Introduction
Sclerosing epithelioid fibrosarcoma (SEF) is a
low-grade variant of fibrosarcoma, which was first described by
Meis-Kindblom et al (1) in
1995 as a rare but clinicopathologically distinct tumor of the soft
tissue. The tumor occurs primarily in the extremities, trunk, head
and neck, and less commonly in the bone and visceral organs
(2). Fewer than 100 cases of SEF have
been reported, with only 3 previously described in the skull
(2). The depiction of its
radiological characteristics is available only in a few scattered
case reports and series in the pathology literature.
The current study reports the case of a patient with
SEF arising from the occipital bone. To the best of our knowledge,
this is the first full description of skull SEF, including its
complete clinical course, imaging findings on computed tomography
(CT) and magnetic resonance imaging (MRI), and pathological
association; although the clinical manifestations, clinical course
and histopathology of skull SEF have been previously reported, its
appearances on CT, MRI and magnetic resonance (MR) venography have
not.
Case report
A 24-year-old Chinese man presented with a 1-year
history of a slowly enlarging and painless mass in the occiput,
which was found incidentally by self-examination. The patient
developed significant dizziness for 5 days, and was referred to the
Neurosurgery Department of the Second Affiliated Hospital of the
School of Medicine, Zhejiang University (Hangzhou, China) in
December 2011 for therapy. A large, firm, non-tender mass was
palpated on physical examination. The patient's past medical
history was unremarkable. Neurological examination and laboratory
investigation revealed normal results.
CT imaging (SOMATOM Sensation 16; Siemens Healthcare
GmbH, Erlangen, Germany) demonstrated an oval-geographic,
osteolytic lesion within the squamous part of the occipital bone,
with a well-demarcated intracranial, calvarial and extracalvarial
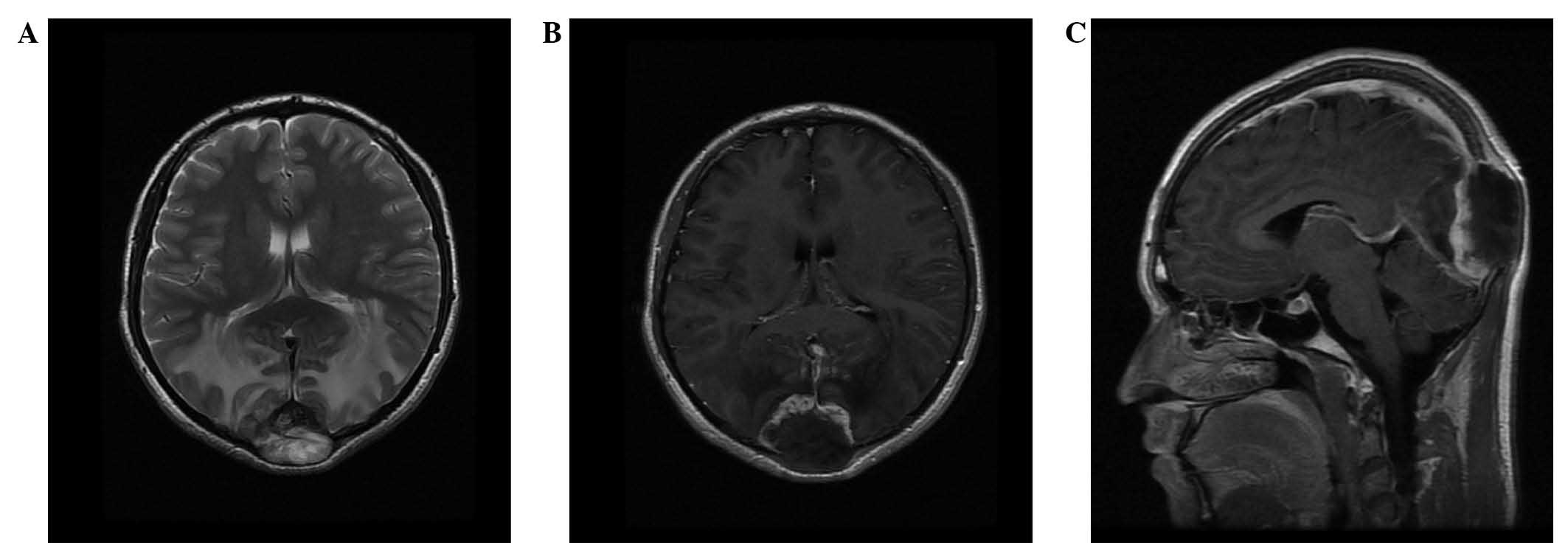
soft tissue mass (Fig. 1). MRI (Signa
HDxt 1.5T; GE Healthcare, Fairfield, CT, USA) revealed a focal,
5.0×4.5×3.5-cm mass with bilateral occipital lobe invasion. The
mass exhibited hypo- and iso-signal intensity on T1-weighted
imaging and mixed-signal intensity on T2-weighted imaging.
