Introduction
Hepatocellular carcinoma (HCC) accounts for 85–90%
cases of primary liver cancer (1). It
is the fifth most common malignancy and the third leading cause of
cancer-related mortality worldwide (1–3). The
epidemiological characteristics of liver cancer differ between
various countries; for example, ~85% of cases of liver cancer occur
in developing countries and 54% occur in China (4). Furthermore, >80% of patients with HCC
have a history of hepatitis or cirrhosis, and HCC generally has a
poor prognosis (1,5–7). The
majority of patients with HCC are present and are diagnosed in the
advanced stages of the disease (5),
and no effective chemotherapy or radiotherapy exists for the
advanced disease. Surgical resection is effective only in the early
stages of HCC, however, the 5-year survival rate is as low as
25–39% following surgery (8). It has
been demonstrated that multiple genes are altered during the
process of hepatocarcinogenesis (9).
Understanding the molecular mechanisms that induce
hepatocarcinogenesis may improve the screening, prevention and
treatment of patients with HCC (10).
Oxidored-nitro domain-containing protein 1 (NOR1)
gene is a nitroreductase (NTR) gene that was initially isolated
from nasopharyngeal carcinoma (NPC) (11). The NOR1 gene encodes two transcripts
and acts as a candidate tumor repressor gene associated with NPC.
It has a similar activity to bacterial NTR, which converts
5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954), a monofunctional
alkylating agent, into a toxic form. A previous study supported the
hypothesis that NOR1 is involved in the chemical carcinogenesis of
hepatic cancer. NOR1 overexpression increased the expression levels
of growth factor receptor-bound protein 2 (Grb2) mRNA and protein
in HepG2 cells, and activated mitogen-activated protein kinase
(MAPK) signal transduction, thus leading to enhanced CB1954-induced
cell killing in HepG2 cells (12).
Furthermore, DNA microarray data suggested that overexpression of
NOR1 protein results in altered gene expression profiles in HepG2
cells, including 59 upregulated genes and 103 downregulated genes
(13). These findings indicate that
the NOR1 gene may have an important role in the development of
HCC.
The present study examined, for the first time, the
expression levels of NOR1 mRNA and protein in specimens of human
normal liver, hepatitis, cirrhosis and HCC, together representing
the process of HCC development. In addition, the association,
between NOR1 expression and clinicopathological parameters of HCC
patients was investigated. The present study may facilitate in
elucidating the role of NOR1 expression in liver carcinogenesis.
The study was approved by the Ethics Committee of The Third Xiangya
Hospital, Central South University (Changsha, Hunan, China).
Materials and methods
Tissue microarrays
Sections (5 µm) of formalin-fixed, paraffin-embedded
tissue microarrays (cat. no. LV20812) were purchased from US
Biomax, Inc. (Rockville, MD, USA). The tissue microarray contained
16 samples of normal human liver, 24 samples of hepatitis, 32
samples of cirrhosis and 32 samples of HCC. Sections were arranged
in duplicate cores per sample.
In situ hybridization (ISH)
The formalin-fixed paraffin-embedded sections were
baked at 60°C for 30 min, then deparaffinized by immersing in
xylene (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) for
15 min twice. After immersing in 100% ethanol (Sinopharm Chemical
Reagent Co., Ltd.) for 5 min, the slides were air-dried and then
incubated with pepsin (Coolaber Technology Co., Ltd., Beijing,
China) at 37°C for 30 min. Digoxigenin-labeled RNA probes were
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The
sequences were as follows:
5′-CTAAGTTCTTTGATGATCCAGACACATGAGTTTCCACAGGCTGATTCACGCT-3′dig. The
probes were diluted in prewarmed hybridization buffer (Wuhan Boster
Biological Tehnology Ltd.., Wuhan, Hubei, China) to a concentration
of 10 ng/µl and added to the slides for incubation at 37°C
overnight. The slides were washed with saline-sodium citrate and
Tris-buffered saline (both Coolaber Technology Co., Ltd.,), then
incubated with mouse anti-digoxygenin monoclonal antibody (1:200
dilution; cat. no. ab119345; Abcam, Cambridge, MA, USA) at 4°C
overnight. For signal development, nitroblue tetrazolium and
bromochloroindolyl phosphate were used for staining at 37°C for 30
min, and nuclear fast red dye served as the counterstain at 37°C
for 1–2 min (all Sigma-Aldrich, St. Louis, MO, USA).
