Introduction
Head and neck squamous cell carcinoma is the sixth
most common malignancy in the world (1), and accounts for 6–7% of all malignant
tumors (2). Hypopharyngeal squamous
cell carcinoma (HSCC) accounts for ~5% of all head and neck
malignant tumors (3). Although the
incidence of HSCC is low, the location of the primary tumor tends
to be concealed, thus being difficult to locate accurately, which
results in a late diagnosis for the majority of patients, once a
tumor located near a neck lymph node has metastasized (4). At present, the majority of patients with
HSCC are surgically treated with radiation therapy or a
comprehensive chemical treatment method (5). However, these approaches result in a
poor prognosis, and ~1/2 of patients experience a recurrence within
1 year (3,6,7).
Additionally, patient's word pronunciation, breathing and
swallowing functions are severely affected or lost, thus seriously
impacting their quality of life and long-term survival (8). Therefore, the identification of novel
tumor markers may aid the earlier detection and improved treatment
of this disease (9).
Tight junctions (TJs) exist between epithelial and
endothelial cells, and function as a barrier to maintain a cellular
steady-state and cell polarity (10).
These junctions are mainly composed of three types of membrane
proteins, including occludin, which is a member of the claudin
family of proteins and junctional adhesion molecules (11). Claudin proteins are major barrier
proteins (12) whose numbers and
distribution directly affects the structure and function of TJs
within the cell membrane (13). In
various tumors, abnormal claudin expression alters TJ structure and
function, causing a disruption in cell polarity and a decrease in
cell adhesion, thus enhancing the invasive and metastatic potential
of tumor cells (14). Among the
claudin proteins, claudin-1 has been observed to be expressed at
different levels in different tumors (15). It has been reported that claudin-1 is
overexpressed and promotes tumor development in renal cell
carcinoma (16), ovarian cancer
(17), gastric adenocarcinoma
(18) and colorectal cancer (19,20).
However, in breast cancer (21,22) and
lung adenocarcinoma (23), claudin-1
expression was previously reported to be significantly reduced.
These differences in expression levels have been confirmed by other
studies, and closely correlate with tumor occurrence and
development (24,25).
The cell cycle in normal cells is regulated by
specific cyclins and cyclin-dependent kinases (CDKs), and exhibits
an orderly start and end (26). By
contrast, malignant cells are characterized by uncontrolled
proliferation due to a loss of cell cycle regulation (27,28).
Cyclin B1 is a cell cycle protein involved in checkpoint control,
promotion of the G2/M phase transition and acceleration of the cell
cycle (29). Previous studies have
demonstrated that cyclin B1 is overexpressed in numerous
malignancies, including breast (30),
gastric (31), early non-small cell
lung (32), colorectal (33) and prostate cancer (34). Furthermore, uncontrolled cyclin B1
expression is closely associated with transformation and malignant
proliferation of tumor cells (35,36), and
its overexpression is associated with poor prognosis in esophageal
(37,38), tongue and lung cancer (39). These findings suggest that cyclin B1
may be a useful tumor diagnostic marker.
However, the potential associations between the
expression levels of claudin-1 and cylin B1 in HSCC and the
clinical stage, pathological grade and prognosis of patients remain
to be examined. Therefore, the present study examined the
expression levels of cyclin B1 and claudin-1 in 97 HSCC tissue
samples and 90 adjacent noncancerous tissue samples using
immunohistochemistry (IHC), in order to determine the associations
between the expression levels of these proteins and the patients'
clinical stage, pathological features and prognosis. The present
findings may aid the early diagnosis and prognosis of HSCC, and may
guide future studies on targeted tumor therapy.
Materials and methods
Patients and sample collection
A total of 97 malignant tumors and 90 adjacent
noncancerous tissue specimens (mucosae) from patients who had
undergone surgical treatment for primary HSCC at Qilu Hospital of
Shandong University (Jinan, China) were obtained from January 2008
to April 2010. All specimens were formalin-fixed (Hubei Xingfa
Chemicals Group Co., Ltd., Hubei, China) and paraffin-embedded
(Beijing Yanshan Yanya Chemical Sales Center, Beijing, China). The
patients enrolled in the present study had not received neoadjuvant
chemotherapy or radiation therapy prior to surgery. All individuals
were treated by standard radical surgery with negative margins, and
were administered 50–65 Gy radiotherapy post-operatively (KDS
Medical Linear Accelerator; Siemens, Munich, Germany). The
tumor-node-metastasis (TNM) classification of tumor samples was
conducted in accordance with the guidelines published by the
International Union Against Cancer in 2002 (40). Patients were followed-up for 3–5
years, and TNM staging was monitored during this period. The mean
age of patients was 58 years, and the age range was 37–78 years.
Patients' age, gender, pathological grade and clinical stage are
presented in Tables I and II. Pathological grade was independently
evaluated by two experienced pathologists (Department of Pathology,
Tai'an City Central Hospital, Ti'an, China). The present study was
approved by the Ethics Committee of Qilu Hospital of Shandong
University (Jinan, China; approval no. 103-2008), and written
informed consent was obtained from the patients' families.
 | Table I.Claudin-1 protein expression and
tumor index correlation analysis. |
Table I.
