Introduction
IgG4-related disease (IgG4-RD) is a newly recognized
clinical condition, which was initially described in 2003 as the
cause of type 1 autoimmune pancreatitis (1). IgG4-related inflammatory pseudotumors
are part of the spectrum of systemic IgG4-RD, which is
characterized by diffuse organ swelling or mass formation, a dense
lymphoplasmacytic infiltrate rich in IgG4-positive plasma cells
with fibrosis and typically an increased serum IgG4 concentration
(2). The most common organs involved
in IgG4-RD include the pancreas, biliary duct, salivary gland,
ocular and lymph nodes, although it has been reported that
IgG4-related inflammatory pseudotumors occur in a range of organs
(3,4).
Renal involvement is uncommon (5). To
the best of our knowledge, <5 cases of IgG4-related renal
inflammatory pseudotumors have been reported in the English
literature (5). The present report
describes a case of IgG4-related inflammatory pseudotumor of the
kidney mimicking renal malignant carcinoma. Written informed
consent was obtained from the patient.
Case report
An 80-year-old woman was referred to Peking Union
Medical College Hospital (Beijing, China) with a low echo-level
lesion in the left kidney, which was identified incidentally by
abdominal ultrasonography during a routine medical examination. The
patient's previous medical history included well-controlled
hypertension and asthma, and her medications included amlodipine
besylate and an inhaler for asthma. The physical examination and
laboratory investigations were normal. Serum creatinine
concentration was 63 µmol/l (normal range, 40–84 µmol/l) and
glomerular filtration rate was 91.1 ml/min/1.73 m2
(normal range, 90–120 ml/min/1.73 m2; 35.9 ml/min/1.73
m2 in left kidney; 55.2 ml/min/1.73 m2 in
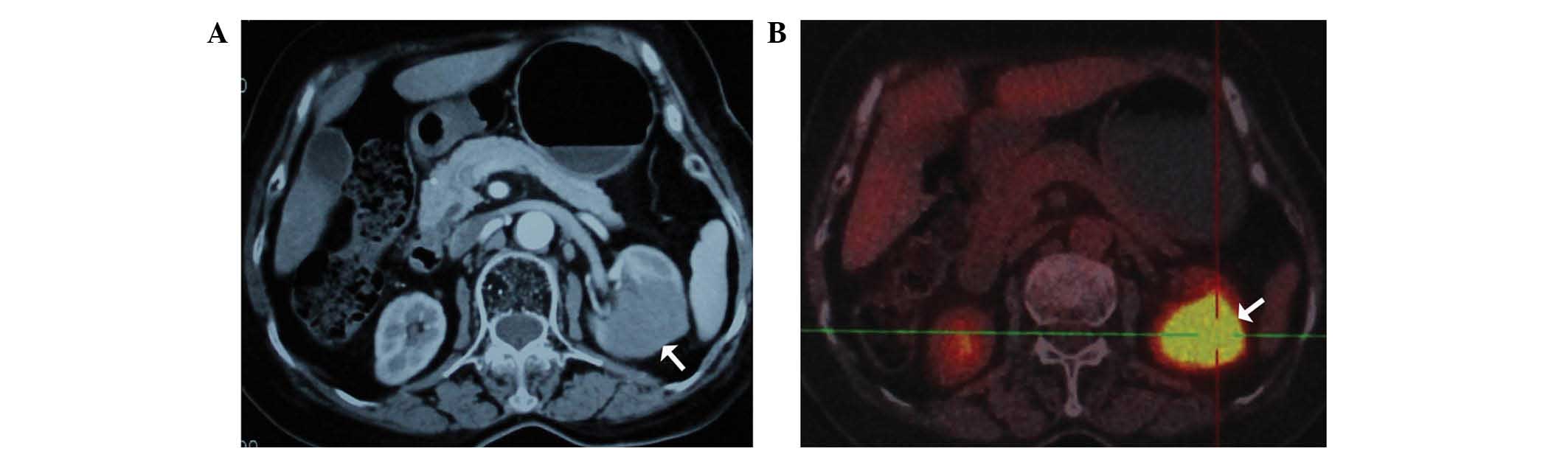
right kidney). Computed tomography (CT; SOMATOM Sensation; Siemens
Healthcare, Erlangen, Germany) revealed a mass of 3.9×3.7 cm
located at the inferior portion of the left kidney, with contrast
enhancement, which suggested a malignant tumor (Fig. 1A). Subsequent positron emission
tomography (PET)-CT (Biograph mCT; Siemens Healthcare) revealed
increased 18F-fluorodeoxyglucose uptake of the renal
mass (Fig. 1B). Based on the clinical
and radiological findings, renal cell carcinoma was diagnosed. A
retroperitoneoscopic left radical nephrectomy was performed under
the diagnosis of renal cell carcinoma.