Immunohistochemistry (IHC)
The sections were baked at 60°C for 30 min followed
by deparaffinization in xylene and rehydrated with graded ethanol.
Antigen retrieval was performed by heating the sections in 0.01 M
citrate buffer (Coolaber Technology Co., Ltd.) for 2 min, then
adding 3% H2O2/methanol solution (Sinopharm
Chemical Reagent Co., Ltd.) to quench endogenous peroxidase. The
sections were subsequently incubated with goat anti-NOR1 polyclonal
antibody (1:50 dilution; cat. no. sc-161980; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C overnight. After
washing with 0.1% Tween-20/phosphate-buffered saline (Coolaber
Technology Co., Ltd.), Polymer Helper (ZSGB-BIO, Beijing, China)
was added to the sections and incubated at room temperature for 15
min. The sections were washed for three times and then incubated
with horseradish peroxidase-conjugated anti-goat IgG (1:2,000
dilution; cat. no. PV-9003; ZSGB-BIO) at room temperature for 15
min. The slides were stained using a DAB staining kit (Fuzhou
Maixin Biotech Co., Ltd., Fuzhou, China) and counterstained with
hematoxylin (Beyotime Institute of Biotechnology, Shanghai, China)
at 37°C for 3–5 min.
Scoring method
The present study used the same semiquantitative
scoring method for ISH and IHC (14,15). The
sections were semiquantitatively analyzed independently by two
pathologists. All staining was observed using an E200 microscope
(Nikon, Tokyo, Japan). The staining intensity was scored as 0
(negative), 1 (weak), 2 (moderate) or 3 (strong). The staining
density score was based on the percentage of positive cells, as
follows: 0 (0%), 1 (1–10%), 2 (11–50%), 3 (51–80%) and 4 (81–100%).
The staining intensity and density scores were multiplied and used
as the overall score. An overall score of >3 was considered to
indicate positive NOR1 expression; scores of ≤3 indicated negative
NOR1 expression.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 19.0; IBM SPSS, Chicago, IL, USA). Data are
representative of at least three independent experiments. Pearson's
