Introduction
Cervical cancer, a malignant neoplasm of the uterine
cervix, is one of the most prevalent gynecological cancers
worldwide. Although cervical cancer screening has been globally
popularized, there are still large numbers of cases of advanced
disease, the majority of which occur in the developing countries
(1,2).
Therefore, studying the mechanisms of tumor invasion and
metastasis, and possible methods of blocking these pathways, has
the potential to greatly improve the prognosis of patients with
cervical cancer.
Secreted protein acidic and rich in cysteine (SPARC)
is a 43 kDa protein that was first identified by Termine et
al (3). As a calcium-binding
matricellular glycoprotein, SPARC is able to interact with various
extracellular matrix macromolecules and regulate cell adhesion,
proliferation and migration (4).
SPARC is overexpressed in the stroma and cancer cells in certain
types of cancer, including breast cancer (5), melanoma (6), and glioma (7), affecting tumor development, invasion and
metastasis. The importance of SPARC in the development of cancers
and its potential role in cancer therapy have generated
considerable interest in recent years (8,9).
The epithelial-mesenchymal transition (EMT) is the
process of transformation of polar epithelial cells into
mesenchymal cells with the ability to invade and migrate. During
this process, malignant cells disseminate from the primary
epithelial neoplasm and invade the local tissue and blood vessels
(10). EMT is closely associated with
the invasion and metastasis of cancer cells (11). An important sign of EMT is the shift
from the expression of E-cadherin to the expression of N-cadherin,
which facilitates the metastatic dissemination ability of malignant
cells. Vimentin is a mesenchymal marker, and its upregulated
expression is frequently associated with EMT (12).
In a previous study, compared with the
low-invasiveness subclones, SPARC was found to be overexpressed in
the highly invasive subclones. Additionally, knockdown of SPARC
significantly inhibited cervical cancer cell proliferation,
invasion and metastasis, accompanied by upregulated E-cadherin
expression (13). Therefore, the
current study aimed to investigate the expression of SPARC in
cervical cancer specimens and assess the association between SPARC
and the prognosis of cervical cancer patients, as well as to
further study the role of SPARC in EMT.
Materials and methods
Cell lines
Human cervical cancer cells were cultured in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified atmosphere of 5% CO2. Subclones of HeLa and
SiHa human cervical carcinoma cells (Shanghai Institute for
Biological Sciences, Chinese Academy of Sciences, Shanghai, China)
were selected according to their differential invasiveness, as
described in previous report (13).
RNA interference
The expression of SPARC was knocked down in cells
using small hairpin RNAs (shRNAs; GeneChem, Co., Ltd., Shanghai,
China), containing a cytomegalovirus-driven green fluorescent
protein (GFP). The sequences for SPARC and the negative control
were as follows: SPARC, 5′-AACAAGACCTTCGACTCTTCC-3′; control,
5′-TTCTCCGAACGTGTCACGT-3′. The invasive subclone cells, HeLa-1 and
SiHa-1, were seeded into six-well plates and then infected with
lentiviral vector, which contained a multiple cloning site for
insertion of shRNA constructs to be driven by an upstream U6
promoter (GeneChem, Co., Ltd.). In order to obtain the best
transfection effect, varying concentration gradients were tested,
and it was determined that one cell transfection required 60 viral
units, which meant that the multiplicity of infection value was 60.
After 24 h, fresh complete medium replaced the medium containing
lentivirus. After another 4 days, >80% GFP-positivity in the
cells was observed using fluorescence microscopy, indicating
successful transfection.
Tissue specimens
Patient specimens were obtained from the Department
of Obstetrics and Gynecology of Shandong Provincial Hospital
affiliated to Shandong University (Jinan, China) between June 2006
and June 2010. Patients were treated consecutively with
conventional radiotherapy and chemotherapy, as follows: Paclitaxel
(Bristol-Myers Squibb, New York, NY, USA) administered
intravenously at a dose of 175 mg/m2 over a period of 3
h on day 1 of a 21-day cycle, plus a carboplatin (Bristol-Myers
Squibb) dose of 360 mg/m2, also administered
intravenously on day 1 of the 21-day cycle, for 6 cycles. All
patients received regular follow-up. During the study period, there
were 9 patients who lost contact and 25 mortalities. The duration
of follow-up was 2–7 years by the end of 2012. The study was
approved by the Institutional Medical Ethics Committee of Shandong
University.
Blood samples
All of the participants provided written informed
consent indicating the willingness to donate their blood for
research. Blood samples were collected in
ethylenediaminetetraacetic acid (EDTA) tubes from the same 230
cervical cancer patients [60 cervical intraepithelial neoplasia
(CIN) cases, 140 squamous cell carcinoma cases and 30
adenocarcinoma cases]. The EDTA-plasma samples from 40 healthy
individuals were also obtained for comparison.
