Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common type of non-Hodgkin lymphoma (NHL) accounting for ~30% of
all NHL cases (1). The addition of
rituximab to the chemotherapy regimen of cyclophosphamide,
doxorubicin, vincristine and prednisone (RCHOP) has significantly
improved the clinical outcome of patients with various forms of
indolent and aggressive CD20-positive NHL, including DLBCL
(2). The mechanisms of rituximab
action remain a matter for debate, but are considered to include
the antibody-dependent cellular cytotoxicity (ADCC) utilizing Fcγ
receptors (FcγR), complement-dependent cytotoxicity, and induction
of apoptosis in B lymphocytes (3).
Rituximab is a monoclonal antibody directed against
the CD20 antigen expressed on the surface of normal and malignant B
lymphocytes (4). Upon binding, it
bridges CD20-positive B lymphocytes with FcγR present on the
effector cells, such as natural killer cells and macrophages
(5). Several distinct FcγR classes
have been described. FcγRI, FcγRIIa, FcγRIIc and FcγRIIIa function
as activating receptors, while FcγRIIb and FcγRIIIb function as
inhibitory receptors (6).
It has been shown that polymorphisms in the
FcγRIIa and FcγRIIIa genes can impair binding of
rituximab to FcγR, thereby compromising its ADCC effects (5,7–9). Four polymorphisms in the FcγRIIa
and FcγRIIIa genes (FcγRIIa-27, FcγRIIa-131,
FcγRIIIa-48, FcγRIIIa-158) have garnered particular
attention and have been described to have variant allele present
with the frequency of >10% in Caucasians (10). Individuals homozygous for His at
FcγRIIa-131 show much more effective phagocytosis of
IgG-opsonized particles than those homozygous for Arg (7). Individuals homozygous for Val at
FcγRIIIa-158 demonstrate tighter FcγR binding to IgG
compared to those homozygous for Phe (8). The FcγRIIa-27 and
FcγRIIIa-48 substitutions, on the other hand, do not affect
the receptor affinity for IgG (8,11). Despite
FcγRIIa-27 and FcγRIIIa-48 polymorphisms being
nonfunctional, they are genetically linked to the
FcγRIIa-131 and FcγRIIIa-158 polymorphisms (10). Available data on the association
between polymorphisms in the FcγR gene and the clinical
outcome in various lymphoma types are inconclusive (12–16). The
objective of our prospective study was to investigate the
distribution of FcγRIIa and FcγRIIIa polymorphisms in
Slovene DLBCL patients. The present study aimed to evaluate the
impact of the 4 most common polymorphisms in the FcγRIIa and
FcγRIIIa genes on the response to RCHOP therapy in
previously untreated Slovenian DLBCL patients. Furthermore, the
association between FcγRIIa and FcγRIIIa
polymorphisms and survival outcomes, including overall survival
(OS) and progression free survival (PFS) were analyzed.
Materials and methods
Patient population
A total of 29 patients with newly diagnosed
CD20-positive DLBCL, who were treated at the Institute of Oncology
Ljubljana (Ljubljana, Slovenia) were enrolled in the study. All
patients received 8 cycles of RCHOP regimen every three weeks
according to the National guidelines for the treatment of NHL
(17). To minimize adverse drug
reactions associated with the treatment regimen, patients were
pre-medicated with clemastine, acetaminophen, and granisetron,
followed by rituximab at a dose of 375 mg/m2.
Subsequently, patients received chemotherapy regimen consisting of
cyclophosphamide, doxorubicin, vincristine and prednisone. Patient
exclusion criteria were as follows: History of central nervous
system lymphomatous disease, other malignancies, infections, or any
other medical condition that would preclude treatment according to
the protocol.
Clinical response was evaluated according to the
revised response criteria for malignant lymphoma proposed by
International Harmonization Project (18). Complete response (CR) was defined as a
complete absence of all detectable evidence of disease. Partial
response (PR) was defined as ≥50% decrease in the sum of the
products of the greatest diameters (SPD) of the six largest nodal
masses. Progressive disease (PD) was defined as ≥50% increase from
nadir in the SPD of any previously identified abnormal node or
appearance of new lesion. Stable disease (SD) was defined as less
than PR, but not PD. Overall response rate (ORR) was defined as the
proportion of patients with reduction in tumor burden (CR+PR).