Claudin-1 protein expression and
tumor index correlation analysis.
|
| Claudin-1
expression |
|---|
|
|
|
|---|
|
Characteristics | No. of cases
(%) | Low (%) | High (%) | P-value |
|---|
| Age, years |
|
|
| 0.175 |
|
<60 | 54 (55.7) | 17 (17.5) | 37 (38.1) |
|
|
≥60 | 43 (44.3) | 9 (9.3) | 34 (35.1) |
|
| Gender |
|
|
| 0.055 |
|
Female | 9 (9.3) | 5 (5.2) | 4 (4.1) |
|
|
Male | 88 (90.7) | 21 (21.6) | 67 (69.1) |
|
| Tobacco
smoking |
|
|
| 0.085 |
| None or
limited | 19 (19.6) | 8 (8.2) | 11 (11.3) |
|
|
Excessively | 78 (80.4) | 18 (18.6) | 60 (61.9) |
|
| Alcohol
consumption |
|
|
| 0.069 |
| None or
limited | 35 (36.1) | 13 (13.4) | 22 (22.7) |
|
|
Excessively | 62 (63.9) | 13 (13.4) | 49 (50.5) |
|
| Degree of
differentiation |
|
|
| 0.004 |
|
Well | 25 (25.8) | 13 (13.4) | 12 (12.4) |
|
|
Moderate | 37 (38.1) | 7 (7.2) | 30 (30.9) |
|
|
Poor | 35 (36.1) | 6 (6.2) | 29 (29.9) |
|
| TNM stage |
|
|
| 0.221 |
|
I+II | 18 (18.6) | 3 (3.1) | 15 (15.5) |
|
|
III+IV | 79 (81.4) | 23 (23.7) | 56 (57.7) |
|
| Lymph node
metastasis |
|
|
| 0.026 |
| No | 35 (36.1) | 14 (14.4) | 21 (21.6) |
|
|
Yes | 62 (63.9) | 12 (12.4) | 50 (51.5) |
|
 | Table II.Cyclin B1 protein expression and
tumor index correlation analysis. |
Table II.
Cyclin B1 protein expression and
tumor index correlation analysis.
|
| Cyclin B1
expression |
|---|
|
|
|
|---|
|
Characteristics | No. of cases
(%) | Low (%) | High (%) | P-value |
|---|
| Age, years |
|
|
| 0.135 |
|
<60 | 54 (55.7) | 22 (22.7) | 32 (33.0) |
|
|
≥60 | 43 (44.3) | 12 (12.4) | 31 (32.0) |
|
| Gender |
|
|
| 0.162 |
|
Female | 9 (9.3) | 5 (5.2) | 4 (4.1) |
|
|
Male | 88 (90.7) | 29 (29.9) | 59 (60.8) |
|
| Tobacco
smoking |
|
|
| 0.322 |
| None or
limited | 19 (19.6) | 8 (8.2) | 11 (11.3) |
|
|
Excessively | 78 (80.4) | 26 (26.8) | 52 (53.6) |
|
| Alcohol
consumption |
|
|
| 0.077 |
| None or
limited | 35 (36.1) | 16 (16.5) | 19 (19.6) |
|
|
Excessively | 62 (63.9) | 18 (18.6) | 44 (45.4) |
|
| Degree of
differentiation |
|
|
| <0.001 |
|
Well | 25 (25.8) | 18 (18.6) | 7 (7.2) |
|
|
Moderate | 37 (38.1) | 8 (8.2) | 29 (29.9) |
|
|
Poor | 35 (36.1) | 8 (8.2) | 27 (27.8) |
|
| TNM stage |
|
|
| 0.548 |
|
I+II | 18 (18.6) | 6 (6.2) | 12 (12.4) |
|
|
III+IV | 79 (81.4) | 28 (28.9) | 51 (52.6) |
|
| Lymph node
metastasis |
|
|
| 0.161 |
| No | 35 (36.1) | 15 (15.5) | 20 (20.6) |
|
|
Yes | 62 (63.9) | 19 (19.6) | 43 (44.3) |
|
IHC staining
Immunostaining was performed with rabbit anti-human
polyclonal antibodies against claudin-1 (catalogno. ZA-0365; 1:100)
and cyclin B1 (catalog no. ZA-0384; 1:100), which were purchased
from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.
(Beijing, China), according to the manufacturer's protocol. Tissue
sections were incubated at 62°C for 30 min, deparaffinized in
xylene (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.)
and rehydrated in graded alcohol prior to pretreatment with 3%
hydrogen peroxide in phosphate-buffered saline (PBS) for 15 min to
block endogenous peroxidase activity. Next, sections were washed 3
times in PBS, and heated in a microwave oven in the presence of
0.01 M citric acid buffer pH 6.0 (in the case of cyclin B1; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.) or
ethylenediaminetetraacetic acid buffer pH 8.0 (in the case of
claudin-1; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.)
for 15 min, and gradually cooled down to room temperature. The
sections were then incubated overnight with the appropriate
anti-cyclin B1 or anti-claudin-1 antibodies in a humidified chamber
at 4°C. The sections were then washed 3 times with PBS, incubated
for 20 min at room temperature in a humidified chamber with reagent
1 (polymer auxiliary agent; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.), washed again with PBS, and incubated for
30 min at 37°C with poly-horseradish peroxidase anti-mouse
immunoglobulin G (catalog no. PV-9000; ready to use; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.). Next, the
sections were washed with a developing solution containing 0.06%
3,3′-diaminobenzidine (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.), counterstained with hematoxylin (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.), and mounted.
