Introduction
Primary central nervous system lymphoma (PCNSL) is a
predominantly extranodal non-Hodgkin's B-cell lymphoma (NHL) of the
brain, spinal cord, leptomeninges and eyes, with an incidence of
0.47 cases per 100,000 person-years (1). Traditional therapies for systemic NHL,
such as the cyclophosphamide, doxorubicin, vincristine and
prednisone (CHOP) regimen, have proven to be ineffective in PCNSL,
presumably due to poor drug penetration across the blood-brain
barrier (BBB) (2). In the past,
whole-brain radiation therapy (WBRT) was considered to be the
first-line treatment for PCNSL due to high complete response rates
(CRRs) of up to 90% (3). However, the
median overall survival (OS) time and 2-year survival rate were
only 12–17 months and 28–40%, respectively (4,5).
Subsequent trials demonstrated improved median OS times with
combined WBRT and methotrexate (MTX)-based chemotherapy, prolonging
the OS time from 11.5 months with WBRT alone (4) to 33–60 months with combined therapy
(6–8).
However, this regimen carries a significant risk of delayed
neurotoxicity (7,9), and therefore WBRT is usually delayed or
avoided and reserved for cases of tumor recurrence. Several trials
have investigated the efficacy of single-agent high-dose MTX
(HD-MTX) and found variable CRRs ranging from 29–52% (10,11). There
is now increasing evidence that MTX-based polychemotherapy is
superior to HD-MTX alone, for instance, when MTX is combined with
HD-cytarabine (Ara-C) or ifosfamide (12,13).
Furthermore, rituximab (RTX) is now frequently combined with
single-agent HD-MTX or MTX-based polychemotherapy, based on
observations that the addition of RTX results in improved response
rates (14–18). The present study reports the
experience of a single institution as a retrospective case series
of 12 patients with newly diagnosed PCNSL treated with HD-MTX and
RTX.
Patients and methods
Patient selection criteria
Following Institutional Review Board (IRB) approval
by the University of Washington Medical Center (Seattle, WA, USA),
a retrospective chart review was performed of patients with PCNSL
at our institution between 2007 and 2011. Inclusion criteria
consisted of an age ≥18 years, a Karnofsky performance status (KPS)
of ≥50%, magnetic resonance imaging (MRI) evidence of disease and a
histologically proven diagnosis. Exclusion criteria consisted of
the presence of other types of malignancy and an immunocompromised
state. All patients underwent staging with computed tomography (CT)
of the chest, abdomen and pelvis, as well as an ophthalmological
examination. In addition, all males underwent a testicular
examination and ultrasound to exclude the presence of testicular
lymphoma. A diagnosis of PCNSL was made by histopathological
confirmation of either biopsy or cerebrospinal fluid (CSF)
studies.
Treatment regimen
Patients underwent induction therapy with combined
biweekly HD-MTX (8 g/m2/dose) and weekly RTX (375
mg/m2/dose). HD-MTX was administered intravenously over
4 h with anti-emetic premedication and with or without concurrent
dexamethasone. All patients received aggressive hydration prior to
and after HD-MTX infusion (sterile water + 150 mEq/l of NaHCO3+20
mEq/l of KCl infused at 150–200 cc/h over 4 h) to maintain a urine
output of ≥100 ml/h and a urinary pH of >7, until serum MTX
levels had decreased to ≤0.1 µM/l. Serum MTX levels were monitored
daily. Leucovorin rescue was initiated 24 h after completion of MTX
infusion and administered at 25 mg every 6 h until serum MTX levels
were ≤0.1 µM/l. Leucovorin dosing was increased in cases of slow
MTX clearance. All patients were instructed to continue leucovorin
at 25 mg orally every 6 h for 2 days following discharge. HD-MTX
during induction was administered until a CR was achieved by MRI
criteria. In the absence of a CR, MTX was administered for a
maximum of eight biweekly doses. RTX was provided for a total of
eight weekly doses.
Responders to the induction regimen received
consolidation treatment with 2 additional cycles of biweekly
HD-MTX, followed by maintenance treatment with monthly HD-MTX for
up to 12 months. The duration of maintenance treatment was
primarily limited by drug toxicity according to the National Cancer
Institute Common Toxicity Criteria (version 4.0; http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf).