Gadolinium-enhanced images revealed prominent perilesional
enhancement, particularly in the region adjacent to the brain.
Irregular hypointense areas within the mass were visible on
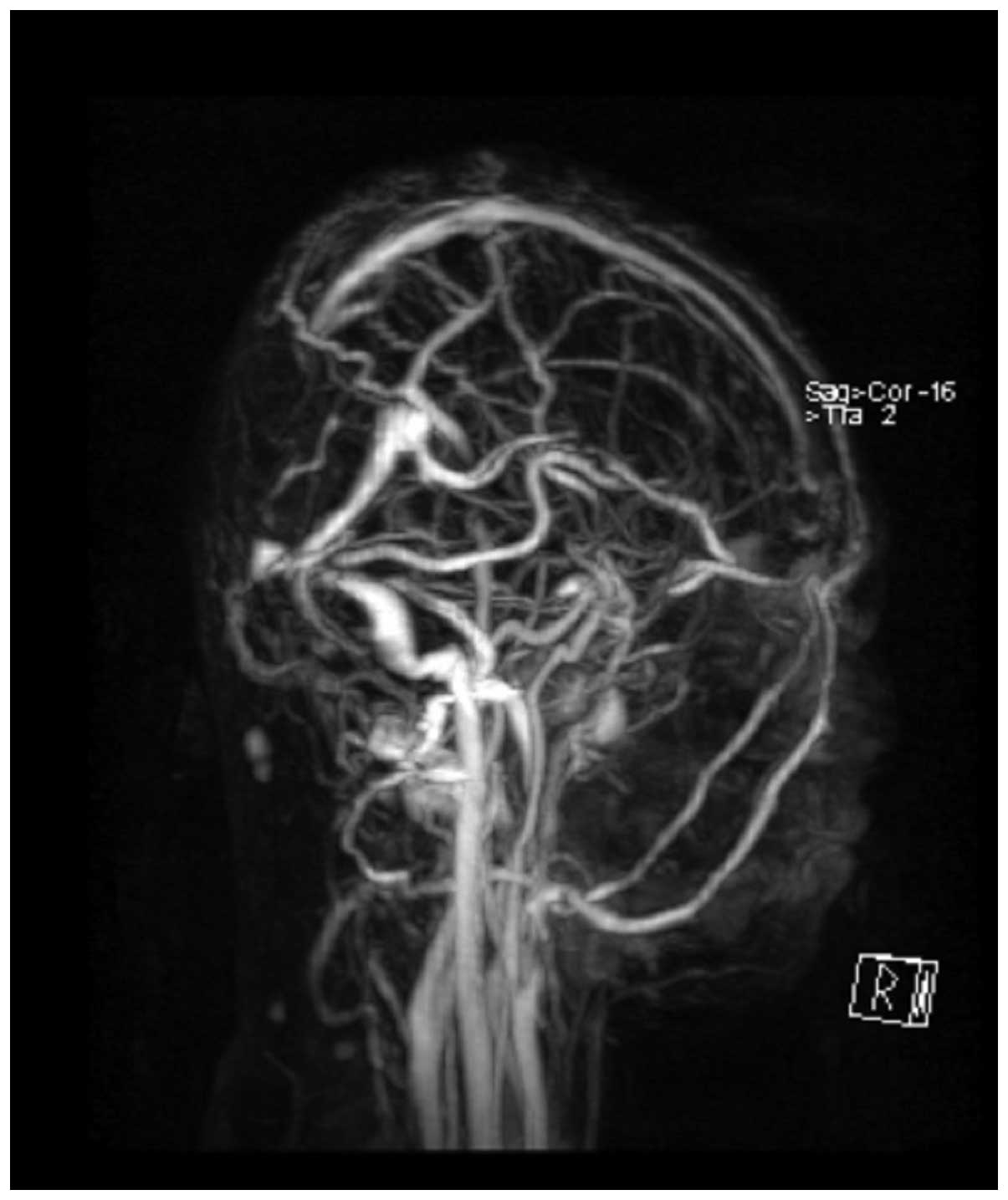
T2-weighted imaging, without obvious enhancement (Fig. 2). MR venography (Sonata 1.5T; Siemens
Healthcare GmbH) was also applied to evaluate the cerebral vein
system. MR venography and MRI indicated that the superior sagittal
sinus and torcular herophili had been invaded, and associated
vasogenic cerebral edema due to mass effect was noted (Figs. 2 and 3).