χ2 test and Fishers exact test were performed to compare
differences between groups. P<0.05 was considered to indicate a
statistically significant.
Results
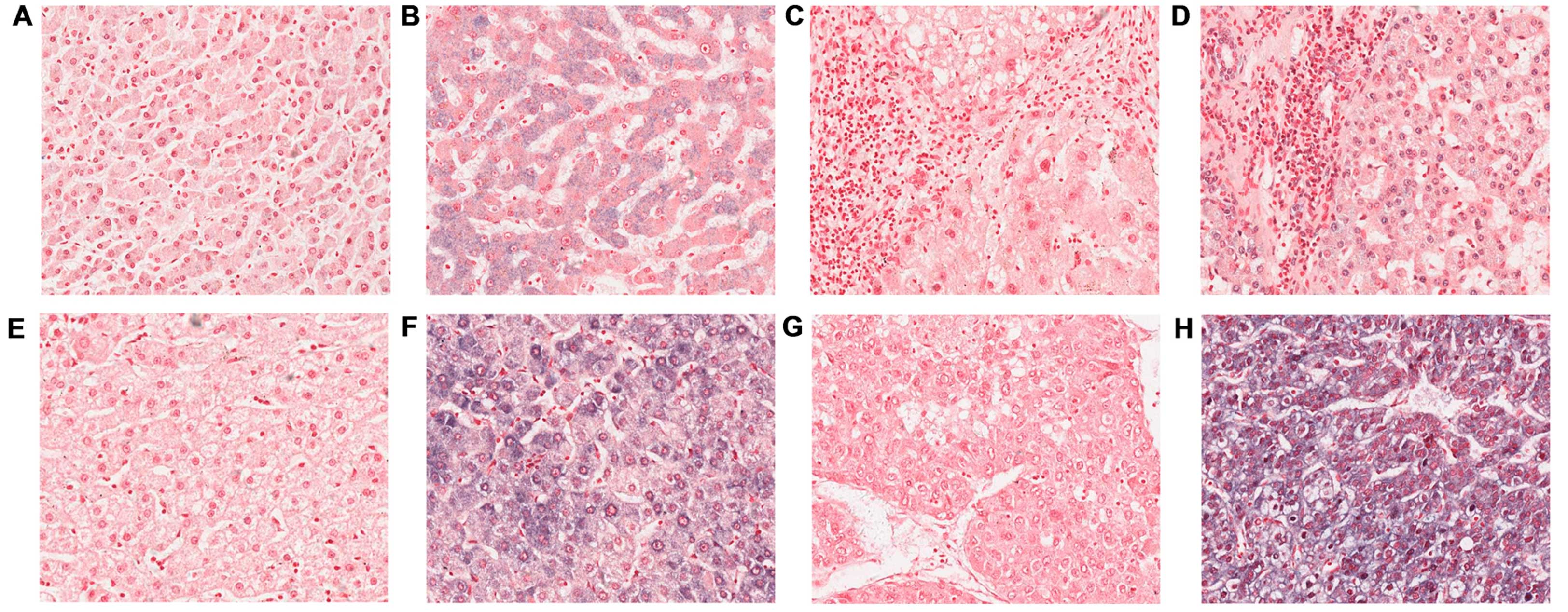
Expression of NOR1 mRNA expression in
normal liver, hepatitis, cirrhosis and HCC tissues
NOR1 mRNA ISH was performed on all sections
representing human normal liver, hepatitis, cirrhosis and HCC.
Representative staining patterns for NOR1 mRNA are indicated in
Fig. 1. Positive signals were
detected in the cytoplasm and/or nucleus. Table I indicates the expression of NOR1 mRNA
in normal liver, hepatitis, cirrhosis and HCC. The positive rate of
NOR1 mRNA expression in normal liver, hepatitis and cirrhosis was
43.8, 58.3 and 65.6%, respectively. The expression of NOR1 mRNA in
hepatitis and cirrhosis did not appear to be significantly
different from the normal liver (P>0.05). By contrast, positive
expression of NOR1 mRNA was exhibited in 25 (78.1%) HCC cases and
negative expression of NOR1 mRNA was observed in the remaining 7
cases (21.9%). The positive rate of NOR1 mRNA in patients with HCC
was significantly higher compared with the normal control
(P=0.017).
 | Table I.Expression of NOR1 mRNA in normal
liver, hepatitis, cirrhosis and HCC. |
Table I.
Expression of NOR1 mRNA in normal
liver, hepatitis, cirrhosis and HCC.
|
|
| NOR1 mRNA expression,
n (%) |
|
|
|---|
|
|
|
|
|
|
|---|
| Specimen type | n | Negative | Positive | χ2 | P-valuea |
|---|
| Normal | 16 | 9
(56.2) | 7
(43.8) |
|
|
| Hepatitis | 24 | 10 (41.7) | 14 (58.3) | 0.819 | 0.366 |
| Cirrhosis | 32 | 11 (34.4) | 21 (65.6) | 2.100 | 0.147 |
| HCC | 32 | 7
(21.9) | 25 (78.1) | 5.672 | 0.017 |
Expression of NOR1 protein expression
normal liver, hepatitis, cirrhosis and HCC tissues
IHC was performed to examine the expression of NOR1
protein in human normal liver, hepatitis, cirrhosis and HCC.
Fig. 2 shows representative staining
patterns of NOR1 protein. The results indicated that NOR1 protein
was expressed at variable levels, and localized within the cellular
nuclei and cytoplasm.