Endpoints and response criteria
The primary end point was radiographic response,
assessed by contrast-enhanced MRI every 2 cycles during induction
and every 2 months thereafter.
Response was classified according to standard
radiographic criteria as a CR, partial response (PR), stable
disease (SD) or progressive disease (PD). A CR was defined as
complete resolution of all contrast-enhanced lesions. A PR was
defined as a >50% reduction in size of contrast-enhancing
lesions, and PD was defined as an increase in size by >25%. All
other situations were classified as SD.
OS time was measured as the time between the
pathological diagnosis and mortality or the time of last follow-up.
Progression-free survival (PFS) time was defined as the time
between the start of treatment and the day of radiographic
progression.
Results
Demographics
A total of 12 patients were included in the present
study (6 females and 6 males). The median patient age at the time
of diagnosis was 62.5 years (range, 19–78 years). The median KPS
was 80% (range, 50–100%). Patient characteristics are listed in
Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Case no. | Gender | Age, years | Diagnosis of
PCNSL | Number of MTX
cycles | Response | PFS, months | Mortality | Site of enhancement
on MRI | Molecular
pathologya | Salvage therapy |
|---|
| 1 | F | 59 | Bx | 15 | CR | 27.7 | No | R thalamus, R
temporal lobe | IHC:
CD19/CD20+ with κ light chain restriction | HD-MTX, IT
cytarabine |
| 2 | M | 77 | Bx | 12 | CR | 6 | Yes | Periventricular WM,
crossing CC; R frontal WM; R occipital horn of lateral
ventricle | IHC:
CD20+; FC: CD20+ with κ light chain
restriction | RTX; RTX + TMZ; gamma
knife; WBRT |
| 3 | F | 66 | Bx | 4 | CR | 5.4+ | No | L frontoparietal
dura | Not performed | None |
| 4 | F | 70 | STR | 8 | PR | 8.5+ | No | L temporal lobe | IHC:
CD20+ | IT cytarabine |
| 5 | F | 68 | Bx | 14 | CR | 5.5+ | No | Patchy areas in
cortical and subcortical WM and cerebellum | IHC:
CD20+ | None |
| 6 | M | 74 | Bx | 12 | PR | 8.4+ | No | Third ventricle,
occipital horn of R lateral ventricle, fourth ventricle | FC:
CD19/CD20/CD45+ with λ light chain restriction | None |
| 7 | M | 78 | Bx | 3 | PR | 2.9+ | No | R posterior
parieto-occipital lobe | FC:
CD19/CD20/CD45+ with λ light chain restriction | None |
| 8 | F | 59 | Bx | 11 | PR | 17.9+ | No | CC, septum pellucidum
and surrounding WM tracts | FC:
CD19/CD20+ with κ light chain restriction | IT cytarabine |
| 9 | F | 19 | CSF | 2 | N/A | N/A | No | CC, L inferior
cerebellar hemisphere | FC: decreased
CD10/CD20, increased CD28 with κ light chain restriction, normal
CD19/45 | N/A |
| 10 | M | 55 | Bx | 9 | CR | 22.1 | No | R frontoparietal
lobe | IHC:
CD20+ | HD-MTX; ASCT |
| 11 | M | 42 | Bx | 11 | CR | 16.6+ | No | Diffusely in
frontal, parietal, temporal and occipital lobes; splenium of
CC | IHC:
CD20+; FC: CD19/CD20+ | ASCT |
| 12 | M | 50 | Bx | 8 | CR | 13.5+ | Yes | L basal ganglia, L
cerebral peduncle | FC:
CD19/CD20+ with λ light chain restriction | IT cytarabine;
ASCT |
Neurological manifestations
The most common symptoms were cognitive dysfunction
(n=5), including word-finding difficulties and altered mental
status, and ataxia and incoordination (n=4). Furthermore, 3
patients experienced vision changes and focal weakness. Less
commonly observed symptoms consisted of headaches (n=2), sensory
changes (n=2) and seizures (n=1).
Tissue diagnosis and imaging
Tissue diagnosis was made either by biopsy (n=10),
resection (n=1; case 4) or CSF analysis (n=1; case 9). Biopsy alone
was diagnostic in 83% of patients (n=10) and revealed diffuse large
B-cell lymphoma (DLBCL), based on characteristic cellular
morphology and antigen expression profile, including positivity for
CD19, CD20 and CD45. CSF analysis in case 9 showed a clonal
population of abnormal B cells. In total, 2 patients underwent a
biopsy after non-diagnostic flow cytometry and cytology from CSF
analysis (cases 3 and 6).