The preoperative diagnosis was invasive meningioma,
and the patient underwent a craniotomy with subtotal tumor
resection. Gross pathological examination revealed a
5.0×4.0×3.0-cm, heterogeneous mass, part of which was rich in blood
supply and appeared as gray-red fragment of tissue. Resected
tissues were paraffin (Fuzhou Maixin Biotech. Co., Ltd., Fuzhou,
China)-embedded, fixed in 10% buffered-formalin (Fuzhou Maixin
Biotech. Co., Ltd.), cut into 3–5 µm sections and stained with
hematoxylin and eosin (Fuzhou Maixin Biotech. Co., Ltd.).
Histological examination of the tumor following hematoxylin and
eosin staining revealed cells arranged in nests or clusters, some
with a central open area, that were separated by prominent collagen
bands. The cells had scant to moderate cytoplasm and generally
round nuclei with moderately hyperchromatic chromatin. A sizeable
area of ischemic necrosis was present, comprising ~50% of the
tissue sections (Fig. 4).
Immunohistochemical staining with the following monoclonal
antibodies (ZSGB-Bio, Beijing, China) for 30 min at room
temperature was also performed: Mouse anti-CD99 (#ZM-0296; dilution
1:100-1:200), mouse anti-CD34 (#ZM-0046; 1:100-1:200), mouse
anti-CD10 (#ZM-0283, 1:100-1:200; rabbit anti-CD1a (#ZA-544;
1:100-1:200); mouse anti-CD31 (#ZM-0044; 1:50-1:100); rabbit
anti-F8 (#ZA-0543; 1:100-1:200); mouse anti-Myogenin (#ZM-0402;
1:25-1:50); HMB45 (#ZM-0187; 1:100-1:200); mouse anti-P63
(#ZM-0406; 1:100-1:200); mouse anti-epithelial membrane antigen
(EMA;#ZM-0095; 1:100-1:200); mouse anti-S100 (#ZM-0224;
1:100-1:200); mouse anti-α-smooth muscle actin (#ZM-0003;
1:50-1:200); mouse anti-glial fibrillary acidic protein (GFAP;
#ZM-117; 1:100-1:200); mouse anti-cytokeratin (AE1/AE3) (#ZM-0069;
1:100-1:200); mouse anti-vimentin (#ZM-0260; 1:100-1:200); mouse
anti-Ki-67 (#ZM-0167; 1:200); and rabbit anti-myogenic
differentiation 1 (MyoD1; #ZA-0585; 1:100). This revealed that the
lesional cells were negative for HMB45, P63, EMA, S-100, α-smooth
muscle actin, GFAP, cytokeratin (AE1/AE3), CD99, CD34, CD10, CD1a,
CD31, F8, myogenin and MyoD1, and positive for vimentin. The Ki-67
staining index was estimated to be 10–15% focally, and 5–10%
overall. A fluorescence in situ hybridization study revealed
no rearrangement of the FUS gene. A diagnosis of SEF was
subsequently determined.