Negative expression for NOR1 protein was observed in
all normal liver samples, while the positive rate was 12.5% in
hepatitis and 15.6% in cirrhotic liver samples. However, no
significant difference in positive expression rate was observed
between normal liver and hepatitis/cirrhotic liver samples
(P>0.05).
NOR1 protein was expressed differentially between
the normal liver and HCC. In a total of 32 cases of HCC, the
expression of NOR1 protein was positive in 21 (65.6%) cases and
negative (34.4%) in the remaining 11 cases. The positive rate of
NOR1 protein expression was significantly higher in HCC tissues
compared with the normal liver tissues (P<0.001; Table II).
 | Table II.Expression of NOR1 protein in normal
liver, hepatitis, cirrhosis and HCC. |
Table II.
Expression of NOR1 protein in normal
liver, hepatitis, cirrhosis and HCC.
|
|
| NOR1 protein
expression, n (%) |
|
|
|---|
|
|
|
|
|
|
|---|
| Specimen type | n | Negative | Positive | χ2 | P-valuea |
|---|
| Normal | 16 | 16
(100.0) | 0 (0.0) |
|
|
| Hepatitis | 24 | 21 (87.5) | 3
(12.5) |
2.162 |
0.141 |
| Cirrhosis | 32 | 27 (84.4) | 5
(15.6) |
2.791 |
0.095 |
| HCC | 32 | 11 (34.4) | 21 (65.6) | 18.667 | <0.001 |
Correlation between NOR1 expression
and clinicopathological parameters of patients with HCC
The present study also analyzed the association
between NOR1 expression and clinicopathological parameters of
patients with HCC. The results in Table
III indicate that there was no statistically significant
association between NOR1 mRNA expression and any of the
clinicopathological parameters investigated (P>0.05). However,
Table IV demonstrates that NOR1
protein expression was correlated with the differentiation degree
and tumor-node-metastasis (TNM) (16)
tumor stage of patients with HCC. The NOR1 protein positive protein
expression rate was significantly higher in HCC patients with a low
differentiation degree and a high TNM stage (P<0.05).
 | Table III.Association between NOR1 mRNA
expression and clinicopathological parameters of patients with
HCC. |
Table III.
Association between NOR1 mRNA
expression and clinicopathological parameters of patients with
HCC.
|
|
| NOR1 mRNA expression,
n (%) |
|
|
|---|
|
|
|
|
|
|
|---|
| Feature | n | Negative | Positive | χ2 | P-value |
|---|
| Age, years |
|
|
|
|
|
|
<50 | 15 | 4 (57.1) | 11 (44.0) | 0.379 | 0.678 |
| ≥50 | 17 | 3 (42.9) | 14 (56.0) |
|
|
| Gender |
|
|
|
|
|
|
Male | 24 | 4 (57.1) | 20 (80.0) | 1.524 | 0.327 |
|
Female | 8 | 3 (42.9) | 5 (20.0) |
|
|
| HBsAg |
|
|
|
|
|
|
(+) | 16 | 3 (27.3) | 13 (61.9) | 3.463 | 0.135 |
|
(−) | 16 | 8 (72.7) | 8 (38.1) |
|
|
| Pathological
stage |
|
|
|
|
|
| I | 3 | 2 (28.6) | 1 (4.0) | 4.355 | 0.113 |
| II | 19 | 4 (57.1) | 15 (60.0) |
|
|
|
III | 10 | 1 (14.3) | 9 (36.0) |
|
|
| TNM status |
|
|
|
|
|
| I | 2 | 1 (14.3) | 1 (4.0) | 1.234 | 0.745 |
| II | 19 | 4 (57.1) | 15 (60.0) |
|
|
|
III | 10 | 2 (28.6) | 8 (32.0) |
|
|
| IV | 1 | 0 (0.0) | 1 (4.0) |
|
|
 | Table IV.Association between NOR1 protein
expression and clinicopathological parameters of patients with
HCC. |
Table IV.