Negative control sections were incubated with PBS instead of
anti-cyclin B1 or anti-claudin-1 antibodies.
Evaluation of IHC staining and
scoring
Sections were microscopically examined by two
pathologists, and scored according to the fraction of stained tumor
cells and the staining intensity (Table
III), with claudin-1+ cells appearing yellow and
cyclin B1+ cells appearing tan or light yellow. For
staining grading, the improved Kojima method was used as previously
described (15). Cells in 10 randomly
selected fields were examined at ×400 magnification. The staining
intensity and proportion of positive cells were semi-quantitatively
determined, with those samples exhibiting a score <2 being
considered negative (−); those with a score of 2 being
considered weakly positive (+); those with a score of
3–4 being considered moderately positive (++); and those
with a score of 5–6 being considered strongly positive
(+++). All analyses conducted were double-blind, with ≥3
points for high expression and ≤2 points for lower expression.
Microscopy was performed using an Olympus microscope (IX71; Olympus
Corporation, Tokyo, Japan).
 | Table III.Stained tumor cells and staining
intensity for claudin-1. |
Table III.
Stained tumor cells and staining
intensity for claudin-1.
| Staining | Score |
|---|
| Intensity |
|
|
Negative | 0 |
| Weak
intensity | 1 |
|
Moderate intensity | 2 |
| Strong
intensity | 3 |
| Percentage of
positive cells |
|
|
<10% | 0 |
|
10–40% | 1 |
|
40–70% | 2 |
|
>70% | 3 |
Statistical analysis
Data were analyzed with SPSS statistical software,
version 13.0 (SPSS, Inc., Chicago, IL, USA). Mann-Whitney 2-tailed
test was performed to compare the expression levels of claudin-1
and cyclin B1 in tumor tissues vs. adjacent noncancerous mucosae.
Correlations between these potential markers and the patients'
clinicopathological features were analyzed with Pearson's
correlation χ2 test. Survival analyses were conducted
with the log-rank test method. P<0.05 was considered to indicate
a statistically significant difference.
Results
IHC analysis of claudin-1 and cyclin
B1 expression in HSCC tissues and noncancerous mucosae