The patient was treated with one cycle of
postoperative chemotherapy (ifosfamide, 2 g/day, days 1–4;
etoposide 0.1 g/day, days 1–5). At 10 months post surgery,
follow-up MRI of the original site revealed tumor recurrence. A
chest CT scan performed 15 months after the surgery demonstrated
multiple nodules in the bilateral lungs, suggesting metastasis.
After considering the option of radiotherapy, the patient decided
on palliative care only due to financial reasons, and succumbed to
the disease 3 years after his initial presentation.
Discussion
SEF is a rare yet distinct tumor, which was
previously considered to be a low-grade variant of fibrosarcoma
with low cellularity, mild pleomorphy, scarce mitotic figures and a
densely sclerotic hyaline matrix (1,2). A
previous systematic review of 90 cases of SEF suggested a local
recurrence rate of 36%, a distant metastasis rate of 83%, and a
mortality rate of 34% from the disease after a mean of 46 months
(3). However, a follow-up study of ≥1
year in 14 cases revealed local recurrence, metastasis and
mortality rates of 50, 86 and 57%, respectively, suggesting a
higher degree of malignancy (2).
Subsequently, a small number of case reports verified that SEF was
a clinically high-grade tumor with full malignant potential
(4–9).
The skull is an extremely infrequent location for
the development of SEF. Since it was first described in 1995, only
3 cases of skull SEF have been reported in the English literature
(Table I) (2). The current case represents the 4th
reported case of skull SEF, and resembles the other reported cases
with regard to its clinical course. These 3 cases combined with the
present case indicate that SEF in the skull tends to have
aggressive behavior, leading to a poor prognosis.
 | Table I.Clinical features of 4 skull
sclerosing epithelioid fibrosarcomas (SEFs). |
Table I.
Clinical features of 4 skull
sclerosing epithelioid fibrosarcomas (SEFs).
| Author | Age,
years/gender | Site | Size, cm | Type of surgery | Time to
LRa | Time to
METSa (location) |
Follow-upa | Refs. |
|---|
| Antonescu et
al | 14/F | Right posterior
fossa, supra-tentorial space, temporal bone | 6.7 | Subtotal
craniotomy | – | 35 mo (bone,
lung) | DOD, 47 mo | (2) |
|
| 52/F | Right frontoparietal
area, intra-extra cranial, bone | 6 | WLE | 12 mo | 13 mo (bone) | DOD, 26 mo |
|
|
| 41/F | Skull base | >15 | WLE | 46 mo | – | AWD, 65 mo |
|
| Present case | 24/M | Occipital area
intra-extra cranial, occipital bone | 5 | Subtotal
craniotomy | 10 mo | 15 mo (lung) | DOD, 24 mo |
|
Radiological findings, although scarce in the
literature, were distinctive in the current case. The imaging
findings demonstrated a well-defined, heterogeneous, intracranial,
calvarial and extracalvarial mass with bone destruction. The
osteolytic lesion presented sharp borders, a lack of bony sclerosis
and a paucity of periosteal reaction. The adjacent brain and venous
sinus were invaded. These appearances may reflect the malignant
biological behavior of the tumor. On T2-weighted MRI without
enhancement, irregular areas of low signal intensity were visible,
a characteristic which may be observed in areas of decreased
cellularity and dense fibrous tissue or collagen deposition
(10). High signal intensity on
T2-weighted images without enhancement is considered to indicate
necrosis (11,12). Intense perilesional enhancement of the
tumor-adjacent brain region on gadolinium-enhanced MR images may be
associated with histopathological changes, which include cellular
and vascular proliferation, peritumoral desmoplastic reaction and
inflammatory cell infiltration. This enhancement pattern may also
be indicative of malignant tumors (13). Therefore, in the current case, imaging
findings successfully suggested a tumor of high malignancy
containing necrosis and fibrous tissue.
Although radiology may provide biological and
histopathological information relating to SEF, the radiological
differential diagnosis of skull SEF is challenging due to its
rarity. A variety of malignant neoplasms in this site must be
considered if an intracranial, calvarial and extracalvarial mass is
detected in the occiput; such neoplasms include malignant
meningioma, metastatic disease, osteosarcoma, plasmacytoma,
chondrosarcoma, malignant fibrous histiocytoma and bone Langerhans
cell histiocytosis (14,15).