Association between NOR1 protein
expression and clinicopathological parameters of patients with
HCC.
|
|
| NOR1 protein
expression, n (%) |
|
|
|---|
|
|
|
|
|
|
|---|
| Feature | n | Negative | Positive | χ2 | P-value |
|---|
| Age, years |
|
|
|
|
|
|
<50 | 15 | 4 (36.4) | 11 (52.4) | 0.744 | 0.472 |
|
≥50 | 17 | 7 (63.6) | 10 (47.6) |
|
|
| Gender |
|
|
|
|
|
|
Male | 24 | 6 (54.5) | 18 (85.7) | 3.740 | 0.088 |
|
Female | 8 | 5 (45.5) | 3 (14.3) |
|
|
| HBsAg |
|
|
|
|
|
|
(+) | 16 | 4 (57.1) | 12 (48.0) | 0.183 | 1.000 |
|
(−) | 16 | 3 (42.9) | 13 (52.0) |
|
|
| Pathological
stage |
|
|
|
|
|
| I | 3 | 3 (27.3) | 0 (0.0) | 6.709 | 0.035 |
| II | 19 | 6 (54.5) | 13 (61.9) |
|
|
|
III | 10 | 2 (18.2) | 8 (38.1) |
|
|
| TNM status |
|
|
|
|
|
| I | 2 | 2 (18.2) | 0 (0.0) | 11.002 | 0.012 |
| II | 19 | 9 (81.8) | 10 (47.6) |
|
|
|
III | 10 | 0 (0.0) | 10 (47.6) |
|
|
| IV | 1 | 0 (0.0) | 1 (4.8) |
|
|
Discussion
The NOR1 gene was initially identified in NPC by Nie
et al in 2003 (11). The
expression of NOR1 was downregulated in the CNE1 NPC cell line in
comparison to normal nasopharyngeal epithelial cells; however,
enzymatic activity was higher in the CNE1 cells compared with the
normal nasopharyngeal epithelial cells. NOR1 is regulated by heat
shock factor 1 and nuclear respiratory factor 1 (17), which are two stress-responsive
transcription factors that have important roles in carcinogenesis
(18,19). During the last decade, research
regarding the association between NOR1 expression and human cancer
has predominantly focused on NPC (20–22). It
has been demonstrated that NOR1 is able to regulate NPC cell
proliferation, apoptosis, autophagy and metabolism (20,21). In
addition, a small number of studies have reported that NOR1
overexpression is associated with prostate (23) and cervical (21) cancer.
Certain in vitro studies also suggest that
NOR1 may be a candidate gene for hepatocarcinogenesis. NOR1
overexpression may induce Grb2 and E-selectin expression in HepG2
cells, and activate MAPK signal transduction (12,13,24). These
studies indicated that NOR1 may be important in the formation of
chemical carcinogens and carcinogenesis of HCC.
The development of HCC is a multistep process with
the involvement of a multifactorial etiology. It is
well-established that HCC frequently arises in the setting of
cirrhosis, and cirrhosis is attributable to chronic hepatitis B or
C virus infection. This trend has been observed in cases in almost
all countries (25–27).
In the current study, the expression of NOR1 was
determined in specimens of normal liver, hepatitis, cirrhosis and
HCC obtained as tissue array slides. ISH was used to detect the
expression of NOR1 mRNA, whereas the expression of NOR1 protein was
examined by IHC. The results indicated that NOR1 was primarily
localized within the nuclei and cytoplasm.
By performing a western blot assay, Xiang et
al identified that human livers exhibit an absence of NOR1
protein. Furthermore, tissue sections from the liver did not stain
positive for NOR1 in an IHC assay (28). Consistent with the results in the
literature, the IHC results of the present study demonstrated
negative expression for NOR1 protein in all 16 normal liver
specimens analyzed. However, the ISH assay revealed that the
positive rate of NOR1 mRNA was 43.8% in normal liver specimens.