IHC was performed to evaluate the expression levels
of claudin-1 and cyclin B1 in 97 carcinomas (HSCCs) and 90 adjacent
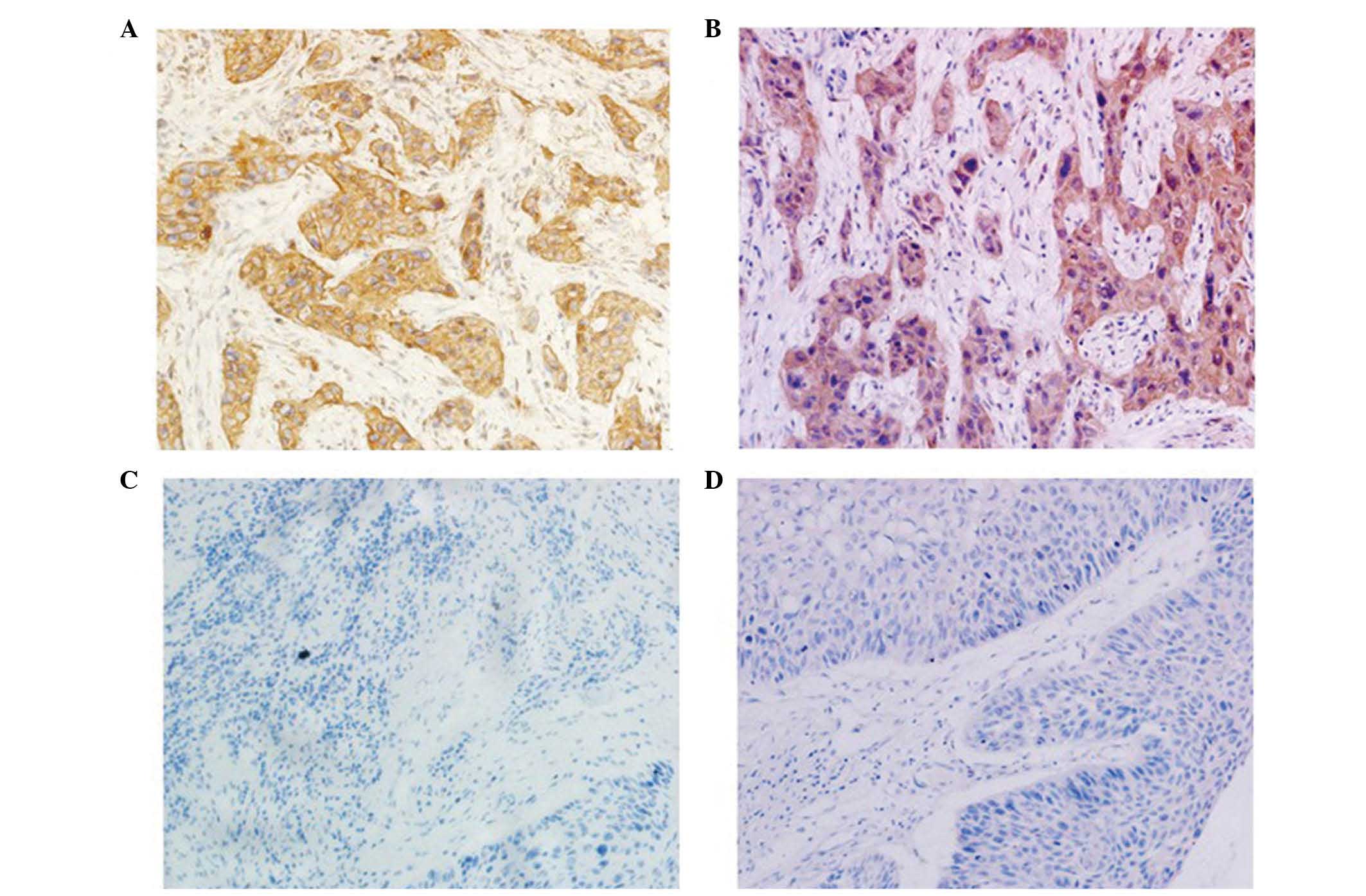
noncancerous mucosae. The results indicate that claudin-1 was
expressed in the cell membrane and cytoplasm, although mainly in
the cytoplasm around the nuclear membrane (Fig. 1A). Claudin-1 expression was
heterogenous, with certain regions being diffuse and others
presenting a focal area, while cyclin B1 expression was detected in
the cytoplasm (Fig. 1B). Low
expression of claudin-1 was not detected in the cell membrane or
cytoplasm (Fig 1C), and low
expression of cyclin B1 was not detected in the cytoplasm (Fig 1D). High expression levels of claudin-1
were observed in 73.2% (71/97) of tumors and 30% (27/90) of
adjacent noncancerous mucosae, while high expression levels of
cyclin B1 were observed in 64.9% (63/97) of tumors and 23.3%
(21/90) of adjacent noncancerous mucosae. These differences were
observed to be statistically significant.
Claudin-1 and cyclin B1 protein
expression and tumor index correlation analysis
The results of Pearson's correlation χ2
test revealed that claudin-1 expression was associated with tumor
differentiation degree (P=0.004) and lymph node metastasis
(P=0.026; Table I), while cyclin B1
expression was associated with tumor differentiation degree
(P=0/002; Table II) in patients with
HSCC. No correlations were observed between other
clinicopathological indices and cyclin B1 or claudin-1
expression.
Claudin-1 and cyclin B1 protein
expression and patient survival rate
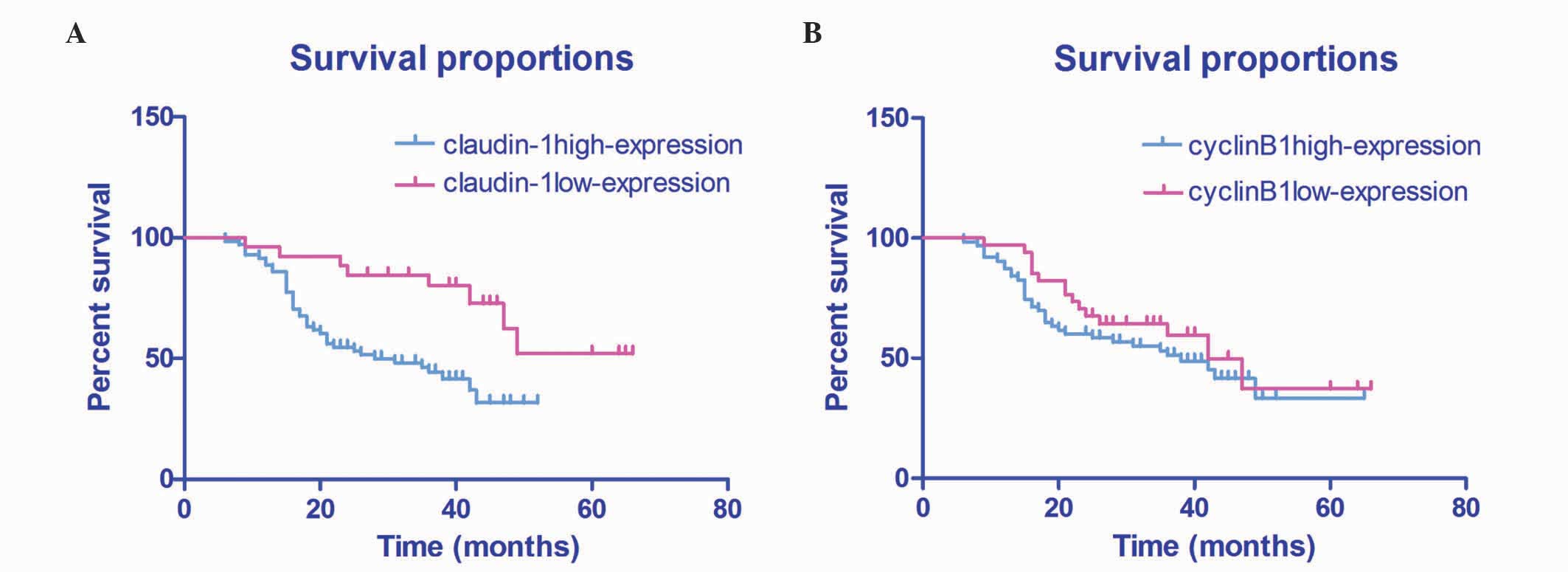
Log-rank test of 97 HSCC patients who were
followed-up for 3–5 years demonstrated that the patient survival
rate was significantly lower in patients with high expression
levels of claudin-1, compared with patients with low expression
levels of claudin-1 (51.71±4.02% vs. 31.78±2.09%; P=0.003; Fig. 2A). Furthermore, patients with high
expression levels of cyclin B1 exhibited a lower survival rate,
compared with patients with those exhibiting low expression levels
of cyclin B1 (43.06±4.25% vs. 38.06±3.11%; P=0.305), although this
was not statistically significant (Fig.