Enzyme-linked immunosorbent assay
(ELISA)
Sandwich ELISA (Human SPARC ELISA kit; #LS-F12653;
LifeSpan BioSciences, Inc., Seattle, WA, USA) was used to measure
the levels of SPARC in different serum samples. Serum was diluted
with enzyme immunoassay (EIA) buffer (containing 1% bovine serum
albumin and 0.05% Tween 20 in phosphate buffer) from the ELISA kit
and incubated for 2 h at 37°C. After 4 washes with EIA buffer,
mouse anti-human IgG horseradish peroxidase-conjugated antibodies
(1:2,500 dilution) from the ELISA kit were added and incubated for
30 min at 4°C. After a further 4 washes, 100 µl
tetramethylbenzidine solution (Sigma-Aldrich, St. Louis, MO, USA)
was added and incubated for 30 min at room temperature. The
reaction was subsequently stopped with 100 µl 1 N sulfuric acid
(Sigma-Aldrich). The experimental procedure was conducted according
to the instruction manual and the results were measured using a
SpectraMax® Plus 384 Microplate Reader (Molecular
Devices, LLC, Sunnyvale, CA, USA) at 450 nm.
Immunohistochemistry (IHC)
IHC experiments were performed according to standard
streptavidin-biotin-peroxidase complex procedures.
Paraffin-embedded, 5-µm thick sections were dewaxed in xylene,
rehydrated in ethanol and fixed in 4% paraformaldehyde for 30 min.
Antigen retrieval was then performed in 0.01 M citrate buffer at pH
6.0 for 15 min in a microwave oven. Paraffin, xylene, ethanol,
paraformaldehyde and citrate buffer were all purchased from
Sigma-Aldrich. The sections were incubated with goat polyclonal IgG
anti-human SPARC (dilution, 1:500; #AF941; R&D Systems, Inc.,
Minneapolis, MN, USA), rabbit polyclonal IgG anti-human E-cadherin
(dilution, 1:200; #sc-7870; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), goat polyclonal IgG anti-human N-cadherin (dilution,
1:200; #sc-31031; Santa Cruz Biotechnology, Inc.) and goat
polyclonal IgG anti-human vimentin (dilution, 1:200; #sc-7557;
Santa Cruz Biotechnology, Inc.) primary antibodies at 4°C
overnight. Subsequently, sections were incubated for 1 h at room
temperature with the following secondary antibodies: Mouse
anti-goat IgG-B (dilution, 1:500; #sc-2489; Santa Cruz
Biotechnology, Inc.) for sections incubated with the goat
polyclonal primary antibodies, and mouse anti-rabbit IgG-B
(dilution, 1:500; #sc-2491; Santa Cruz Biotechnology, Inc.) for
sections incubated with the rabbit polyclonal primary antibody. and
stained with the enzyme substrate 3′,3-diaminobenzidine
tetrahydrochloride (Sigma-Aldrich). Rabbit IgG (#sc-2027; Santa
Cruz Biotechnology, Inc.) was used as a negative control in the
place of the primary antibody. An inverted microscope (IV953;
Unico, Dayton, NJ, USA) was used to observe the sections. Brown
granules in the cytoplasm or stroma were considered to be positive
expression of SPARC.
IHC analysis
Immunohistochemical expression was evaluated using
Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA) to
detect photodensity. In brief, 5 positive fields within a section
were selected at random and read using Image-Pro Plus 6.0. The mean
densities were subsequently calculated. According to the intensity
and percentage of positive staining, a semi-quantitative scoring
system was used to evaluate SPARC expression (14). The staining intensity of SPARC was
scored as 0 (negative), 1 (weak), 2 (moderate) or 3 (strong); while
the percentage of positively stained cells was scored from 0 to 4
(score 0, 0% cells stained; score 1, 1–25%; score 2, 26–50%; score
3, 51–75%; or score 4, 76–100%). The final staining score (0–7) was
calculated by adding together the intensity and percentage scores,
and the scores of 0, 1–3, 4–5, and 6–7 were converted into the sum
indices -, +, ++ and +++, respectively. For statistical analysis,
low SPARC expression was defined as indices of - or +, while high
SPARC expression was considered to be indicated by indices of ++ or
+++. Each tissue section was independently analyzed by three
pathologists.
Reverse transcription
(RT)-quantitative polymerase chain reaction (qPCR)
Total RNA was extracted using Invitrogen™ Trizol
reagent (Thermo Fisher Scientific, Inc.) from human cervical cancer
tissues and subclone cells. RNA was reverse transcribed into cDNA
using the SuperScript® VILO™ cDNA Synthesis kit (Thermo
Fisher Scientific, Inc.) following the manufacturer's protocols.