The study was approved by the Institutional Review
Board at the Institute of Oncology Ljubljana (no. 03-Z/KSOPKR-22)
and National Medical Ethics Committee of Republic of Slovenia (no.
38/10/09). Written informed consent was obtained from all patients
prior to inclusion in the study.
FcγRIIa and FcγRIIIa genotyping
Genomic DNA was extracted using an innuPREP DNA
blood kit (Analytik Jena AG, Jena, Germany) according to the
manufacturer's instructions. The Gln to Trp substitution at
position 27 and the Arg to His substitution at position 131 of the
FcγRIIa gene were amplified using the following primers: Gln>Trp
27, F 5′-TGTAAAACGACGGCCAGTCCGCTGCAAGTACAGATCTT-3′ and R
5′-CAGGAAACAGCTATGACCTCCTCCACTGACCGGAAAGC-3′; Arg>His 131, F
5′-TGTAAAACGACGGCCAGTACGTACCTCTGAGACTGAAAA −3′ and 5′-
CAGGAAACAGCTATGACCATCTTGGCAGACTCCCCATA-3′ (10). EXPRESS SYBR® GreenER™ qPCR
SuperMix Universal was used for amplification of DNA sequences with
the following components: Master Mix, water, forward and reverse
primers and genomic DNA (ThermoFisher Scientific, Waltham,
Massachusetts, US). PCR conditions were as follows: 10 min of
initial denaturation at 95°C, followed by 45 cycles at 95°C for 45
sec, 65°C for 45 sec and 72°C for 55 sec. PCR purification was
performed using ExoSap IT (Affymetrix, Santa Clara, CA, USA).
Sequencing was performed using BigDye Terminator v3.1 Cycle
Sequencing Kit (ThermoFisher Scientific) with the following
conditions: Denaturation at 96°C for 1 min, followed by 30 cycles
at 96°C for 10 sec, 50°C for 5 sec and 60°C for 4 min. Sequence
clean-up was conducted using ethanol and EDTA, and sequences were
detected on an Analyzer 3500 (ThermoFisher Scientific).
The Leu to His or Arg substitution at position 48 of
the FcγRIIIa was amplified using the following primers: F
5′-TGTAAAACGACGGCCAGTCACCAAGCATGGGTTTGCAAT-3′and R
5′-CAGGAAACAGCTATGACCGCACCTGTACTCTCCACTGTCGTC-3′ (10). The PCR reaction, purification,
sequencing and detection were performed with the same reagents
under the same conditions as described above.
The Val to Phe substitution at position 158 of the
FcγRIIIa was amplified using the following primers: F
5′-ATATTTACAGAATGGCACAGG-3′ and R
5′-CAGGAAACAGCTATGACCCCAACTCAACTTCCCAGTGTGATT-3′ primers (10,19). The
LightCycler® 480 High Resolution Melting Master (Roche
Applied Science, Mannheim, Germany) was used for amplification of
specific DNA sequences with the following components: Master Mix,
MgCl2, water, forward and reverse primers and genomic
DNA. PCR conditions were as follows: 10 min of initial denaturation
at 95°C followed by 45 cycles at 95°C for 35 sec, 65°C for 55 sec
(decreased 0.5°C per cycle), and 72°C for 45 sec. PCR purification,
sequencing and detection was conducted in a similar manner as
described above.
Statistical analysis
Summary statistics were used to describe patients'
characteristics. Observed genotype frequencies were tested for
deviation from Hardy-Weinberg equilibrium with the Chi-square test.
Categorical variables were compared using the Chi-square test.
Survival rates according to FcγR polymorphisms were analyzed using
the Kaplan-Meier method and log-rank test was used to compare
survival between the groups. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using the SPSS Statistics 22.0 software package (IBM
SPSS, Armonk, New York, USA).
Results
Patient characteristics
The study population consisted of 13 women and 16
men of Caucasian ethnic origin with DLBCL. Baseline characteristic
of participants, including Ann Arbor clinical stage, International
prognostic Index (IPI), presence of bulky disease and irradiation
in first line therapy are presented in Table I. The median age at the start of
treatment was 62 years. A total of 19 patients were low to
low-intermediate risk group according to IPI, whereas 10 patients
had bulky disease. All patients were treated with RCHOP
chemotherapy. A total of 16 patients with residual disease were
additionally irradiated as a part of the first line treatment.
 | Table I.Patients' characteristics. |
Table I.