2B).
Correlation of claudin-1 and cyclin B1
protein expression
Among the 71 HSCC cases with high expression levels
of claudin-1 identified in the present study, there were 56 cases
with high expression levels of cyclin Bl. Furthermore, of the 34
cases with low cyclin B1 expression analyzed in the present study,
19 cases also exhibited low claudin-1 expression. The results of
Pearson's correlation indicated that cyclin B1 and claudin-1
expression were significantly correlated (r=0.482; P=0.0003;
Table IV).
 | Table IV.Claudin-1 and cyclin B1 correlation
analysis in head and neck squamous cell carcinoma tissues. |
Table IV.
Claudin-1 and cyclin B1 correlation
analysis in head and neck squamous cell carcinoma tissues.
|
| Cyclin B1
expression, n |
|
|---|
|
|
|
|
|---|
| Claudin-1
expression | Low | High | Total | P-value |
|---|
| Low | 19 | 7 | 26 |
|
| High | 15 | 56 | 71 |
|
| Total | 34 | 63 | 97 | 0.0003 |
Discussion
Cyclin B1 is important in controlling cells in the
G2/M phase and in regulating the mitotic entry by forming a complex
with CDK1 (41,42). Under physiological conditions, cyclin
B1 is activated at the beginning of the S phase, and is localized
in the cell cytoplasm during the G2 phase (43). Cyclin B1 is combined with CDK1 to form
the mitosis-promoting factor, which adjusts the G2/M cell cycle
checkpoint to promote the transition from G2 to M phase and
initiate mitosis (44). In the middle
and late phases of mitosis, cyclin B1 is degraded by the anaphase
promoting complex via the ubiquitin proteasome pathway, which
results in chromosome depolymerization, nucleoli and nuclear
membrane regeneration, and completion of the cell cycle following
cytokinesis (45). During the
interphase, cyclin B1 is localized in the cytoplasm, mainly around
the nuclear membrane, and its orientation is influenced by cell
retention signals (46).
A previous study demonstrated that elevated cyclin
B1 expression altered the spindle checkpoint via different
mechanisms, caused chromosomal instability, led to an abnormal cell
division and promoted tumor development in squamous cell carcinoma
(47). Previous studies have also
noted increased levels of cyclin B1 in malignant tumors, including
breast (30), gastric (31), early non-small cell lung (32), colorectal (33), prostate (34), esophageal (37,38),
tongue and lung cancer (15), which
suggests that cyclin B1 may be a potential tumor marker. The
present results revealed that cyclin B1 expression was mainly
localized in the cytoplasm of malignant cells, with 63/97 HSCC
patients displaying positive expression for cyclin B1, compared
with the levels observed in normal tissues adjacent to the
carcinomas (P<0.05). Additionally, cyclin B1 expression was
observed to be associated with the degree of differentiation of the
tumor (P<0.05), but not with the index of clinical pathology.
These findings were consistent with previous studies reporting that
cyclin B1 overexpression led to cell cycle disorders or G2/M
checkpoint dysregulation, causing uncontrolled proliferation and
eventually tumor formation (48). In
the present study, Kaplan-Meier analysis revealed that the survival
rates of HSCC patients with high expression levels of cyclin B1
were not statistically different from those exhibited by patients
with low cyclin B1 expression levels. However, in previous studies
on esophageal (37), tongue (39) and non-small cell lung cancer (32), cyclin B1 overexpression was observed
to be associated with patient survival. Considering that multiple
factors may influence survival, and that differential expression of
cyclin B1 has been reported in different tissues, the potential
correlation between cyclin B1 expression and survival may be
different depending on each tissue. Therefore, cyclin B1 cannot be
used independently as an index of prognosis in patients with
HSCC.
The mechanisms leading to claudin overexpression in
tumor tissues are of particular interest. Abnormalities in claudins
expression or localization result in abnormal intercellular
signaling and promote tumor formation and metastasis (49). Altered claudin-1 expression leads to
the destruction of epithelial permeability and barrier function,
loss of cell polarity, decrease in intercellular adhesion force and
tumor development (16,23). A previous study has reported that
increased claudin-1 expression may inhibit E-cadherin expression,
thus promoting an increased in the expression levels of the
components of the E-cadherin/T-cell factor (TCF) signaling pathway
(50). Changes in claudin-1
localization within the cell membrane, alongside a decrease in
E-cadherin expression and an increase in the expression of the
components of the E-cadherin/TCF signaling pathway, may promote
tumor development (50). Claudin-1
expression is elevated in various types of tumors (16–20). In
oral squamous cell carcinoma, previous studies noted that elevated
claudin-1 expression was associated with vascular and peripheral
nerve invasion by the tumor tissue (51). In the present study, claudin-1
expression was significantly higher in HSCC tissues, compared with
the expression levels in adjacent tissues, and claudin-1 protein
expression was associated with tumor differentiation degree
(P=0.004) and lymph node metastasis (P=0.026), but not with
clinicopathological index. Kaplan-Meier analysis demonstrated that
patients with elevated claudin-1 expression exhibited a
significantly lower survival rate than patients with low claudin-1
expression. Previous studies have demonstrated that increased
claudin-1 expression promotes cell migration and matrix
metalloproteinase-2 (MMP-2) activation (52), which leads to an increased tumor
invasiveness and often poor prognosis (53). Therefore, claudin-1 may participate in
the development of HSCC and lymph node metastasis. The present
findings may contribute to improve diagnostics, prognosis
assessment and selection of treatment plan in patients with HSCC.
