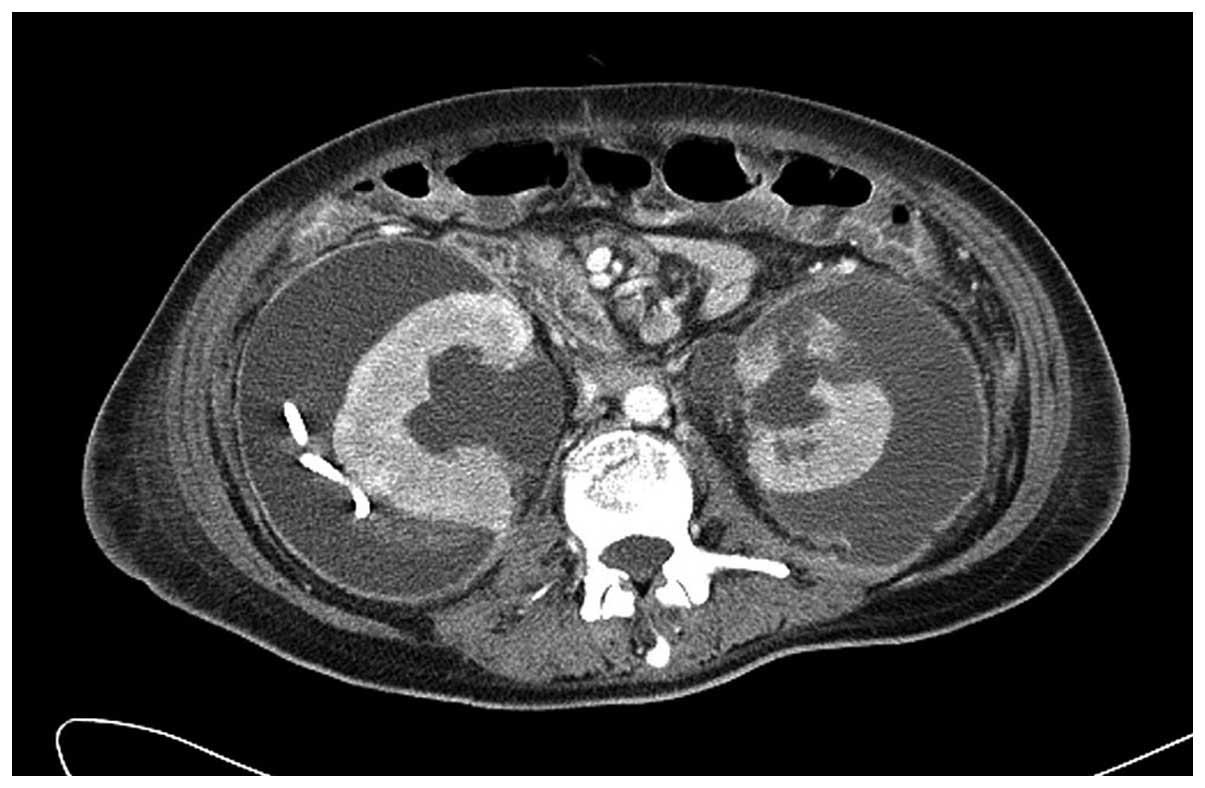
CT scan 3 weeks subsequent to hemodialysis revealed that the right
perirenal hematoma had increased in size and a left perirenal
hematoma with renal parenchyma disruption and laceration had
developed (Fig. 3). The patient
underwent ultrasonography-guided percutaneous catheter drainage for
the right perirenal hematoma. However, intervention on the left
side was terminated, due to the patient exhibiting unstable vital
signs [systolic blood pressure, 192 mmHg (normal range <120
mmHg); heart rate, 130 beats/min (normal range, 60–100 beats/min);
blood oxygen saturation, 79% (normal range, 95–100%)]. An
additional CT scan was performed 4 weeks subsequent to the previous
CT scan, 7 weeks subsequent to the termination of bevacizumab
treatment, which demonstrated that neither of the perirenal
hematomas had progressed in size (Fig.
4). The patient received the best supportive care, but
succumbed 4 weeks later to the rapidly progressing cancer.
Discussion
Perirenal hematomas commonly arise from a traumatic
renal injury (4). However,
spontaneous perirenal hematomas are rare (4). In a meta-analysis of 47 publications and
165 cases in 2002, Zhang et al (6) analyzed the etiology of spontaneous
perirenal hematomas. The most common etiology was malignancy,
including renal cell carcinoma in 33.3% of cases, followed by a
non-malignant tumor, including angiomyolipoma in 24.4% of the
cases, an arteriovenous malformation in 17.9% of the cases, an
infection in 10.3%, of the cases, nephritis in 5.1% of the cases
and hematological disease in 5.1% of the cases. There are a few
cases of perirenal hematomas that are idiopathic (7). Among dialysis patients, spontaneous
perirenal hematomas have been infrequently reported in the
literature. It is possible that hemorrhage may occur as a result of
acquired cystic change (8). The
present patient had no pathological renal conditions, including
renal primary cancer, renal metastasis, vascular diseases and
acquired cystic kidney disease.
Bevacizumab was the first anti-angiogenic agent
approved for use in cancer by the USA FDA in 2004 (9). Angiogenesis inhibitors, in particular
inhibitors of the VEGF pathway, have been associated with
significant bleeding complications (9). In a meta-analysis of 20 randomized
controlled trials, which consisted of 12,617 patients with a
variety of solid tumors, bevacizumab was identified to
significantly increase the risk of bleeding, with a relative risk
(RR) of 2.48 [95% confidence interval (CI), 1.93–3.18] compared
with control groups (10). However,
among the various bleeding complications associated with
bevacizumab treatment, perirenal hematoma has only been previously
reported by one study. Hayashi et al (4) reported a case of a 59-year-old woman
with metastatic rectal cancer treated with bevacizumab, who
developed lower-back pain following 11 cycles of chemotherapy. A CT
scan revealed the presence of a perirenal hematoma, and the
discontinuation of bevacizumab treatment resulted in an improvement
of symptoms.