Patients' characteristics.
| Patients'
characteristics | Number of patients
(n=29) | % |
|---|
| Gender |
|
|
| Male | 16 | 55.2 |
|
Female | 13 | 44.8 |
| Age |
|
|
| >60
years | 17 | 58.6 |
| ≤60
years | 12 | 41.4 |
| Ann Arbour clinical
stage |
|
|
|
I–II | 11 | 37.9 |
|
III–IV | 18 | 62.1 |
| IPI |
|
|
|
0–2 | 19 | 65.5 |
|
3–5 | 10 | 34.5 |
| Bulky disease |
|
|
|
Yes | 10 | 65.5 |
| No | 19 | 34.5 |
| Irradiation first
line |
|
|
|
Yes | 16 | 55.2 |
| No | 13 | 44.8 |
| Response |
|
|
| CR | 25 | 86.2 |
| PR | 2 |
6.9 |
|
PD/SD | 2 |
6.9 |
| ORR
(CR+PR) | 27 | 93.1 |
FcγRIIa and FcγRIIIa genotype
frequencies
The genotype distribution of FcγRIIa and FcγRIIIa
alleles is presented in Table II.
There were no significant differences between genotype groups in
terms of gender, age, clinical stage, IPI and presence of bulky
disease. The observed genotype frequencies did not deviate from
those expected under the Hardy-Weinberg equilibrium
(P>0.05).
 | Table II.Genotype frequency. |
Table II.
Genotype frequency.
|
| Number of patients
(%) |
|---|
|
|
|
|---|
|
|
FcγRIIa-27 |
FcγRIIa-131 |
FcγRIIIa-48 |
FcγRIIIa-158 |
|---|