To improve overall survival rates, HSCC patients who exhibit
elevated claudin-1 expression may require particularly aggressive
interventions and intensive combined therapy.
Yoon et al (54) observed that claudin-1 expression was
able to enhance the expression and activity of MMP-2, while cyclin
Bl was also able to promote the secretion of MMP-2, which results
in MMP-2-mediated interstitial degradation, thus facilitating the
transition into a cancerous cell (55). Elevated claudin-1 expression may lead
to the activation of TCF-lymphoid enhancer-binding
factor/beta-catenin (52), which
contains a transcription factor capable of inducing the expression
of genes involved in cell proliferation, survival and invasiveness
(56), thus regulating the cell cycle
in cancer cells. Of the HSCC patients examined in the present
study, 71 cases exhibited increased claudin-1 expression, and 63
cases exhibited elevated cyclin B1 expression, which were
significantly correlated (P<0.001). These findings indicate that
claudin-1 may be involved in cell cycle regulation and cellular
differentiation.
In the present study, IHC analysis confirmed that
claudin-1 and cyclin B1 exhibited significantly high expression in
HSCC tissues, compared with matched adjacent tissues. Further
studies on a larger number of specimens are required in order to
validate these preliminary observations. In addition, the messenger
RNA expression levels of claudin-1 and cyclin B1 in HSCC and
matched adjacent tissues should be analyzed in future studies via
reverse transcription-quantitative polymerase chain reaction to
further support the present results. Furthermore, the percentage of
expression of claudin-1 and cyclin B1 in HSCC specimens should be
assessed in future studies in order to evaluate the use potential
use of claudin-1 and cyclin B1 as HSCC tumor markers.
In conclusion, the present study demonstrated an
elevated cyclin B1 and claudin-1 expression in HSCC specimens, thus
suggesting the use of these proteins as tumor markers. Cyclin B1
expression is associated with the degree of tumor differentiation
in patients with HSCC, while claudin-1 expression was associated
with the degree of tumor differentiation and lymph node metastasis
in these patients. Furthermore, the expression of cyclin B1 and the
expression of claudin-1 are significantly correlated in patients
with HSCC. Therefore, joint detection of claudin-1 and cyclin B1
may aid to guide cancer therapy and prognosis determination in
these patients. Additionally, claudin-1 is associated with
survival, and may be used as a monitoring indicator. Overall, the
present findings suggest the use of cyclin B1 and claudin-1 as
potential novel targets for the treatment of HSCC.
Acknowledgements
The present study was supported by the Shandong
Provincial Natural Science Foundation (Jinan, China; grant no.
ZR2013HM107). The authors would like to thank all the patients who
participated in the study, in addition to LetPub (www.letpub.com; Woburn, MA, USA) for the linguistic
assistance provided during the preparation of the present
manuscript.
References
|
1
|
Wittekindt C, Wagner S, Mayer CS and
Klußmann JP: Basics of tumor development and importance of human
papilloma virus (HPV) for head and neck cancer.
Laryngorhinootologie. 91(Suppl 1): S1–S26. 2012.(In German).
PubMed/NCBI
|
|
2
|
Landis SH, Murray T, Bolden S and Wingo
PA: Cancer statistics, 1999. CA Cancer J Clin. 49:8–31. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hall SF, Groome PA, Irish J and O'Sullivan
B: The natural history of patients with squamous cell carcinoma of
the hypopharynx. Laryngoscope. 118:1362–1371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu S, Gu JC, Liang J, Yu T and Dong XH:
Expression and clincal significance of Survivin and PTEN in
hypopharyngeal squamous cell carcinoma. Guangdong Yi Xue.
33:1958–1960. 2012.(In Chinese).
|
|
5
|
Lang S, Wollenberg B, Dellian M, et al:
Clinical and epidemiological data of patients with malignomas of
the head and neck. Laryngorhinootologie. 81:499–508. 2002.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boyle P and Ferlay J: Cancer incidence and
mortality in Europe, 2004. Ann Oncol. 16:481–488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rose BS, Jeong JH, Nath SK, Lu SM and Mell
LK: Population-based study of competing mortality in head and neck
cancer. J Clin Oncol. 29:3503–3509. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takes RP, Strojan P, Silver CE, Bradley
PJ, Haigentz M Jr, Wolf GT, Shaha AR, Hartl DM, Olofsson J,
Langendijk JA, et al: International Head and Neck Scientific Group:
Current trends in initial hypopharyngeal cancer: The declining use
of open surgery. Head Neck. 34:270–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Srinivas PR, Kramer BS and Srivastava S:
Trends in biomarker research for cancer detection. Lancet Oncol.
2:698–704. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
González-Mariscal L, Betanzos A, Nava P
and Jaramillo BE: Tight junction proteins. Prog Biophys Mol Biol.