All patients underwent MRI of the brain and entire
spine. Characteristic imaging findings included homogenously
enhancing periventricular white matter lesions with associated
diffusion restriction, although exceptions to these features were
observed as well. Systemic involvement of lymphoma was excluded in
10 subjects by CT of the chest, abdomen and pelvis. Cases 2 and 4
underwent whole-body positron emission tomography (PET)-CT instead
of CT of the chest, abdomen and pelvis. CT in case 9 revealed
pulmonary nodules, but subsequent PET-CT excluded
18F-fluorodeoxyglucose avidity. None of the patients
presented with intra-ocular involvement of lymphoma.
Treatment and treatment response
A total of 109 cycles of MTX were administered (104
induction and 5 maintenance cycles), with a mean of 8.6 cycles and
a median of 11 cycles (range, 2–15 cycles).
The most commonly encountered side effects were
fatigue (n=4), anemia, neutropenia, nausea and renal dysfunction
(n=3 each). While 2 individuals experienced transient
transaminitis, 1 patient presented with thrombocytopenia and 1 with
an RTX-related infusion reaction. Grade 3 toxicities were
identified in 2 patients: Case 7 was affected by severe mucositis,
febrile neutropenia, anemia and thrombocytopenia, requiring
transfusion of blood products; case 5 was also transfused due to
anemia. No toxicity was reported in 1 patient (case 11) and data
was unavailable for another (case 9). No grade 4 toxicities were
observed.
Salvage therapy was administered to 7 patients; 4 of
these (cases 1, 4, 8 and 12) received intrathecal Ara-C alone or in
combination with HD-MTX. Case 2 was re-challenged with 8 cycles of
RTX and 1 cycle of temozolomide (TMZ), followed by gamma-knife
stereotactic radiosurgery and WBRT. Case 10 received HD-MTX as
salvage treatment prior to undergoing autologous peripheral blood
stem cell transplantation (ASCT). Two additional patients (cases 11
and 12) underwent ASCT after achieving a CR.
The mean overall response rate (ORR) to treatment
was 91.7% (11 out of 12 patients). A CR was achieved in 7 patients
(58.3%) and a PR in 4 patients (33.3%). Survival data was not
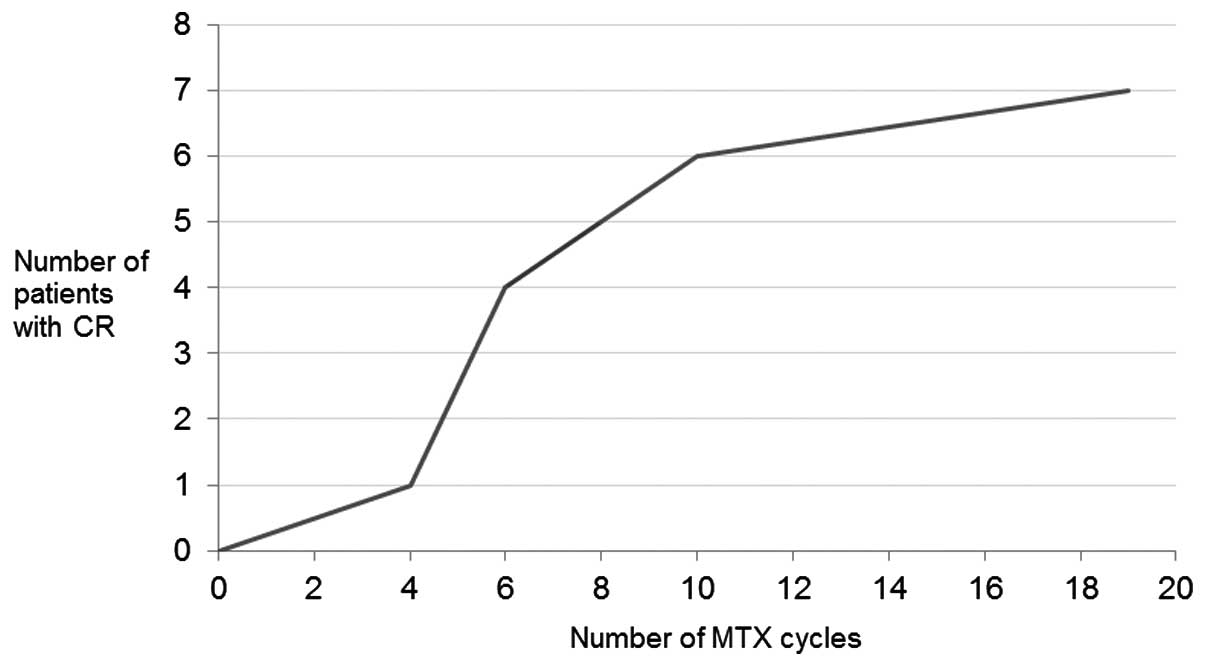
available for case 9. A median of 6 cycles of MTX (range, 4–19
cycles) had to be administered to achieve a CR (Fig. 1). In total, 3 patients (cases 1, 2 and
10) had recurrent disease after a median PFS time of 22.1 months
(range, 6–27.7 months; Fig. 2). Seven
patients did not experience disease progression after achieving a
CR or PR (cases 3–8 and 11). At the time of data analysis, 10 out
of 12 patients were alive after a median time of 8.5 months (range,
2.9–17.9 months) following treatment response. Case 2 succumbed to