In summary, the current study reports an extremely
rare instance of SEF arising within the occipital bone and
simulating a high-grade tumor. Imaging features may provide
important biological and histopathological information to make an
accurate diagnosis. Radiologists must consider SEF as a possible
diagnosis for a patient presenting with a slowly enlarging mass in
this location that radiologically appears as a malignant tumor with
necrosis and fibrous tissue, and exhibits an intense perilesional
enhancement pattern.
References
|
1
|
Meis-Kindblom JM, Kindblom LG and Enzinger
FM: Sclerosing epithelioid fibrosarcoma: A variant of fibrosarcoma
simulating carcinoma. Am J Surg Pathol. 19:979–993. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antonescu CR, Rosenblum MK, Pereira P,
Nascimento AG and Woodruff JM: Sclerosing epithelioid fibrosarcoma.
A study of 16 cases and confirmation of a clinicopathologically
distinct tumor. Am J Surg Pathol. 25:699–709. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ossendorf C, Studer GM, Bode B and Fuchs
B: Sclerosing epithelioid fibrosarcoma: Case presentation and a
systematic review. Clin Orthop Relat Res. 466:1485–1491. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elkins CT and Wakely PE Jr: Sclerosing
epithelioid fibrosarcoma of the oral cavity. Head Neck Pathol.
5:428–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bai S, Jhala N, Adsay NV and Wei S:
Sclerosing epithelioid fibrosarcoma of the pancreas. Ann Diagn
Pathol. 17:214–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chow LT, Lui YH, Kumta SM and Allen PW:
Primary sclerosing epithelioid fibrosarcoma of the sacrum: A case
report and review of the literature. J Clin Pathol. 57:90–94. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tomimaru Y, Nagano H, Marubashi S,
Kobayashi S, Eguchi H, Takeda Y, Tanemura M, Kitagawa T, Umeshita
K, Hashimoto N, et al: Sclerosing epithelioid fibrosarcoma of the
liver infiltrating the inferior vena cava. World J Gastroenterol.
15:4204–4208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Folk GS, Williams SB, Foss RB and
Fanburg-Smith JC: Oral and Maxillofacial sclerosing epithelioid
fibroscarcoma: Report of five cases. Head Neck Pathol. 1:13–20.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grunewald TG, von Luettichau I, Weirich G,
Wawer A, Behrends U, Prodinger PM, Jundt G, Bielack SS, Gradinger R
and Burdach S: Sclerosing epithelioid fibrosarcoma of the bone: A
case report of high resistance to chemotherapy and a survey of the
literature. Sarcoma. 2010:4316272010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Christensen DR, Ramsamooj R and Gilbert
TJ: Sclerosing epithelioid fiberosarcoma: Short T2 on MR imaging.
Skeletal Radiol. 26:619–621. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ulaner G, Hwang S, Lefkowitz RA, Landa J
and Panicek DM: Musculoskeletal tumors and tumor-like conditions:
Common and avoidable pitfalls at imaging in patients with known or
suspected cancer: Part A: Benign conditions that may mimic
malignancy. Int Orthop. 37:871–876. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ulaner G, Hwang S, Landa J, Lefkowitz RA
and Panicek DM: Musculoskeletal tumours and tumour-like conditions:
Common and avoidable pitfalls at imaging in patients with known or
suspected cancer: Part B: Malignant mimics of benign tumours. Int
Orthop. 37:877–882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Semelka RC, Hussain SM, Marcos HB and
Woosley JT: Perilesional enhancement of hepatic metastases:
Correlation between MR imaging and histopathologic findings -
initial observations. Radiology. 215:89–94. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gangadhar K and Santhosh D:
Radiopathological evaluation of primary malignant skull tumors: A
review. Clin Neurol Neurosurg. 114:833–839. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitsuya Kl, Nakasu Y, Horiguchi S, Harada
H, Nishimura T, Yuen S, Asakura K and Endo M: Metastatic skull
tumors: MRI features and a new conventional Classification. J
Neurooncol. 104:239–245. 2011. View Article : Google Scholar : PubMed/NCBI
|