81:1–44. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gumbiner BM: Cell adhesion: The molecular
basis of tissue Architecture and morphogenesis. Cell. 84:345–357.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heiskala M, Peterson PA and Yang Y: The
roles of claudin superfamily proteins in paracellular transport.
Traffic. 2:93–98. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harhaj NS and Antonetti DA: Regulation of
tight junctions and loss of barrier function in pathophysiology.
Int J Biochem Cell Biol. 36:1206–1237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishida M, Kushima R and Okabe H: Claudin
expression in rectal well-differentiated endocrine neoplasms
(carcinoid tumors). Oncol Rep. 21:113–117. 2009.PubMed/NCBI
|
|
16
|
Fritzsche FR, Oelrich B, Johannsen M,
Kristiansen I, Moch H, Jung K and Kristiansen G: Claudin-1 protein
expression is a prognostic marker of patient survival in renal cell
carcinomas. Clin Cancer Res. 14:7035–7042. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soini Y and Talvensaari-Mattila A:
Expression of claudins 1, 4, 5, and 7 in ovarian tumors of diverse
types. Int J Gynecol Pathol. 25:330–335. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Resnick MB, Gavilanez M, Newton E, Konkin
T, Bhattacharya B, Britt DE, Sabo E and Moss SF: Claudin expression
in gastric adenocarcinomas: A tissue microarray study with
prognostic correlation. Hum Pathol. 36:886–892. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huo Q, Kinugasa T, Wang L, Huang J, Zhao
J, Shibaguchi H, Kuroki M, Tanaka T, Yamashita Y, Nabeshima K, et
al: Claudin-1 protein is a major factor involved in the
tumorigenesis of colorectal cancer. Anticancer Res. 29:851–857.
2009.PubMed/NCBI
|
|
20
|
de Oliveira SS, de Oliveira IM, De Souza W
and Morgado-Díaz JA: Claudins upregulation in human colorectal
cancer. FEBS Lett. 579:6179–6185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krämer F, White K, Kubbies M, Swisshelm K
and Weber BH: Genomic organization of claudin-1 and its assessment
in hereditary and sporadic breast cancer. Hum Genet. 107:249–256.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tokés AM, Kulka J, Paku S, Szik A, Páska
C, Novák PK, Szilák L, Kiss A, Bögi K and Schaff Z: Claudin-1, −3
and −4 proteins and mRNA expression in benign and malignant breast
lesions: A research study. Breast Cancer Res. 7:R296–R305. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chao YC, Pan SH, Yang SC, Yu SL, Che TF,
Lin CW, Tsai MS, Chang GC, Wu CH, Wu YY, et al: Claudin-1 is a
metastasis suppressor and correlates with clinical outcome in lung
adenocarcinoma. Am J Respir Crit Care Med. 179:123–133. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Swisshehn K, Macek R and Kubbies M: Role
of claudins in tumorigenesis. Adv Drug Deliv Rev. 57:919–928. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsueh C, Chang YS, Tseng NM, Liao CT,
Hsueh S, Chang JH, Wu IC and Chang KP: Expression pattern and
prognostic significance of claudins 1, 4, and 7 in nasopharyngeal
carcinoma. Hum Pathol. 41:944–950. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elledge SJ: Cell cycle checkpoints:
Preventing an identity crisis. Science. 274:1664–1672. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karagoz ID, Ozaslan M, Cengiz B, Kalender
ME, Kilic IH, Oztuzcu S, Gogebakan B and Demiryurek AT: CDC 25A
gene 263C/T, −350C/T and −51C/G polymorphisms in breast carcinoma.
Tumour Bio1. 31:597–604. 2010. View Article : Google Scholar
|
|
29
|
Brandeis M, Rosewell I, Carrington M,
Crompton T, Jacobs MA, Kirk J, Gannon J and Hunt T: Cyclin B2-null
mice develop normally and are fertile whereas cyclin B1-null mice
die in utero. Proc Natl Acad Sci USA. 95:4344–4349. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barrett KL, Demiranda D and Katula KS:
Cyclin b1 promoter activity and functional cdk1 complex formation
in G1 phase of human breast cancer cells. Cell Biol Int. 26:19–28.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yasuda M, Takesue F, Inutsuka S, Honda M,
Nozoe T and Korenaga D: Overexpression of cyclin B1 in gastric
cancer and its clinicopathological significance: An
immunohistological study. J Cancer Res Clin Oncol. 28:412–416.
2002. View Article : Google Scholar
|
|
32
|
Soria JC, Jang SJ, Khuri FR, Hassan K, Liu
D, Hong WK and Mao L: Overexpression of cyclin B1 in early-stage
non-small cell lung cancer and its clinical implication. Cancer
Res. 60:4000–4004. 2000.PubMed/NCBI
|
|
33
|
Grabsch H, Lickvers K, Hansen O, Takeno S,
Willers R, Stock W, Gabbert HE and Mueller W: Prognostic value of
cyclin B1 protein expression in colorectal cancer. Am J Clin
Pathol. 122:511–516. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mashal RD, Lester S, Corless C, Richie JP,
Chandra R, Propert KJ and Dutta A: Expression of cell
cycle-regulated proteins in prostate cancer. Cancer Res.