Consequently, there have been only two reported
cases of perirenal hematoma in patients undergoing bevacizumab
treatment, including the present case. In the two studies, the
patients were diagnosed with metastatic colon cancer. The present
study hypothesizes that the occurrence of spontaneous perirenal
hematoma associated with bevacizumab treatment, which is a rare
event, may be due to the high relative risk of bleeding in
colorectal cancer. Patients with other tumors may be at a different
risk of bleeding, due to variations in tumor biology and
treatments. In a previous meta-analysis, the risk of high-grade
bleeding associated with bevacizumab treatment was relatively
increased in patients with NSCLC (RR, 3.41; 95% CI, 1.68–6.91), RCC
(RR, 6.37; 95% CI, 1.43–28.33) and CRC (RR, 9.11; 95% CI,
1.70–48.79) compared with metastatic breast cancer (RR, 1.33; 95%
CI, 0.32–5.46) and metastatic pancreatic cancer (RR, 1.54; 95% CI,
0.51–4.64) (11).
The most common symptom of perirenal hematoma is
flank pain (6). However, the initial
presenting symptom of the present patient was oliguria. Therefore,
the cause of the acute renal failure (ARF) of the present patient
was unclear. In total, 2 weeks prior to presentation, the
creatinine levels of the patient were normal, but then abruptly
increased. Perirenal hematoma is known to cause ARF (12,13). There
was little evidence to suspect any other cause for the sudden
elevation in creatinine levels. Therefore, the present study
concluded that the massive perirenal hematoma likely contributed to
the ARF in the present study.
Notably, in the present study, following the
discontinuation of bevacizumab treatment, the perirenal hematoma
increased in size and a novel perirenal hematoma developed.
According to pharmacokinetic studies of bevacizumab, the half-life
of bevacizumab is relatively long; the mean half-life of
bevacizumab is ~21 days, but the range varies between 11 and 50
days (14). Considering that the two
perirenal hematomas observed in the present patient did not
stabilize until 7 weeks subsequent to the cessation of treatment,
the present study hypothesizes that the residual circulating
bevacizumab aggravated the perirenal hematomas up to 7 weeks
following the cessation of treatment.
Overall, the present study concludes that
bevacizumab is likely to have contributed to the development of the
perirenal hematoma. Although the development of a spontaneous
perirenal hematoma caused by bevacizumab treatment is an extremely
rare event, it may lead to serious complications. Furthermore, due
to the perirenal hematoma and ARF, the patient in the present study
could not receive any additional chemotherapy and the patient
succumbed to uncontrolled colon cancer.
In conclusion, patients undergoing bevacizumab
treatment for cancer should be closely monitored for bleeding
complications. In particular, when colorectal cancer patients
receive bevacizumab-containing chemotherapy, physicians should be
aware of unusual bleeding complications, including perirenal
hematomas.
References
|
1
|
Ranieri G, Patruno R and Ruggieri E:
Vascular endothelial growth factor (VEGF) as a target of
bevacizumab in cancer: from the biology to the clinic. Curr Med
Chem. 13:1845–1857. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hurwitz H and Saini S: Bevacizumab in the
treatment of metastatic colorectal cancer: Safety profile and
management of adverse events. Semin Oncol. 33(Suppl 10): S26–S34.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saif MW: Managing bevacizumab-related
toxicities in patients with colorectal cancer. J Support Oncol.
7:245–251. 2009.PubMed/NCBI
|
|
4
|
Hayashi H, Okamoto I and Nakagawa K:
Perirenal hematoma associated with bevacizumab treatment. Invest
New Drugs. 30:808–809. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual (7th). Springer.
New York, NY: 2010.
|
|
6
|
Zhang JQ, Fielding JR and Zou KH: Etiology
of spontaneous perirenal hemorrhage: A meta-analysis. J Urol.
167:1593–1596. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McDougal WS, Kursh ED and Persky L:
Spontaneous rupture of the kidney with perirenal hematoma. J Urol.
114:181–184. 1975.PubMed/NCBI
|
|
8
|
Milard PR and Oliver D: Acquired cystic
disease of the kidneys: a hazard of long-term intermittent
maintenance haemodialysis. J Clin Pathol. 30:868–877. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Folkman J: Angiogenesis: An organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hapani S, Sher A, Chu D and Wu S:
Increased risk of serious hemorrhage with bevacizumab in cancer
patients: A meta-analysis. Oncology. 79:27–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hang XF, Xu WS, Wang JX, et al: Risk of
high-grade bleeding in patients with cancer treated with
bevacizumab: A meta-analysis of randomized controlled trials. Eur J
Clin Pharmacol. 67:613–623. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gültekin N, Akın F and Küçükateş E:
Warfarin-induced bilateral renal hematoma causing acute renal
failure. Turk Kardiyol Dern Ars. 39:228–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh N, Neubauer BE and Venuto RC: Acute
renal failure during pregnancy secondary to spontaneous perirenal
hematoma. Ren Fail. 29:1053–1054. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gordon MS, Margolin K, Talpaz M, Sledge GW
Jr, Holmgren E, Benjamin R, Stalter S, Shak S and Adelman D: Phase
I safety and pharmacokinetic study of recombinant human
anti-vascular endothelial growth factor in patients with advanced
cancer. J Clin Oncol. 19:843–850. 2001.PubMed/NCBI
|