The ABI PRISM 7500 Real-Time PCR System (Applied Biosystems Inc.;
Thermo Fisher Scientific, Inc.) was used to perform qPCR analysis;
a 20 µl reaction volume contained 10 µl Power SYBR Green PCR Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.), 1 µl of
each primer (5 µmol/l), 1 µl of cDNA template and 8 µl
DNAse/RNAse-free water (Sigma-Aldrich). The PCR cycle began with a
denaturation step (30 sec at 95°C), followed by 40 repeated cycles
of annealing/extension (5 sec at 95°C and 30 sec at 60°C. All
reactions were performed in triplicate. Specific primers were
designed by LightCycler® Probe Design software (Roche
Diagnostics, Basel, Switzerland) and synthesized by Takara
Biotechnology Co., Ltd. (Dalian, China). The sequences of primers
were as follows: N-cadherin forward, 5′-GTGCCATCATTGCCATCCTGCT-3′,
and reverse, 5′-CTGGTCTTCTTCTCCTCCACCTTCT-3′; vimentin forward,
5′-GGAAGGCGAGGAGAGCAGGATT-3′, and reverse,
5′-TTCAAGGTCATCGTGATGCTGAGAAG-3′; E-cadherin forward,
5′-GGATTGCAAATTCCTGCCATTC-3′, and reverse,
5′-AACGTTGTCCCGGGTGTCA-3′; SPARC forward,
5′-ACATAAGCCCAGTTCATCACCA-3′, and reverse,
5′-ACAACCGATTCACCAACTCCA-3′; β-actin forward,
5′-CCACGAAACTACCTTCAACTCCA-3′, and reverse,
5′-GTGATCTCCTTCTGCATCCTGTC-3′. The 2−ΔΔCq method
correlated with efficiency corrected normalized quantification
results, relative quantification of gene expression was obtained by
relative standard curves (15).
Western blot
Cells were washed twice with ice-cold
phosphate-buffered saline and then lysed on ice in
radioimmunoprecipitation assay buffer containing 1 mM
phenylmethylsulfonyl fluoride. Proteins (50 µg/lane) were resolved
by sodium dodecyl sulfate polyacrylamide gel electrophoresis,
transferred to polyvinyl difluoride membranes, and blocked with 5%
bovine serum albumin. The membranes were first incubated with the
aforementioned primary antibodies against SPARC, E-cadherin,
N-cadherin and vimentin at 1:1,000 dilutions overnight at 4°C, and
then incubated with bovine anti-goat IgG-AP (#sc-2351) and bovine
anti-rabbit IgG-AP (#sc-2372) secondary antibodies (dilution,
1:1,000; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Polyclonal goat IgG anti-human glyceraldehyde
3-phosphate dehydrogenase (dilution, 1:1,000; #sc-20357; Santa Cruz
Biotechnology, Inc.) was used as the control antibody incubated
overnight at 4°C. Blots were developed using the enhanced
chemiluminescence method (Pierce™ ECL Western Blotting Substrate;
Thermo Fisher Scientific, Inc.) and Gel-Pro 6.0 Analyzer Software
(Media Cybernetics, Inc., Rockville, MD, USA) was used to analyze
the intensity of the protein bands.
Statistical analysis
SPSS software version 13.0 (SPSS Inc., Chicago, IL,
USA) was used for statistical analysis. A χ2 test was
used to analyze IHC data. Measured data were recorded as the mean ±
standard error. A two-tailed t-test was used to compare the means
between two sets, and a one-way analysis of variation was used to
compare the means among three groups. Kaplan-Meier survival curves
were calculated and analyzed using the log-rank test. Using
Pearson's product-moment correlation coefficient, the associations
between SPARC and E-cadherin, N-cadherin and vimentin were
analyzed. Individual prognosis was defined by multivariate Cox
proportional hazard models. P<0.05 (two-sided) was considered to
indicate statistical significance.
Results
Expression of SPARC in human cervical
cancer tissues
SPARC expression in normal human cervical tissue and
CIN was very low compared with that in cervical carcinomas
(Fig. 1A and B). High SPARC
expression was detected in the cytoplasm of cancer cells and the
stroma of cervical carcinomas (Fig. 1C
and D). Furthermore, high SPARC expression was closely
associated with poor differentiation, advanced stage and lymph node
metastasis of cervical carcinomas (Table
I). Similar results were also obtained through RT-qPCR; high
SPARC mRNA expression was observed in cervical carcinoma, and was
closely associated with their progression (Table II).
 | Table I.Protein expression of SPARC in human
cervical tissues. |
Table I.