complications associated with pneumonia and a pulmonary embolus.
Case 12 underwent an ASCT as part of salvage therapy and succumbed
to respiratory failure associated with lymphocytic alveolitis and a
drug reaction with eosinophilia and systemic symptoms.
Discussion
The optimal treatment for PCNSL is unknown. Previous
studies have demonstrated improved survival with combined WBRT and
MTX-based therapy; however, this regimen is associated with
significant risks of delayed neurotoxicity, particularly in
patients >60 years old, manifesting as cognitive dysfunction,
which can progress to dementia with gait ataxia and incontinence
(19,20). MTX remains the cornerstone of therapy
and mounting evidence suggests that MTX-based polychemotherapy
improves survival (14,16). WBRT tends to be reserved for recurrent
disease or those unable to receive chemotherapy.
RTX is a chimeric monoclonal antibody against the
CD20 antigen on B lymphocytes, which is present in the vast
majority of patients with systemic NHL and PCNSL (21). Administration of RTX improves the
outcome in patients with systemic DLBCL, reduces the rate of CNS
relapse in high-risk disease, and in conjunction with the CHOP
regimen, has become a standard component of treatment for DLBCL
(22). The mechanism of action for
RTX includes complement-mediated and antibody-dependent cellular
cytotoxicity, as well as the antibody-induced inhibition of cell
growth and apoptosis (21). However,
the clinical effects of RTX in PCNSL are unclear. Although RTX has
poor BBB penetration due to its large molecular size, a certain
degree of therapeutic drug level is achieved (~1.5% of serum
concentration) (23), likely due to
disruption of the BBB by the tumor itself. This explains the
rationale for using RTX in the induction phase of treatment only
when BBB permeability is highest due to increased tumor burden.
To date, no randomized controlled trials of combined
HD-MTX and RTX have been conducted, and treatment protocols are
largely institution-dependent. However, multiple prospective and
retrospective studies have indicated that the addition of RTX to
MTX-based chemotherapy may improve response rates and PFS times
compared with single-agent HD-MTX (Table
II). In one prospective single-arm study (16), patients received induction
chemotherapy with an RTX, MTX, vincristine and procarbazine
regimen, and achieved an ORR of 93% and a CRR of 78%. Another study
investigated combined RTX, MTX and ifosfamide therapy, and found
that the addition of RTX resulted in an improved CRR (100%) and
6-month PFS (PFS-6) rate (94%) compared with MTX and ifosfamide
alone (CRR, 68%; PFS-6, 63%) (15).
Two previous studies evaluated combined HD-MTX and RTX (14,24).