56:4159–4163. 1996.PubMed/NCBI
|
|
35
|
Song Y, Zhao C, Dong L, Fu M, Xue L, Huang
Z, Tong T, Zhou Z, Chen A, Yang Z, et al: Overexpression of cyclin
B1 in human esophageal squamous cell carcinoma cells induces tumor
cell invasive growth and metastasis. Carcinogenesis. 29:307–315.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao M, Kim YT, Yoon BS, Kim SW, Kang MH,
Kim SH, Kim JH, Kim JW and Park YW: Expression profiling of cyclin
B1 and D1 in cervical carcinoma. Exp Oncol. 28:44–48.
2006.PubMed/NCBI
|
|
37
|
Murakami H, Furihata M, Ohtsuki Y and
Ogoshi S: Determination of the prognostic significance of cyclin B1
overexpression in patients with esophageal squamous cell carcinoma.
Virchows Arch. 434:153–158. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takeno S, Noguchi T, Kikuchi R, Uchida Y,
Yokoyama S and Müller W: Prognostic value of cyclin B1 in patients
with esophageal squamous cell carcinoma. Cancer. 94:2874–2881.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hassan KA, El-Naggar AK, Soria JC, Liu D,
Hong WK and Mao L: Clinical significance of cyclin B1 protein
expression in squamous cell carcinoma of the tongue. Clin Cancer
Res. 7:2458–2462. 2001.PubMed/NCBI
|
|
40
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumors (7th). Wiley-Blackwell.
Hoboken, NJ: 2009.
|
|
41
|
Yuan J, Krämer A, Matthess Y, Yan R,
Spänkuch B, Gätje R, Knecht R, Kaufmann M and Strebhardt K: Stable
gene silencing of cyclin B1 in tumor cells increases susceptibility
to taxol and leads to growth arrest in vivo. Oncogene.
25:1753–1762. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stark GR and Taylor WR: Control of the
G2/M transition. Mol Biotechnol. 32:227–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nurse P: Ordering S phase and M phase in
the cell cycle. Cell. 79:547–550. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nigg EA: The substrates of the cdc2
kinase. Semin Cell Biol. 2:261–270. 1991.PubMed/NCBI
|
|
45
|
Farhana L, Dawson M, Rishi AK, Zhang Y,
Van Buren E, Trivedi C, Reichert U, Fang G, Kirschner MW and
Fontana JA: Cyclin B and E2F-1 expression in prostate carcinoma
cells treated with the novel retinoid CD437 are regulated by the
ubiquitin-mediated pathway. Cancer Res. 62:3842–3849.
2002.PubMed/NCBI
|
|
46
|
Cukier IH, Li Y and Lee JM: Cyclin B1/Cdkl
binds and phosphorylates filamin A and regulates its ability to
cross-link actin. FEBS Lett. 581:1661–1672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hoffmann TK, Trellakis S, Olulicz K,
Schuler P, Greve J, Arnolds J, Bergmann C, Bas M, Lang S, Lehnerdt
G, et al: Cyclin B1 Expression and p53 Status in Squamous Cell
Carcinomas of the Head and Neck. A NTICANCER R ESEARCH.
31:3151–3158. 2011.
|
|
48
|
Barascu A, Besson P, Le Floch O, Bougnoux
P and Jourdan ML: CDK1-cyclin B1 mediates the inhibition of
proliferation induced by omega-3 fatty acids in MDA-MB-231 breast
cancer cells. Int J Biochem Cell Biol. 38:196–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Krause G, Winkler L, Mueller SL, Haseloff
RF, Piontek J and Blasig IE: Structure and function of claudins.
Biochim Biophys Acta. 1778:631–645. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dhawan P, Singh AB, Deane NG, No Y, Shiou
SR, Schmidt C, Neff J, Washington MK and Beauchamp RD: Claudin-1
regulates cellular transformation and metastatic behavior in colon
cancer. J Clin Invest. 115:1765–1776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dos Reis PP, Bharadwaj RR, Machado J,
Macmillan C, Pintilie M, Sukhai MA, Perez-Ordonez B, Gullane P,
Irish J and Kamel-Reid S: Claudin 1 overexpression increases
invasion and is associated with aggressive histological features in
oral squamous cell carcinoma. Cancer. 113:3169–3180. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Oku N, Sasabe E, Ueta E, Yamamoto T and
Osaki T: Tight junction protein claudin-1 enhances the invasive
activity of oral squamous cell carcinoma cells by promoting
cleavage of laminin-5 gamma2 chain via matrix metalloproteinase
(MMP)-2 and membrane-type MMP-1. Cancer Res. 66:5251–5257. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
de la Peña S, Sampieri CL and León-Córdoba
K: Matrix metalloproteases as molecular markers in gastric cancer.
Med Clin (Barc). 134:123–126. 2010.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yoon CH, Kim MJ, Park MJ, Park IC, Hwang
SG, An S, Choi YH, Yoon G and Lee SJ: Claudin-1 acts through
c-Abl-protein kinase Cdelta (PKCdelta) signaling and has a causal
role in the acquisition of invasive capacity in human liver cells.
J Biol Chem. 285:226–233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kawamoto H, Uozumi T, Kawamoto K, Arita K,
Yano T and Hirohata T: Type IV collagenase activity and cavernous
sinus invasion in human pituitary adenomas. Acta Neurochir (Wien).
138:390–395. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Arabzadeh A, Troy TC and Turksen K: Role
of the Cldn6 cytoplasmic tail domain in membrane targeting and
epidermal differentiation in vivo. Mol Cell Biol. 26:5876–5887.
2006. View Article : Google Scholar : PubMed/NCBI
|