Protein expression of SPARC in human
cervical tissues.
|
|
| SPARC
lowa | SPARC
highb |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Variables | Total | n | % | n | % | χ2 | P-value |
|---|
| Normal | 40 | 39 | 97.5 | 1 | 2.5 | 88.9 | <0.010 |
| Cervical
intraepithelial neoplasia | 60 | 49 | 81.7 | 11 | 18.3 |
|
|
| Carcinoma | 170 | 50 | 29.4 | 120 | 70.6 |
|
|
| Pathology type |
|
|
|
|
| 0.65 | 0.421 |
| Squamous
cell carcinoma | 140 | 43 | 30.7 | 97 | 69.3 |
|
|
|
Adenocarcinoma | 30 | 7 | 23.3 | 23 | 76.7 |
|
|
| Cell
differentiation |
|
|
|
|
| 28.4 | <0.010 |
| High and
Medium | 89 | 42 | 47.2 | 47 | 52.8 |
|
|
| Low | 81 | 8 | 9.9 | 73 | 90.1 |
|
|
| Tumor stage |
|
|
|
|
| 32.6 | <0.010 |
| Stage
I | 60 | 32 | 53.3 | 28 | 46.7 |
|
|
| Stage
II | 59 | 16 | 27.1 | 43 | 72.9 |
|
|
| Stages
III and IV | 51 | 2 | 3.9 | 49 | 96.1 |
|
|
| Nodal status |
|
|
|
|
| 28.3 | <0.010 |
|
Positive | 66 | 4 | 6.1 | 62 | 93.9 |
|
|
|
Negative | 104 | 46 | 44.2 | 58 | 55.8 |
|
|
 | Table II.mRNA expression of SPARC in human
cervical tissues. |
Table II.
mRNA expression of SPARC in human
cervical tissues.
|
| n | SPARCa mRNA | P-value |
|---|
| Control | 40 | 0.0087±0.0028 |
|
| CIN | 60 | 0.0096±0.0034 | 0.168b |
| Carcinoma | 170 | 0.0897±0.0103 |
<0.010c |
| Pathology type |
|
| 0.804 |
|
Squamous cell carcinoma | 140 | 0.0786±0.0157 |
|
|
Adenocarcinoma | 30 | 0.0994±0.0172 |
|
| Cell
differentiation |
|
| <0.010 |
| High
and medium | 89 | 0.0173±0.0083 |
|
|
Low | 81 | 0.0985±0.0117 |
|
| Tumor stage |
|
| <0.010 |
| Stage
I | 76 | 0.0162±0.0076 |
|
| Stage
II | 81 | 0.0632±0.0094 |
|
| Stages
III and IV | 13 | 0.0983±0.0138 |
|
| Nodal status |
|
| <0.010 |
|
Positive | 66 | 0.0986±0.0154 |
|
|
Negative | 104 | 0.0173±0.0067 |
|
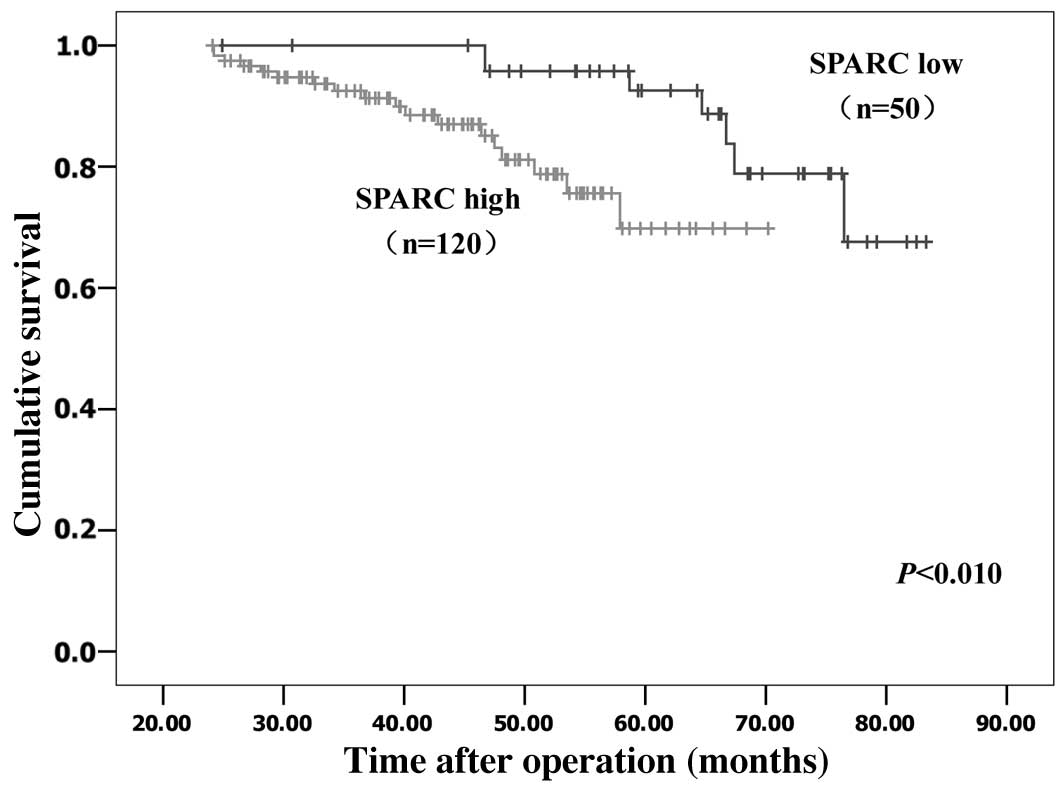
Kaplan-Meier survival analyses were performed to
evaluate the prognostic value of SPARC in cervical cancer. The
results revealed that patients with low SPARC expression had a
significantly more favorable prognosis than those with high SPARC
expression (log rank, P<0.01; Fig.