| Genotype | Gln | 23 (79.3%) | Arg | 5 (17.2%) | Leu | 24 (82.8%) | Val | 3 (10.3%) |
|
| Gln/Trp | 6 (20.7%) | Arg/His | 16 (55.2%) | Leu/His | 4 (13.8%) | Phe/Val | 16 (55.2%) |
|
| Trp | 0 (0.0%) | His | 8 (27.6%) | Leu/Arg | 1 (3.4%) | Phe | 10 (34.5%) |
Linkage disequilibrium between FcγRIIa-27 and
FcγRIIIa-48 alleles was observed. Patients homozygous for
Gln at FcγRIIa-27 were more likely to be homozygous for Leu
at FcγRIIIa-48 (P=0.03).
Response to frontline RCHOP
therapy
Response to frontline therapy was as follows: 25
patients achieved CR, 2 patients PR, while 2 patients had PD
(Table I). The ORR was 93%. No
significant differences were observed between different genotypes
and ORR. With the median follow-up time of 29.7 months (range
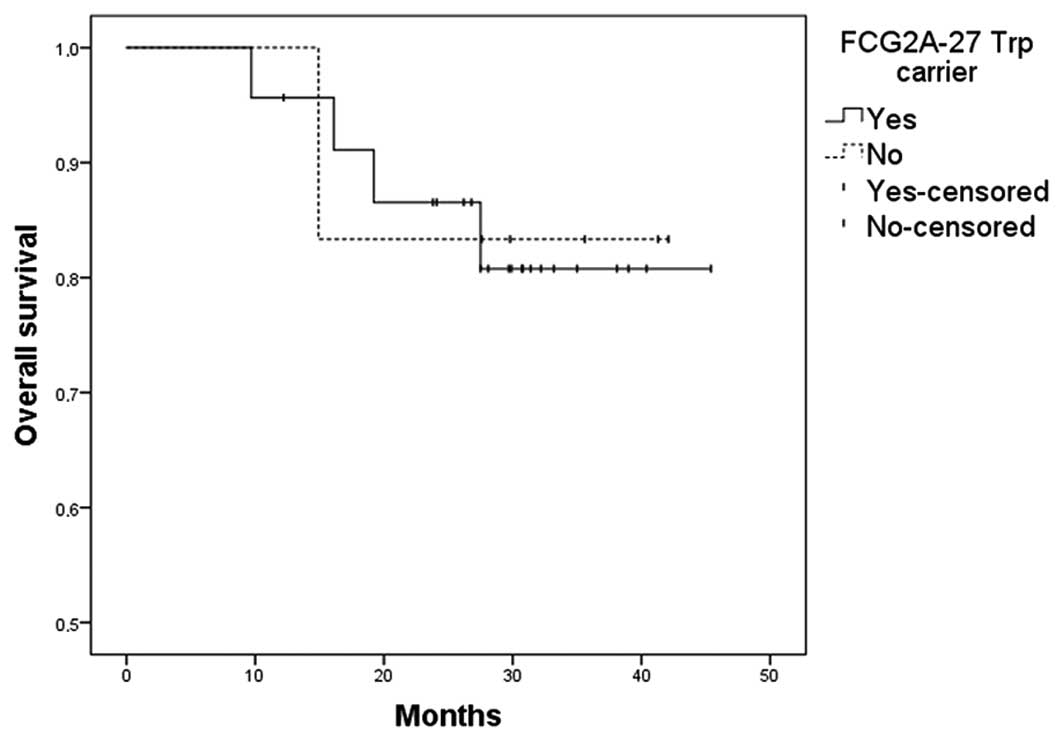
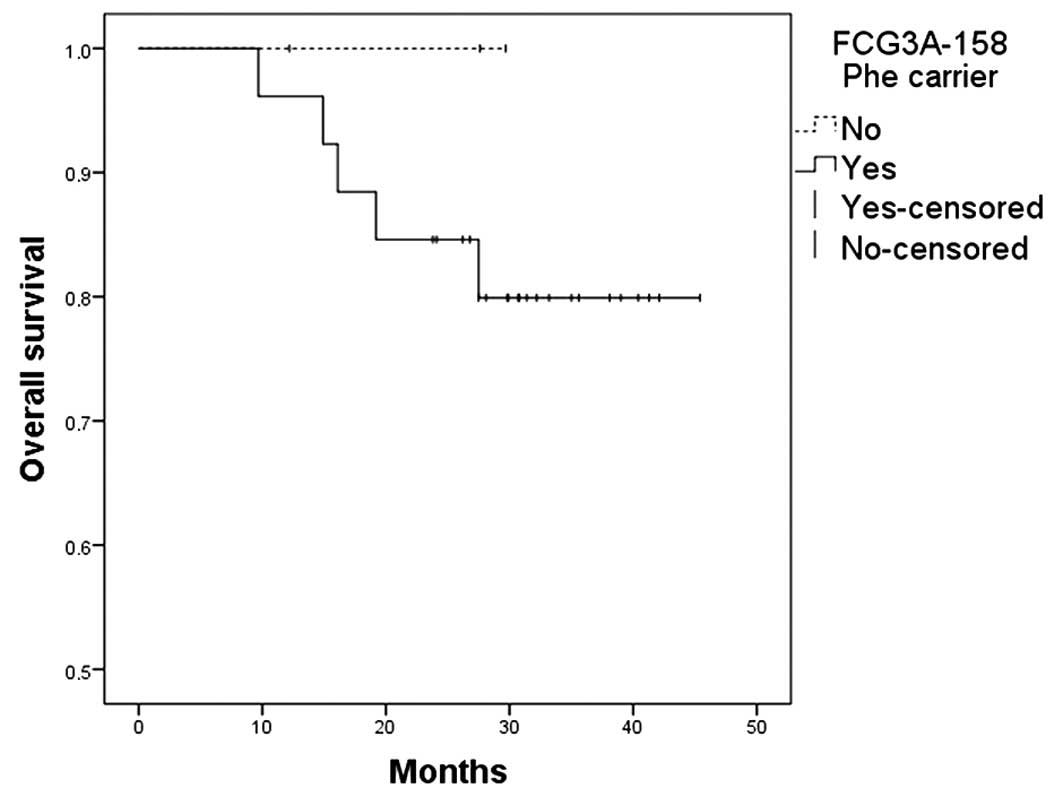
9.7–45.4), the OS and PFS were 83%. No significant differences were
observed among various genotypes and OS (Figs. 1–4) or
PFS (data not shown). At the end of follow-up, 5 patients have
died. Four of the patients had IPI 4 and one had bulky disease.
Patients with IPI equal or <3 were more likely to be alive at
the end of follow-up (P=0.001).