Gross examination revealed a 5.0×3.5 cm-sized white
homogenous mass that was confined to the kidney (Fig. 2A). Resected tissues were fixed in 10%
neutral-buffered formalin (Beijing Dingguo Changsheng Biotechnology
Co., Ltd., Beijing, China), paraffin (Beijing Dingguo Changsheng
Biotechnology Co., Ltd.)-embedded and cut into 5-mm sections. The
sections were then deparaffinized in xylene, rehydrated in an
ethanol series and treated with trypsin (Beijing Dingguo Changsheng
Biotechnology Co., Ltd.) and proteases (Beijing Dingguo Changsheng
Biotechnology Co., Ltd.). Hematoyxlin and eosin (Beijing Dingguo
Changsheng Biotechnology Co., Ltd.) staining was used to observe
morphological changes under a microscope (TS100; Nikon Corporation,
Tokyo, Japan). Tissue sections were incubated with monoclonal mouse
anti-human CD21 (1:100; cat. no. sc-13135; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), monoclonal mouse anti-human
CD3 (1:100; cat. no. sc-20047; Santa Cruz Biotechnology, Inc.),
polyclonal goat anti-human CD34 (1:100; cat. no. sc-7045; Santa
Cruz Biotechnology, Inc.), monoclonal mouse anti-human CD79 (1:100;
cat. no. sc-390372; Santa Cruz Biotechnology, Inc.), monoclonal
mouse anti-human S-100 (1:50; cat. no. sc-53438; Santa Cruz
Biotechnology, Inc.), polyclonal goat anti-mouse Ki-67 (1:50; cat.
no. sc-7846; Santa Cruz Biotechnology, Inc.), polyclonal goat
anti-human IgG (1:50; cat. no. sc-34665; Santa Cruz Biotechnology,
Inc.) and monoclonal mouse anti-human IgG4 (1:50; cat. no.
sc-69919; Santa Cruz Biotechnology, Inc.) primary antibodies at
room temperature for 1 h. The sections were then washed in
phosphate-buffered saline (Beijing Dingguo Changsheng Biotechnology
Co., Ltd.) and incubated with fluorescein isothiocyanate-conjugated
donkey anti-goat IgG (1:240; cat. no. sc-2024; Santa Cruz
Biotechnology, Inc.) or horseradish peroxidase-conjugated goat
anti-mouse IgG (1:200; cat. no. sc-2005; Santa Cruz Biotechnology,
Inc.) secondary antibodies at room temperature for 30 min. Next,
sections were stained with ABC solution ((Beijing Dingguo
Changsheng Biotechnology Co., Ltd.) and 3,3′-diaminobenzidine
(Dako, Carpinteria, CA, USA) and counterstained with Mayer's
hematoxylin (Beijing Dingguo Changsheng Biotechnology Co., Ltd.).
Staining was visualized using a microscope (TS100; Nikon
Corporation). Microscopic examination revealed diffuse
lymphoplasmacytic infiltration with myofibroblastic proliferation
(Fig. 2B). Immunohistochemical
staining was positive for anaplastic lymphoma kinase, B-cell
lymphoma 2, cluster of differentiation (CD)21, CD3, CD34
(vascular), CD79, S-100, Ki-67, immunoglobulin (Ig)G and the
IgG4/IgG-positive cell ratio was >40% (Fig. 2C and D). Immunohistochemical staining
was negative for desmin, epithelial membrane antigen and smooth
muscle actin. The histological and immunohistochemical findings
were interpreted to indicate an IgG4-related inflammatory
pseudotumor. Serum IgG4 levels were normal (69.2 mg/dl; normal
range, 4.8–105 mg/dl) at two weeks subsequent to surgery. The
patient outcome was positive, and there was no evidence of IgG4-RD
at 3 months post-surgery. The patient did not undergo any
additional therapy.
Discussion
IgG4-RD is a rare systemic disorder that typically
affects middle-aged men, which may involve the pancreas, bile duct,
salivary gland, retroperitoneum, ureter, kidney, adrenal gland and
prostate, as well as other organs (2). More than 90% of patients with
IgG4-related renal disease have a history of prior extrarenal
lesions, including pancreatitis, retroperitoneal fibrosis and
sialadenitis (5). In the kidney, the
most common form of lesion is tubulointerstitial nephritis;
however, various glomerular lesions, in particular membranous
glomerulonephritis, may develop simultaneously with
tubulointerstitial nephritis (5).
IgG4-RD is very rarely observed in the form of an inflammatory
pseudotumor of the kidney.
Pathophysiologically, a dense lymphoplasmacytic
infiltrate with an increased number of IgG4-positive plasma cells
and fibrosis is a key characteristic in IgG4-RD (2). However, the role of IgG4-positive cells
remains to be elucidated in IgG4-RD. Approximately 80% of
IgG4-related kidney disease patients demonstrate an increased serum
IgG4 concentration (≥135 mg/dl; normal range, 4.8–105.0 mg/dl). In
addition, patients with IgG4-related renal disease typically
exhibit hypergammaglobulinemia, hypocomplementemia or mild renal
dysfunction (5,6).