2). By multivariate analysis, considering all clinical and
pathological factors together, lymph node metastasis (P<0.001;
hazard ratio, 1.019), expression of SPARC (P<0.001; hazard
ratio, 4.225) and stage of tumor (P=0.005; hazard ratio, 1.930)
were significant prognostic factors (Table III).
 | Table III.Predictive factors of survival by
multivariate analysis (Cox proportional hazards model). |
Table III.
Predictive factors of survival by
multivariate analysis (Cox proportional hazards model).
| Prognostic
factor | Hazard ratio (95%
CI) | P-value |
|---|
| SPARC (low vs.
high) | 4.225
(2.325–7.678) | <0.001 |
| Pathology type (SCC
vs. ACA) | 0.978
(0.429–2.230) | 0.957 |
| Cell
differentiation (high + medium vs. low) | 0.999
(0.998–1.000) | 0.075 |
| Tumor stage (stage
III–IV vs. I–II) | 1.930
(1.218–3.059) | 0.005 |
| Lymph node
metastasis (positive vs. negative) | 1.019
(1.009–1.029) | <0.001 |
| Tumor size | 1.001
(0.996–1.005) | 0.771 |
| Age | 1.263
(0.593–2.687) | 0.545 |
Serum levels of SPARC in patients with
cervical neoplasia and healthy controls
The mean serum levels of SPARC in healthy controls
and CIN patients were markedly lower than that in patients with
cervical carcinoma (124.73±76.28 and 138.29±84.57 vs. 486.58±135.84
ng/ml; P<0.010; Table IV). There
was no significant difference between healthy controls and CIN
patients (P>0.05). Furthermore high SPARC serum levels were
closely associated with poor differentiation, advanced stage and