Chamberlain and Johnston (14)
conducted a prospective phase II trial using this regimen and
reported a ORR and CRR of 80 and 60%, respectively. These results
are similar to those of the present study (ORR, 91%; CRR, 58%),
although there were certain differences in trial design. While the
patients in the present study received weekly RTX for a maximum of
8 cycles or until a radiographic CR was achieved, patients in the
previous study were administered a median of 4 cycles of RTX
(range, 4–6 cycles) every 2 weeks (14). It is unknown whether more frequent
administration of RTX is associated with improved survival, as
median OS time had not been attained in the present cohort at the
time of data analysis. Notably, toxicity from RTX was limited to
grade 3 or less in the patients, suggesting that additional cycles
of RTX were relatively well tolerated. Holdhoff et al
(24) treated 27 patients with the
same combination regimen as in the present study and compared the
response with that of patients who received single-agent HD-MTX at
their institution. The study found that the addition of RTX
resulted in an improved CRR (89%) and PFS time (27 months). Similar
to the present study, OS had not been reached in those receiving
HD-MTX and RTX.
 | Table II.Selected studies of single-agent
HD-MTX vs. MTX-based polychemotherapy with RTX. |
Table II.
Selected studies of single-agent
HD-MTX vs. MTX-based polychemotherapy with RTX.
| First author/s
(year) | n | MTX dose,
g/m2 | RTX dose,
mg/m2 | Other drugs | ORR (CRR),
%a | Median PFS,
months | Median OS,
months | Comments | (Ref.) |
|---|
| Single-agent
HD-MTX |
|
|
|
|
|
|
|
|
|
|
Herrlinger et al
(2002) | 37 | 8 | – | – | 34
(29) | 10 | 25 | Prospective | (11) |
|
Batchelor et al
(2003) | 25 | 8 | – | – | 74
(52) | 12 | >23 | Prospective | (10) |
| Combined HD-MTX and
RTX |
|
|
|
|
|
|
|
|
|
| Shah
et al (2007) | 30 | 3.5 | 500 | Vincristine,
procarbazine | 93
(78) | N/A; 57% estimated
2-year PFS rate | 40 (estimated) | Prospective
Followed by WBRT (23.4 Gy if CR to induction vs. 45 Gy if less than
CR attained); consolidation with IT cytarabine | (16) |
|
Chamberlain and Johnston
(2010) | 40 | 8 | 375 | – | 80
(60) | 21 | 29 (estimated) | Prospective | (14) |
| Fritsch
et al (2011) | 28 | 3 | 375 | Procarbazine,
lomustine | 82
(64) | 16 | 17.5 | Prospective; all
patients ≥65 years | (18) |
|
Birnbaum et al
(2012) | 17 | 4 | 375 | Ifosfamide | 100 (59) | 18 | Not reached | Retrospective | (15) |
|
Holdhoff et al
(2014) | 27 | 8 | 375 | – | 89
(73) | 27 | Not reached | Retrospective | (24) |
| Present
study | 12 | 8 | 375 | – | 91
(58) | 22 | Not reached | Retrospective |
|
RTX may also be beneficial in individuals ≥65 years
of age. Treatment of this group is particularly challenging since
WBRT and HD-MTX are notoriously associated with late neurotoxicity
(19,20). In a prospective study of 28 patients
receiving RTX, HD-MTX, procarbazine and lomustine (R-MCP) (18), the ORR (82%) and CRR (64%) were
comparable to response rates in younger subjects. OS and PFS times
were also prolonged with the addition of RTX when compared with a
previous single-arm study (25) using
MCP alone (R-MCP group: OS, 17.5 months and PFS, 16 months; MCP
group: OS, 15.4 months and PFS, 5.9 months).
Lastly, there is inconclusive data on the optimal
treatment for recurrent PCNSL. The median OS time in recurrent or
progressive PCNSL is ~4.5 months (26). Since the majority of patients are
older (>60 years) at the time of relapse, regimens with minimal
toxicity are preferred. Single-agent RTX in 12 patients with
recurrent PCNSL resulted in an ORR and CRR of 36 and 27%,
respectively, and a median OS time of 21 months (27). By contrast, data from two small
retrospective case series suggested that combined RTX and TMZ was
well tolerated and resulted in a higher ORR (53–100%), but a
shorter OS time (8–14 months) (28,29).