All patients (3/3) homozygous for Val at
FcγRIIIa-158 achieved CR without additional irradiation,
whereas only 41% (9/22) Phe carriers did so (Table III). The P-value for this comparison
was approaching statistical significance (P=0.096).
 | Table III.Irradiation and genotype frequency in
patients with complete response (n=25). |
Table III.
Irradiation and genotype frequency in
patients with complete response (n=25).
|
| CR without
irradiation | CR with
irradiation | P-value |
|---|
|
FcγRIIa-27 |
|
|
|
|
Gln | 10 | 10 | 0.541 |
|
Gln/Trp+Trp | 2 | 3 |
|
|
FcγRIIa-131 |
|
|
|
|
Arg | 2 | 2 | 0.672 |
| Arg/His
+ His | 10 | 11 |
|
|
FcγRIIIa-48 |
|
|
|
|
Leu | 11 | 10 | 0.328 |
|
Leu/His+Leu/Arg | 1 | 3 |
|
|
FcγRIIIa-158 |
|
|
|
|
Val | 3 | 0 | 0.096 |
|
Phe/Val+Phe | 9 | 13 |
|
Discussion
Rituximab has been integrated into routine clinical
practice in the treatment of different B-cell lymphomas (2). However, the response among lymphoma
types and among different patients within each type is highly
variable (15). Therefore, there is
an urgent need to identify patients who are likely to respond to
rituximab prior to the start of the treatment.
One of the potential mechanisms of rituximab
activity is ADCC via FcγR on effector cells. Accordingly, it has
been assumed that functional polymorphisms in the FcγRIIa
and FcγRIIIa genes may alter rituximab binding to effector
cells and thereby modify its antitumor effect in the DLBCL patients
(12,16). However, the clinical data supporting
this hypothesis are inconclusive. In a study of 113 patients of
Asian ethnic origin, patients homozygous for Val at
FcγRIIIa-158 had an improved outcome after RCHOP therapy
(16). Conversely, Ahlgrimm and
colleagues detected no statistically significant association
between the response to RCHOP therapy and FcγR alleles in
512 DLBCL Caucasian patients (12).
However, the authors did notice a trend toward superior CR rates
for carriers of Val at FcγRIIIa-158 (12). Three smaller European studies also
failed to detect a link between the RCHOP therapy and FcγR
alleles in DLBCL (20–22). In line with these findings, the
present study likewise reports no significant association between
response to RCHOP therapy and FcγR alleles. If any, there
was a trend for better response to chemotherapy without the need
for additional irradiation in patients homozygous for Val at
FcγRIIIa-158. The difference between the present study in
Caucasian patients and the study with Asian participants may be
partly explained by unidentified genetic differences between races
and a higher number of included patients by Kim and colleagues
(16).
Contrary to aggressive types of lymphoma, studies on
indolent lymphomas treated with rituximab monotherapy have shown
that patients homozygous for Val at FcγRIIIa-158 have better
responses than Phe carriers (13–15). Treon
and colleagues have also shown better responses in patients
carrying Leu and His at FcγRIIIa-48, whereas Weng and
colleagues observed better responses in patients homozygous for His
at FcγRIIa-131. The available evidence suggests that
polymorphisms in FcγR may affect response only when
rituximab is used as monotherapy, as in trials with indolent
lymphomas.
FcγRIIa and FcγRIIIa polymorphisms had
no impact on OS or PFS in the present study. A number of other
previous studies also failed to show significant difference in
survival in regard to FcγR genotypes, in both aggressive and
indolent lymphomas (12–14,16,20–22).
There is only one study in follicular lymphoma that reported
improved PFS in patients homozygous for Val at FcγRIIIa-158
and homozygous for His at FcγRIIa-131 (15).
Finally, no differences in patients' characteristics
or frequency of genotypes were observed in the present patient
population compared to other published data from Europe, Unites
States and Asia (10,16,23,24).
Genetic linkage between FcγRIIa-27 and FcγRIIIa-48
polymorphisms were noted. Genetic linkage among polymorphisms
within and between FcγRIIa and FcγRIIIa has also been
confirmed by Hatjiharissi and colleagues (10). In the present study, only a single
genetic linkage was observed, most likely due to the small number
of patients included, whereas Hatjiharissi et al (10) found extensive genetic linkage between
all four major polymorphisms of FcγRIIa and FcγRIIIa.