lymph node metastasis of cervical carcinomas (P<0.010). No
significant differences were found between different pathological
types of cervical cancer (P>0.05).
 | Table IV.Serum levels of SPARC in patients
with cervical tumors. |
Table IV.
Serum levels of SPARC in patients
with cervical tumors.
|
| n | SPARCa (ng/ml) | P-value |
|---|
| Control | 40 | 124.73±76.28 |
|
| CIN | 60 | 138.29±84.57 | 0.416b |
| Carcinoma | 170 | 486.58±135.84 |
<0.010c |
| Pathology type |
|
| 0.695 |
|
Squamous cell carcinoma | 140 | 463.74±136.22 |
|
|
Adenocarcinoma | 30 | 474.81±157.29 |
|
| Cell
differentiation |
|
| <0.010 |
| High
and medium | 89 | 143.61±83.64 |
|
|
Low | 81 | 496.73±132.78 |
|
| Tumor stage |
|
| <0.010 |
| Stage
I | 76 | 107.16±65.34 |
|
| Stage
II | 81 | 243.19±96.35 |
|
| Stages
III and IV | 13 | 483.27±147.36 |
|
| Nodal status |
|
| <0.010 |
|
Positive | 66 | 473.25±140.08 |
|
|
Negative | 104 | 132.91±86.59 |
|
Associations between SPARC and EMT
markers
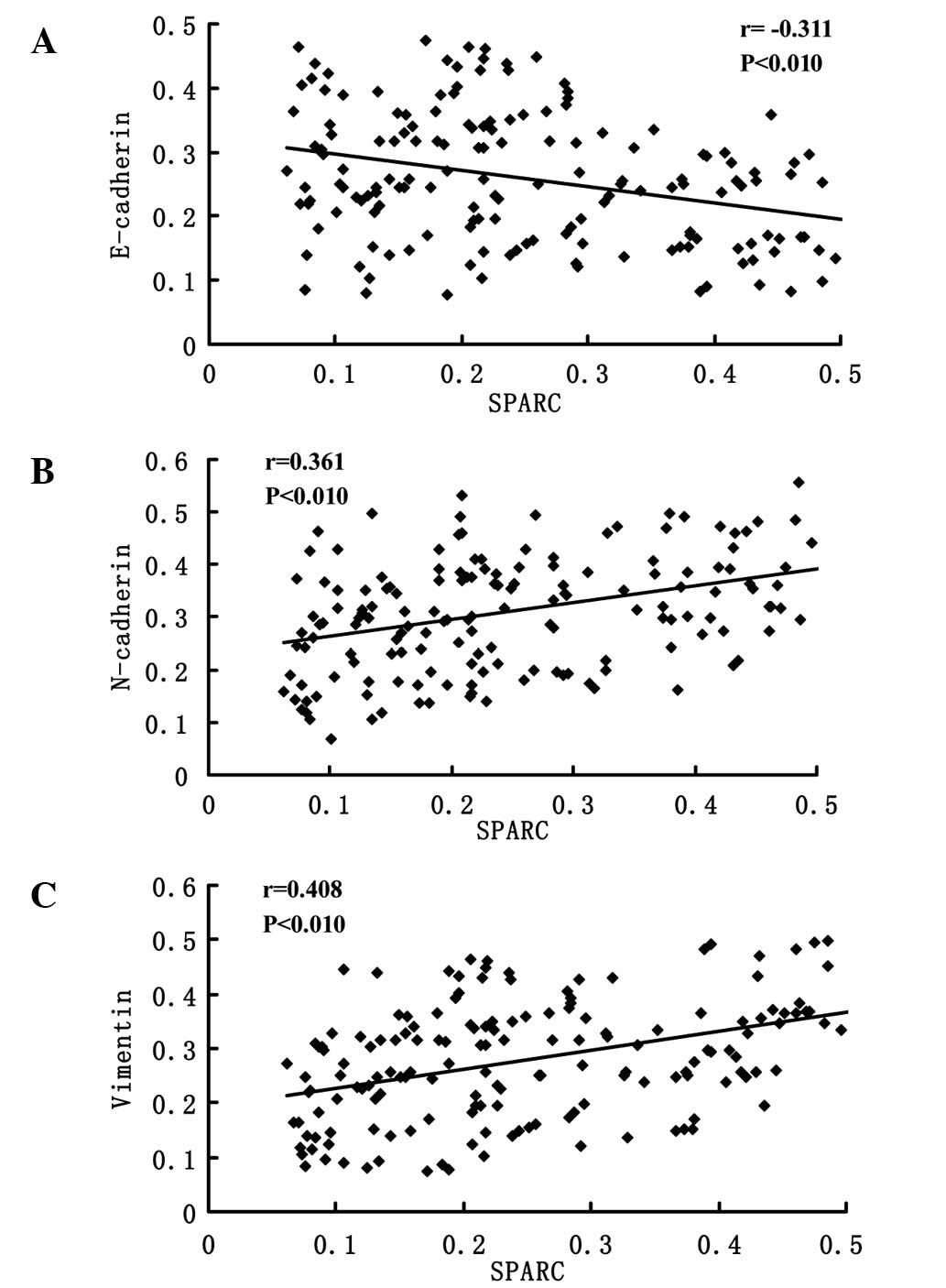
Representative IHC staining images for E-cadherin,
N-cadherin and vimentin expressions are presented in Fig. 3. The photo densities of IHC staining
images were evaluated by Image-Pro Plus 6.0. According to the
Pearson product-moment correlation coefficient, the expression of
E-cadherin and SPARC exhibited a negative correlation (r=−0.311,
P<0.01; Fig. 4A); however, the
expression of N-cadherin (r=0.361, P<0.01; Fig. 4B) and vimentin (r=0.408, P<0.01;
Fig. 4C) vs. SPARC exhibited strong
positive correlations.