The power of the present study is limited by the
small number of patients, the lack of randomized data and its
retrospective nature. Nonetheless, the results support the
observation that addition of RTX to HD-MTX imparts a survival
benefit compared with single-agent HD-MTX. Future randomized
controlled trials will provide further insight into the efficacy of
RTX in PCNSL treatment. Currently, these include the HOVON 105
PCNSL/ALLG NHL24 trial (trial number, EudraCT 2009-014722-42) and
the International Extranodal Lymphoma Study Group (IELSG)-32 trial
(trial number, NCT01011920). The former randomizes patients to
either a MTX, teniposide, carmustine and prednisolone (MBVP)
regimen or a RTX + MBVP regimen, followed by consolidation with
Ara-C. The IELSG-32 trial randomizes patients to either MTX +
Ara-C, MTX + Ara-C + RTX or MTX + Ara-C + RTX + thiotepa. It
further randomizes patients who achieve a CR to any of these
regimens to consolidation treatment with WBRT or HD-chemotherapy +
ASCT.
In conclusion, the addition of RTX to HD-MTX in the
treatment of PCNSL may increase response rates and prolong PFS
times. It remains unclear whether RTX improves long-term outcome
and whether the addition of other chemotherapeutic agents
ameliorates survival. These questions should ideally be evaluated
in prospective trials.
Acknowledgements
Preliminary results were presented as an abstract at
the 10th Annual Meeting of the European Association of
Neuro-Oncology.
References
|
1
|
Villano JL, Koshy M, Shaikh H, Dolecek TA
and McCarthy BJ: Age, gender and racial differences in incidence
and survival in primary CNS lymphoma. Br J Cancer. 105:1414–1418.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mead GM, Bleehen NM, Gregor A, Bullimore
J, Shirley D, Rampling RP, Trevor J, Glaser MG, Lantos P, Ironside
JW, et al: A medical research council randomized trial in patients
with primary cerebral non-Hodgkin lymphoma: Cerebral radiotherapy
with and without cyclophosphamide, doxorubicin, vincristine and
prednisone chemotherapy. Cancer. 89:1359–1370. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Korfel A and Uschlegel: Diagnosis and
treatment of primary CNS lymphoma. Nat Rev Neurol. 9:317–327. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nelson DF, Martz KL, Bonner H, Nelson JS,
Newall J, Kerman HD, Thomson JW and Murray KJ: Non-Hodgkin's
lymphoma of the brain: Can high dose, large volume radiation
therapy improve survival? Report on a prospective trial by the
radiation therapy oncology group (RTOG): RTOG 8315. Int J Radiat
Oncol Biol Phys. 23:9–17. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laperriere NJ, Cerezo L, Milosevic MF,
Wong CS, Patterson B and Panzarella T: Primary lymphoma of brain:
Results of management of a modern cohort with radiation therapy.
Radiother Oncol. 43:247–252. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DeAngelis LM, Yahalom J, Thaler HT and
Kher U: Combined modality therapy for primary CNS lymphoma. J Clin
Oncol. 10:635–643. 1992.PubMed/NCBI
|
|
7
|
Abrey LE, DeAngelis LM and Yahalom J:
Long-term survival in primary CNS lymphoma. J Clin Oncol.
16:859–863. 1998.PubMed/NCBI
|
|
8
|
Morris PG and Abrey LE: Therapeutic
challenges in primary CNS lymphoma. Lancet Neurol. 8:581–592. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Omuro AM, Ben-Porat LS, Panageas KS, Kim
AK, Correa DD, Yahalom J, Deangelis LM and Abrey LE: Delayed
neurotoxicity in primary central nervous system lymphoma. Arch
Neurol. 62:1595–1600. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Batchelor T, Carson K, O'Neill A, Grossman
SA, Alavi J, New P, Hochberg F and Priet R: Treatment of primary
CNS lymphoma with methotrexate and deferred radiotherapy: A report
of NABTT 96–07. J Clin Oncol. 21:1044–9. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herrlinger U, Schabet M, Brugger W,
Kortmann RD, Küker W, Deckert M, Engel C, Schmeck-Lindenau HJ,
Mergenthaler HG, Krauseneck P, et al: German cancer society
neuro-oncology working group NOA-03 multicenter trial of
single-agent high-dose methotrexate for primary central nervous
system lymphoma. Ann Neurol. 51:247–252. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thiel E, Korfel A, Martus P, Kanz L,
Griesinger F, Rauch M, Röth A, Hertenstein B, von Toll T,
Hundsberger T, et al: High-dose methotrexate with or without whole
brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase
3, randomised, non-inferiority trial. Lancet Oncol. 11:1036–1047.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferreri AJ, Reni M, Foppoli M, Martelli M,
Pangalis GA, Frezzato M, Cabras MG, Fabbri A, Corazzelli G,
Ilariucci F, et al: High-dose cytarabine plus high-dose
methotrexate versus high-dose methotrexate alone in patients with
primary CNS lymphoma: A randomised phase 2 trial. Lancet.