This may also provide an explanation for why some polymorphisms at
FcγRIIIa-48 are associated with a better clinical response
to rituximab despite the lack of impact on IgG1 binding, as seen in
the study of Treon and colleagues (14).
The present study has several limitations that merit
consideration. The sample size was quite modest and the follow-up
relatively short. In addition, ADCC may not be the predominant
mechanism of rituximab activity in DLBCL, so investigating
polymorphisms in genes implicated in other pathways of rituximab
activity may be necessary for reliable interpretation of
results.
In summary, the results of the present study
indicate that FcγRIIa and FcγRIIIa polymorphisms do
not impact the survival of DLBCL patients on RCHOP therapy. Due to
a trend for better response to RCHOP therapy in patients homozygous
for Val at FcγRIIIa-158, larger studies are needed to reach
a clear conclusion about the potential predictive and prognostic
value of FcγRIIa and FcγRIIIa polymorphisms.
Acknowledgements
This research was partially supported by Slovenian
Research Agency (Program P3-0321). The authors thank Dr JZ Cerne
for her critical review of the manuscript.
References
|
1
|
Tilly H, Vitolo U, Walewski J, da Silva
MG, Shpilberg O, André M, Pfreundschuh M and Dreyling M: ESMO
Guidelines Working Group: Diffuse large B-cell lymphoma (DLBCL):
ESMO Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 23(Suppl 7): vii78–vii82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molina A: A decade of rituximab: Improving
survival outcomes in non-Hodgkin's lymphoma. Annu Rev Med.
59:237–250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jazirehi AR and Bonavida B: Cellular and
molecular signal transduction pathways modulated by rituximab
(Rituxan, anti-CD20 mAb) in non-Hodgkin's lymphoma: Implications in
chemosensitization and therapeutic intervention. Oncogene.
24:2121–2143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Plosker GL and Figgitt DP: Rituximab: A
review of its use in non-Hodgkin's lymphoma and chronic lymphocytic
leukaemia. Drugs. 63:803–843. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhuang Y, Xu W, Shen Y and Li J: Fcγ
receptor polymorphisms and clinical efficacy of rituximab in
non-Hodgkin' lymphoma and chronic lymphocytic leukemia. Clin
Lymphoma Myeloma Leuk. 10:347–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ravetch JV and Bolland S: IgG Fc
receptors. Annu Rev Immunol. 19:275–290. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Parren PW, Warmerdam PA, Boeije LC, Arts
J, Westerdaal NA, Vlug A, Capel PJ, Aarden LA and van de Winkel JG:
On the interaction of IgG subclasses with the low affinity Fc gamma
RIIa (CD32) on human monocytes, neutrophils, and platelets.
Analysis of a functional polymorphism to human IgG2. J Clin Invest.
90:1537–1546. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koene HR, Kleijer M, Algra J, Roos D, von
dem Borne AE and de Haas M: Fc gammaRIIIa-158V/F polymorphism
influences the binding of IgG by natural killer cell Fc gammaRIIIa,
independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood.
90:1109–1114. 1997.PubMed/NCBI
|
|
9
|
de Haas M, Koene HR, Kleijer M, de Vries
E, Simsek S, van Tol MJ, Roos D and von dem Borne AE: A triallelic
Fc gamma receptor type IIIA polymorphism influences the binding of
human IgG by NK cell Fc gamma RIIIa. J Immunol. 156:2948–2955.
1996.PubMed/NCBI
|
|
10
|
Hatjiharissi E, Hansen M, Santos DD, Xu L,
Leleu X, Dimmock EW, Ho AW, Hunter ZR, Branagan AR, Patterson CJ,
et al: Genetic linkage of Fc gamma RIIa and Fc gamma RIIIa and
implications for their use in predicting clinical responses to
CD20-directed monoclonal antibody therapy. Clin Lymphoma Myeloma.
7:286–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Warmerdam PA, van de Winkel JG, Vlug A,
Westerdaal NA and Capel PJ: A single amino acid in the second
Ig-like domain of the human Fc gamma receptor II is critical for
human IgG2 binding. J Immunol. 147:1338–1343. 1991.PubMed/NCBI
|
|
12
|
Ahlgrimm M, Pfreundschuh M, Kreuz M,
Regitz E, Preuss KD and Bittenbring J: The impact of Fc-γ receptor
polymorphisms in elderly patients with diffuse large B-cell
lymphoma treated with CHOP with or without rituximab. Blood.