 | Figure 3.Immunohistochemical staining of
E-cadherin, N-cadherin and vimentin. Immunohistochemical staining
of E-cadherin in (A) normal human cervical tissue, (B) CIN and (C)
cervical carcinoma (magnification, ×200). Immunohistochemical
staining of N-cadherin in (D) normal human cervical tissue
(magnification, ×100), (E) CIN (magnification, ×100) and (F)
cervical carcinoma (magnification, ×200). Immunohistochemical
staining of vimentin in (G) normal human cervical tissue
(magnification, ×100), (H) CIN (magnification, ×200) and (I)
cervical carcinoma (magnification, ×200). CIN, cervical
intraepithelial neoplasia. |
Effects of SPARC knockdown on
expression of EMT markers
The expression levels of three EMT-related proteins
(E-cadherin, N-cadherin and vimentin) were detected by RT-qPCR and
western blotting in SPARC shRNA-infected cells. As shown in
Fig. 5, E-cadherin was upregulated
and N-cadherin and vimentin were downregulated following SPARC
shRNA infection, in association with the knockdown of SPARC
expression.
 | Figure 5.Effects of SPARC knockdown on the
expression of E-cadherin, N-cadherin and vimentin. (A) Western blot
analysis measuring protein expression. The grey values of SPARC,
E-cadherin, N-cadherin and vimentin in SPARC shRNA-infected cells
were 0.26±0.07, 0.98±0.12, 0.36±0.04 and 0.31±0.06 respectively,
and the grey values of SPARC, E-cadherin, N-cadherin and vimentin
in control shRNA-infected cells were 0.95±0.09, 0.32±0.03,
0.97±0.11 and 0.92±0.08 respectively. There were significant
differences between SPARC shRNA infected cells and control
shRNA-infected cells (P<0.05). (B) Reverse
transcription-quantitative polymerase chain reaction relative to
βactin measuring mRNA expression. SPARC, N-cadherin and vimentin
mRNA expression in control shRNA-infected cells were 10.5±3.06,
13.4±2.56 and 11.6±2.85 times than that in SPARC shRNA infected
cells, however E-cadherin mRNA expression in SPARC shRNA infected
cells was 12.7±2.79 times than that in control shRNA-infected
cells. There were significant differences between SPARC shRNA
infected cells and control shRNA-infected cells (P<0.05). |
Discussion
To the best of our knowledge, the current study is
the first to demonstrate that the expression of SPARC is
significantly associated with poor clinicopathological
characteristics and poor prognosis in human cervical carcinoma
patients, and that it may have a role in the EMT of cervical cancer
cells.
IHC analyses in the present study revealed that
SPARC expression was significantly upregulated in cervical cancer
tissues compared with normal cervical tissues and CIN. RT-qPCR
experiments also confirmed that the mRNA expressions of SPARC was
upregulated in cervical carcinoma tissues. Furthermore, high mRNA
and protein expression levels of SPARC were associated with poor
tissue differentiation, advanced stage and lymph node metastasis in
cervical carcinomas. At present, few studies regarding SPARC and
cervical cancer are available. A genome-wide screening study
conducted by Sova et al in invasive cervical cancer showed
that SPARC was upregulated in multiple cervical cancer cell lines
and was aberrantly methylated; the aberrant methylation of SPARC
was also observed at a high proportion in invasive cervical cancer
clinical samples (16). In a previous
study, we established highly invasive subclones and subclones with
low invasiveness by the single cell cloning technology;
subsequently, we identified that the expression of SPARC in the
highly invasive subclones was much higher than that in those with
low invasiveness. SPARC downregulation significantly suppressed
cell proliferation, caused cell apoptosis, and inhibited cell
invasion and metastasis in cervical cancer. Considering all of
these findings together, we suggest that SPARC may promote the
progression of cervical cancer.
SPARC is widely expressed in cancer and regulates
cell survival, invasiveness and tumor-stroma interactions to
promote tumor progression (17). One
function of SPARC in carcinoma appears to be inhibiting the
expression of E-cadherin and promoting EMT (8). In melanoma, it was demonstrated that
SPARC could downregulate the expression of E-cadherin and
P-cadherin, induce the switch from E-cadherin to N-cadherin, and
enhance the expression of other extracellular proteins involved in
EMT, which contributed to the dissemination of melanoma (18,19). In
lung cancer, ectopic expression of SPARC was observed to induce EMT
with increased expression of vimentin and decreased expression of
E-cadherin (20). EMT is important in
the development of various types of epithelial cancer (21). Thus, the present study focused on
assessing the association between SPARC and three EMT-related
hallmarks, E-cadherin, N-cadherin and vimentin, in cervical cancer.
The results revealed that SPARC was inversely correlated with
E-cadherin and positively correlated with N-cadherin and vimentin;
knockdown of SPARC resulted in increased expression of E-cadherin
and reduced N-cadherin and vimentin expression. SPARC may promote
EMT-associated tumor invasion and contribute to cancer cell
metastasis in cervical cancer.
In summary, the present study demonstrated that the
expression of SPARC is associated with cancer cell invasion and
metastasis, and poor prognosis in cervical cancer patients. We
hypothesize that SPARC may be important during EMT. These
observations support our belief that SPARC is a promising
therapeutic target for the inhibition of metastasis and is a
prognostic biomarker for cervical cancer.