374:1512–1520. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chamberlain MC and Johnston SK: High-dose
methotrexate and rituximab with deferred radiotherapy for newly
diagnosed primary B-cell CNS lymphoma. Neuro Oncol. 12:736–744.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Birnbaum T, Stadler EA, von Baumgarten L
and Straube A: Rituximab significantly improves complete response
rate in patients with primary CNS lymphoma. J Neurooncol.
109:285–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shah GD, Yahalom J, Correa DD, Lai RK,
Raizer JJ, Schiff D, LaRocca R, Grant B, DeAngelis LM and Abrey LE:
Combined immunochemotherapy with reduced whole-brain radiotherapy
for newly diagnosed primary CNS lymphoma. J Clin Oncol.
25:4730–4735. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wieduwilt MJ, Valles F, Issa S, Behler CM,
Hwang J, McDermott M, Treseler P, O'Brien J, Shuman MA, Cha S, et
al: Immunochemotherapy with intensive consolidation for primary CNS
lymphoma: A pilot study and prognostic assessment by
diffusion-weighted MRI. Clin Cancer Res. 18:1146–1155. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fritsch K, Kasenda B, Hader C, Nikkhah G,
Prinz M, Haug V, Haug S, Ihorst G, Finke J and Illerhaus G:
Immunochemotherapy with rituximab, methotrexate, procarbazine and
lomustine for primary CNS lymphoma (PCNSL) in the elderly. Ann
Oncol. 22:2080–2085. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Filley CM and Kleinschmidt-DeMasters BK:
Toxic leukoencephalopathy. N Engl J Med. 345:425–432. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gavrilovic IT, Hormigo A, Yahalom J,
DeAngelis LM and Abrey LE: Long-term follow-up of high-dose
methotrexate-based therapy with and without whole brain irradiation
for newly diagnosed primary CNS lymphoma. J Clin Oncol.
24:4570–4574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cang S, Mukhi N, Wang K and Liu D: Novel
CD20 monoclonal antibodies for lymphoma therapy. J Hematol Oncol.
5:642012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maloney DG, Grillo-López AJ, Bodkin DJ,
White CA, Liles TM, Royston I, Varns C, Rosenberg J and Levy R:
IDEC-C2B8: Results of a phase I multiple-dose trial in patients
with relapsed non-Hodgkin's lymphoma. J Clin Oncol. 15:3266–3274.
1997.PubMed/NCBI
|
|
23
|
Ruhstaller TW, Amsler U and Cerny T:
Rituximab: Active treatment of central nervous system involvement
by non-Hodgkin's lymphoma? Ann Oncol. 11:374–375. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Holdhoff M, Ambady P, Abdelaziz A, Sarai
G, Bonekamp D, Blakeley J, Grossman SA and Ye X: High-dose
methotrexate with or without rituximab in newly diagnosed primary
CNS lymphoma. Neurology. 83:235–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Illerhaus G, Marks R, Müller F, Ihorst G,
Feuerhake F, Deckert M, Ostertag C and Finke J: High-dose
methotrexate combined with procarbazine and CCNU for primary CNS
lymphoma in the elderly: Results of a prospective pilot and phase
II study. Ann Oncol. 20:319–325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gerstner ER and Batchelo TT: Primary
central nervous system lymphoma. Arch Neurol. 67:291–297. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Batchelor TT, Grossman SA, Mikkelsen T, Ye
X, Desideri S and Lesser GJ: Rituximab monotherapy for patients
with recurrent primary CNS lymphoma. Neurology. 76:929–930. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong ET, Tishler R, Barron L and Wu JK:
Immunochemotherapy with rituximab and temozolomide for central
nervous system lymphomas. Cancer. 101:139–145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Enting RH, Demopoulos A, DeAngelis LM and
Abrey LE: Salvage therapy for primary CNS lymphoma with a
combination of rituximab and temozolomide. Neurology. 63:901–903.
2004. View Article : Google Scholar : PubMed/NCBI
|