118:4657–4662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cartron G, Dacheux L, Salles G,
Solal-Celigny P, Bordos P, Colombat P and Watier H: Therapeutic
activity of humanized anti-CD20 monoclonal antibody and
polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood.
99:754–758. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Treon SP, Hansen M, Branagan AR, Verselis
S, Emmanouilides C, Kimbey E, Frankel SR, Touroutoglou N, Turnbull
B, Anderson KC, et al: Polymorphisms in FcgammaRIIIa (CD16)
receptor expression are associated with clinical response to
rituximab in Waldenström's macroglobulinaemia. J Clin Oncol.
23:474–481. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weng WK and Levy R: Two immunoglobulin G
fragment C receptor polymorphisms independently predict response to
rituximab in patients with follicular lymphoma. J Clin Oncol.
21:3940–3947. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim DW, Jung HD, Kim JG, Lee JJ, Yang DH,
Park YH, Do YR, Shin HJ, Kim MK, Hyun MS and Sohn SK: FCGR3A gene
polymorphisms may correlate with response to frontline R-CHOP
therapy for diffuse large B-cell lymphoma. Blood. 108:2720–2725.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Institute of Oncology Ljubljana
[Internet]. Ljubljana, Slovenia: Smernice za obravnavo bolnikov z
malignimi limfomi. https://www.onko-i.si/uploads/media/Doktrina_limfomi_2015.docAccessed.
Feb 11–2015
|
|
18
|
Cheson BD, Horning SJ, Coiffier B, Shipp
MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A,
Hagenbeek A, et al: Report of an international workshop to
standardize response criteria for non-Hodgkin's lymphomas. NCI
Sponsored International Working Group. J Clin Oncol.
17:12441999.PubMed/NCBI
|
|
19
|
Rohtagi S, Gohil S, Kuniholm MH, Schultz
H, Dufaud C, Armour KL, Badri S, Mailliard RB and Pirofski LA: Fc
gamma receptor 3A polymorphism and risk for HIV-associated
cryptococcal disease. MBio. 4:e00573–e00613. 2013.PubMed/NCBI
|
|
20
|
Mitrovic Z, Aurer I, Radman I, Ajdukoviç
R, Sertiç J and Labar B: FCgammaRIIIA and FCgammaRIIa polymorphisms
are not associated with response to rituximab and CHOP in patients
with diffuse large B-cell lymphoma. Haematologica. 92:998–999.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fabisiewicz A, Paszkiewicz-Kozik E,
Osowiecki M, Walewski J and Siedlecki JA: FcγRIIA and FcγRIIIA
polymorphisms do not influence survival and response to rituximab,
cyclophosphamide, doxorubicin, vincristine, and prednisone
immunochemotherapy in patients with diffuse large B-cell lymphoma.
Leuk Lymphoma. 52:1604–1606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Váróczy L, Zilahi E, Gyetvai A, Kajtár B,
Gergely L, Sipka S and Illés A: Fc-gamma-receptor IIIa polymorphism
and gene expression profile do not predict the prognosis in diffuse
large B-cell lymphoma treated with R-CHOP protocol. Pathol Oncol
Res. 18:43–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carlotti E, Palumbo GA, Oldani E, Tibullo
D, Salmoiraghi S, Rossi A, Golay J, Pulsoni A, Foà R and Rambaldi
A: FcgammaRIIIA and FcgammaRIIA polymorphisms do not predict
clinical outcome of follicular non-Hodgkin's lymphoma patients
treated with sequential CHOP and rituximab. Haematologica.
92:1127–1130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hurvitz SA, Betting DJ, Stern HM, Quinaux
E, Stinson J, Seshagiri S, Zhao Y, Buyse M, Mackey J, Driga A, et
al: Analysis of Fcγ receptor IIIa and IIa polymorphisms: Lack of
correlation with outcome in trastuzumab-treated breast cancer
patients. Clin Cancer Res. 18:3478–3486. 2012. View Article : Google Scholar : PubMed/NCBI
|