Acknowledgements
This study was supported by the China Postdoctoral
Science Foundation (no. 2014M551919). The funding organizations had
no role in the experimental design, data collection and analysis,
or the manuscript preparation.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waggoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Termine JD, Kleinman HK, Whitson SW, Conn
KM, McGarvey ML and Martin GR: Osteonectin, a bone-specific protein
linking mineral to collagen. Cell. 26:99–105. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bornstein P and Sage EH: Matricellular
proteins: Extracellular modulators of cell function. Curr Opin Cell
Biol. 14:608–616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsiao YH, Lien HC, Hwa HL, Kuo WH, Chang
KJ and Hsieh FJ: SPARC (osteonectin) in breast tumors of different
histologic types and its role in the outcome of invasive ductal
carcinoma. Breast J. 16:305–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Botti G, Scognamiglio G, Marra L, Collina
F, Di Bonito M, Cerrone M, Grilli B, Anniciello A, Franco R,
Fulciniti F, et al: SPARC/osteonectin is involved in metastatic
process to the lung during melanoma progression. Virchows Arch.
465:331–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seno T, Harada H, Kohno S, Teraoka M,
Inoue A and Ohnishi T: Downregulation of SPARC expression inhibits
cell migration and invasion in malignant gliomas. Int J Oncol.
34:707–715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng J and Tang L: SPARC in Tumor
Pathophysiology and as a Potential Therapeutic Target. Curr Pharm
Des. 20:6182–6190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang B, Chen K, Xu W, Chen D, Tang W and
Xia TS: Integrative genomic analyses of secreted protein acidic and
rich in cysteine and its role in cancer prediction. Mol Med Rep.
10:1461–1468. 2014.PubMed/NCBI
|
|
10
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steinestel K, Eder S, Schrader AJ and
Steinestel J: Clinical significance of epithelial-mesenchymal
transition. Clin Transl Med. 3:172014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thiery JP, Chua K, Sim WJ and Huang R:
Epithelial mesenchymal transition during development in fibrosis
and in the progression of carcinoma. Bull Cancer. 97:1285–1295.
2010.(In French). PubMed/NCBI
|
|
13
|
Chen J, Shi D, Liu X, Fang S, Zhang J and
Zhao Y: Targeting SPARC by lentivirus-mediated RNA interference
inhibits cervical cancer cell growth and metastasis. BMC Cancer.
12:4642012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soumaoro LT, Uetake H, Higuchi T, Takagi
Y, Enomoto M and Sugihara K: Cyclooxygenase-2 expression: A
significant prognostic indicator for patients with colorectal
cancer. Clin Cancer Res. 10:8465–8471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmittgen TD, Zakrajsek BA, Mills AG,
Gorn V, Singer MJ and Reed MW: Quantitative reverse
transcription-polymerase chain reaction to study mRNA decay:
Comparison of endpoint and real-time methods. Anal Biochem.
285:194–204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sova P, Feng Q, Geiss G, Wood T, Strauss
R, Rudolf V, Lieber A and Kiviat N: Discovery of novel methylation
biomarkers in cervical carcinoma by global demethylation and
microarray analysis. Cancer Epidemiol Biomarkers Prev. 15:114–123.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arnold SA and Brekken RA: SPARC: A
matricellular regulator of tumorigenesis. J Cell Commun Signal.
3:255–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Girotti MR, Fernández M, López JA,
Camafeita E, Fernández EA, Albar JP, Benedetti LG, Valacco MP,
Brekken RA, Podhajcer OL and Llera AS: SPARC promotes cathepsin
B-mediated melanoma invasiveness through a collagen I/α2β1 integrin
axis. J Invest Dermatol. 131:2438–2447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fenouille N, Tichet M, Dufies M, Pottier
A, Mogha A, Soo JK, Rocchi S, Mallavialle A, Galibert MD, Khammari
A, et al: The epithelial-mesenchymal transition (EMT) regulatory
factor SLUG (SNAI2) is a downstream target of SPARC and AKT in
promoting melanoma cell invasion. PLoS One. 7:e403782012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miao L, Wang Y, Xia H, Yao C, Cai H and
Song Y: SPOCK1 is a novel transforming growth factor-β target gene
that regulates lung cancer cell epithelial-mesenchymal transition.
Biochem Biophys Res Commun. 440:792–797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wendt MK, Balanis N, Carlin CR and
Schiemann WP: STAT3 and epithelial-mesenchymal transitions in
carcinomas. JAKSTAT. 3:e289752014.PubMed/NCBI
|