In a previous study by Xiang et al, the
expression of human NOR1 protein expression was examined in various
normal and cancerous tissues. It was observed that NOR1 expression
was weak or negative in the majority of malignant cells, however,
moderate to strong expression of NOR1 was displayed in liver cancer
cells (29). Similarly, the present
study identified a trend for increased positive rate of NOR1
protein and mRNA expression from normal liver samples to hepatitis,
cirrhosis and HCC samples. However, positive NOR1 protein and mRNA
expression observed in the normal liver samples was not
significantly different to the hepatitis and cirrhotic liver
samples. By contrast, the positive rate of NOR1 mRNA and protein
expression in patients with HCC was significantly higher in
comparison to the normal control. The aforementioned findings
indicate a possible role of NOR1 in the progression of HCC.
Furthermore, the current study identified that NOR1 protein
expression correlates with certain clinicopathological parameters
of HCC, including pathological stage and TNM status. The positive
rate of NOR1 expression was higher in HCC patients with poor
pathological differentiation grade and high TNM stage.
In conclusion, the present study examined the mRNA
and protein expression of NOR1 in normal human liver, hepatitis,
cirrhosis and HCC specimens, which together represent the process
of HCC development. NOR1 expression was increased in HCC and its
expression was correlated with clinicopathological parameters of
patients with HCC. Thus, NOR1 may be involved in HCC progression
and could be employed as a predictive biomarker in HCC
development.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 30300383, 81072270
and 81101828) and the Fundamental Research Funds for the Central
Universities (grant no. 2011JQ030).
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altekruse SF, McGlynn KA and Reichman ME:
Hepatocellular carcinoma incidence, mortality and survival trends
in the United States from 1975 to 2005. J Clin Oncol. 27:1485–1491.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang W, Shu XO, Li H, Yang G, Cai H, Ji
BT, Gao J, Gao YT, Zheng W and Xiang YB: Vitamin intake and liver
cancer risk: A report from two cohort studies in China. J Natl
Cancer Inst. 104:1173–1181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teo EK and Fock KM: Hepatocellular
carcinoma: An Asian perspective. Dig Dis. 19:263–268. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu AX: Systemic therapy of advanced
hepatocellular carcinoma: How hopeful should we be? Oncologist.
11:790–800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El-Serag HB and Mason AC: Rising incidence
of hepatocellular carcinoma in the United States. N Engl J Med.
340:745–750. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thomas MB and Zhu AX: Hepatocellular
carcinoma: The need for progress. J Clin Oncol. 23:2892–2899. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shiraha H, Yamamoto K and Namba M: Human
hepatocyte carcinogenesis (review). Int J Oncol. 42:1133–1138.
2013.PubMed/NCBI
|
|
10
|
Hagymási K and Tulassay Z: Epidemiology,
risk factors and molecular pathogenesis of primary liver cancer.
Orv Hetil. 149:541–548. 2008.(In Hungarian). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nie X, Zhang B, Li X, Xiang J, Xiao B, Ma
J, Zhou M, Zhu S, Lu H, Gui R, et al: Cloning, expression and
mutation analysis of NOR1, a novel human gene down-regulated in
HNE1 nasopharyngeal carcinoma cell line. J Cancer Res Clin Oncol.
129:410–414. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gui R, Li D, Qi G, Suhad A and Nie X:
Inhibition of Grb2-mediated activation of MAPK signal transduction
suppresses NOR1/CB1954-induced cytotoxicity in the HepG2 cell line.
Oncol Lett. 4:566–570. 2012.PubMed/NCBI
|
|
13
|
Shen CM, Nie XM and Li DQ: Screening of
genes differentially expressed in HepG2 cells transfected with NOR1
gene using DNA microarray. Practical Preventive Medicine.
14:609–611. 2007.(In Chinese).
|
|
14
|
Gu X, Fu M, Ding Y, Ni H, Zhang W, Zhu Y,
Tang X, Xiong L, Li J, Qiu L, et al: TIMP-3 expression associates
with malignant behaviors and predicts favorable survival in HCC.
PLoS One. 9:e1061612014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Friedrichs K, Gluba S, Eidtmann H and
Jonat W: Overexpression of p53 and prognosis in breast cancer.