It is difficult to discriminate between IgG4-related
renal inflammatory pseudotumor and malignant lesions based on
imaging studies (5). Abdominal
ultrasonography may reveal low echoic lesions or parenchymal
swelling, and contrast-enhanced CT frequently reveals isolated or
multiple low-density lesions (7). On
magnetic resonance imaging (MRI), renal masses demonstrate iso- or
hypointensity on T1-weighted images and hypointensity on
T2-weighted images, and demonstrate mild contrast enhancement
following contrast medium administration (8). The present case was initially approached
as renal cell carcinoma due to ultrasound, contrast-enhanced CT and
PET-CT findings. To the best of our knowledge, the present case
report is the first to include ultrasound, contrast-enhanced CT and
positron emission tomography findings to reveal apparent renal cell
tumor-like lesions.
Corticosteroids are the first line treatment regimen
for IgG4-RD, although no controlled trial has been performed and
the treatment protocol has varied among countries or institutions
(9). In the few previous reports of
renal involvement with IgG4-RD, the renal lesions have been
observed to decrease in size following corticosteroid therapy, with
some disappearing and others recurring subsequent to cessation of
treatment (10–12). Furthermore, corticosteroids have been
reported to improve renal function and decease serum IgG4 levels
after 1 month of therapy; however, renal function may not be
totally recovered (10).
The present report described a case of IgG4-related
inflammatory pseudotumor of the kidney that was distinguished from
a renal malignant tumor. If a subperiosteal tumor-like mass is
observed, it is important to consider the possibility of an
IgG4-related inflammatory pseudotumor. If the present lesion had
been diagnosed as an IgG4-related renal inflammatory pseudotumor
prior to surgery, it may have been appropriate to perform a needle
biopsy and administer steroid therapy. The present case highlights
the importance of recognizing that IgG4-related diseases may
involve the kidney and may subsequently result in the development
of masses that simulate a renal cell carcinoma.
References
|
1
|
Kamisawa T, Funata N, Hayashi Y, Eishi Y,
Koike M, Tsuruta K, Okamoto A, Egawa N and Nakajima H: A new
clinicopathological entity of IgG4-related autoimmune disease. J
Gastroenterol. 38:982–984. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stone JH, Zen Y and Deshpande V:
IgG4-related disease. N Engl J Med. 366:539–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamoto H, Yamaguchi H, Aishima S, Oda Y,
Kohashi K, Oshiro Y and Tsuneyoshi M: Inflammatory myofibroblastic
tumor versus IgG4-related sclerosing disease and inflammatory
pseudotumor: A comparative clinicopathologic study. Am J Surg
Pathol. 33:1330–1340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moriarty MA, Dahmoush L and Nepple KG:
IgG4 related disease of the ureter (inflammatory pseudotumor). J
Urol. 191:1126–1127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saeki T and Kawano M: IgG4-related kidney
disease. Kidney Int. 85:251–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsubata Y, Akiyama F, Oya T, Ajiro J,
Saeki T, Nishi S and Narita I: IgG4-related chronic
tubulointerstitial nephritis without autoimmune pancreatitis and
the time course of renal function. Intern Med. 49:1593–1598. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuroda N, Nao T, Fukuhara H, Karashima T,
Inoue K, Taniguchi Y, Takeuchi M, Zen Y, Sato Y, Notohara K and
Yoshino T: IgG4-related renal disease: Clinical and pathological
characteristics. Int J Clin Exp Pathol. 7:6379–6385.
2014.PubMed/NCBI
|
|
8
|
Tan TJ, Ng YL, Tan D, Fong WS and Low AS:
Extrapancreatic findings of IgG4-related disease. Clin Radiol.
69:209–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kamisawa T, Okazaki K, Kawa S, Shimosegawa
T and Tanaka M: Research Committee for Intractable Pancreatic
Disease and Japan Pancreas Society: Japanese consensus guidelines
for management of autoimmune pancreatitis: III. Treatment and
prognosis of AIP. J Gastroenterol. 45:471–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saeki T, Kawano M, Mizushima I, amamoto M,
Wada Y, Nakashima H, Homma N, Tsubata Y, Takahashi H, Ito T, et al:
The clinical course of patients with IgG4-related kidney disease.
Kidney Int. 84:826–833. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizushima I, Yamada K, Fujii H, Inoue D,
Umehara H, Yamagishi M, Yamaguchi Y, Nagata M, Matsumura M and
Kawano M: Clinical and histological changes associated with
corticosteroid therapy in IgG4-related tubulointerstitial
nephritis. Mod Rheumatol. 22:859–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dhobale S, Bedetti C, Killian P, Ilyas M,
Liput J, Jasnosz K and Giclas P: IgG4 related sclerosing disease
with multiple organ involvements and response to corticosteroid
treatment. J Clin Rheumatol. 15:354–357. 2009. View Article : Google Scholar : PubMed/NCBI
|