Cancer. 72:3641–3647. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual (7th). Springer.
New York: 2010.
|
|
17
|
Li W, Li X, Wang W, Li X, Tan Y, Yi M,
Yang J, McCarthy JB, Xiong W, Wu M, et al: NOR1 is an HSF1- and
NRF1-regulated putative tumor suppressor inactivated by promoter
hypermethylation in nasopharyngeal carcinoma. Carcinogenesis.
32:1305–1314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Z, Chen L, Leung L, Yen TS, Lee C and
Chan JY: Liver-specific inactivation of the Nrf1 gene in adult
mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia.
Proc Natl Acad Sci USA. 102:4120–4125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai C, Whitesell L, Rogers AB and
Lindquist S: Heat shock factor 1 is a powerful multifaceted
modifier of carcinogenesis. Cell. 130:1005–1018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li W, Li X, Wang W, Yi M, Zhou Y, Zheng P,
Xiong W, Yang J, Peng S, McCarthy JB, et al: Tumor suppressor gene
Oxidored-nitro domain-containing protein 1 regulates nasopharyngeal
cancer cell autophagy, metabolism and apoptosis in vitro. Int J
Biochem Cell Biol. 45:2016–2026. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ouyang J, Wu M, Huang C, Cao L and Li G:
Overexpression of oxidored-nitro domain containing protein 1
inhibits human nasopharyngeal carcinoma and cervical cancer cell
proliferation and induces apoptosis: Involvement of mitochondrial
apoptotic pathways. Oncol Rep. 29:79–86. 2013.PubMed/NCBI
|
|
22
|
Wang W, Li X, Zhang W, Li W, Yi M, Yang J,
Zeng Z, Wanshura Colvin LE, McCarthy JB, Fan S, et al:
Oxidored-nitro domain containing protein 1 (NOR1) expression
suppresses slug/vimentin but not snail in nasopharyngeal carcinoma:
Inhibition of EMT in vitro and in vivo in mice.
Cancer Lett. 348:109–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shan Z, Hou Q, Zhang N, Guo L, Zhang X, Ma
Y and Zhou Y: Overexpression of oxidored-nitro domain containing
protein 1 induces growth inhibition and apoptosis in human prostate
cancer PC3 cells. Oncol Rep. 32:1939–1946. 2014.PubMed/NCBI
|
|
24
|
Li YJ, Wang WW and Li DQ: Effect of NOR1
Gene on Level of E-selectin in HepG2 Cells and its Mechanism. The
Practical Journal of Cancer. 25:9–12. 2010.(In Chinese).
|
|
25
|
Perz JF, Armstrong GL, Farrington LA,
Hutin YJ and Bell BP: The contributions of hepatitis B virus and
hepatitis C virus infections to cirrhosis and primary liver cancer
worldwide. J Hepatol. 45:529–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Davila JA, Morgan RO, Shaib Y, McGlynn KA
and El-Serag HB: Hepatitis C infection and the increasing incidence
of hepatocellular carcinoma: A population-based study.
Gastroenterology. 127:1372–1380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanwal F, Hoang T, Kramer JR, Asch SM,
Goetz MB, Zeringue A, Richardson P and El-Serag HB: Increasing
prevalence of HCC and cirrhosis in patients with chronic hepatitis
C virus infection. Gastroenterology. 140:1182–1188. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiang B, Wang W, Li W, Li X, Li X and Li
G: Differential expression of oxidored nitro domain containing
protein 1 (NOR1), in mouse tissues and in normal and cancerous
human tissues. Gene. 493:18–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiang B, Yi M, Wang L, Liu W, Zhang W,
Ouyang J, Peng Y, Li W, Yin D, Zhou M, et al: Preparation of
polyclonal antibody specific for NOR1 and detection of its
expression pattern in human tissues and nasopharyngeal carcinoma.
Acta Biochim Biophys Sin (Shanghai). 41:754–762. 2009. View Article : Google Scholar : PubMed/NCBI
|