Introduction
Gastrointestinal stromal tumors (GIST) are
mesenchymal neoplasms of the gastrointestinal (GI) tract, the
incidence of which is low 10–20 per 100,000 individuals in China
(1,2).
The mainstay of treatment is resection, and anticancer and
biological therapy may be used in cases of extensive metastasis.
One of the characteristic features of GISTs is the expression of
CD117, a c-Kit proto-oncogene protein. The tumors most commonly
occur in the GI tract from the esophagus to the anus (3–5); however,
tumors expressing CD117 occasionally arise in locations adjacent
to, but outside of, the GI tract. GISTs have been reported in the
omentum, mesentery, gallbladder, prostate and retroperitoneum
(6–9).
GISTs arising outside of the GI track are classified as
extra-gastrointestinal stromal tumors (EGISTs) (9). EGIST is rare, with an estimated
incidence of 1.5–6.0% worldwide (1,2). However,
due to the rarity of EGIST, mortality rates remain unclear. EGIST
refers to tumors derived from the omentum, mesentery or
retroperitoneal soft tissue; however, these tumors originate in the
stroma and possess no definitive type of cell differentiation, and
are not associated with intestinal and digestive tract serosa
(7). EGIST has been reported in the
omentum, mesentery, bladder, gallbladder, pancreas, uterus and
retroperitoneum (1,6,7,10). The treatment for EGISTs is resection,
if possible. Primary pancreatic EGISTs are rare in the clinic and
in the literature, with only ~20 cases reported to date (11–28).
Similarly, hepatic EGIST is also rare (29–31). The
occurrence of a primary pancreatic EGIST accompanied by a primary
hepatic EGIST in the same patient is, therefore, considered to be
an extremely rare event. The present study reports a case of
primary pancreatic EGIST accompanied by an apparent primary hepatic
EGIST.
Case report
A 56-year-old female patient was admitted to The
First Hospital of Jilin University (Changchun, China) in July 2014,
10 days after solid lesions were detected in the pancreas and liver
during a routine physical examination at the same hospital.
Findings from general examination upon admission included: No skin
or sclera jaundice, abdominal pain, bloating, nausea, vomiting,
discomfort or palpable abdominal mass. There was also no history of
pancreatitis or abdominal trauma. A 5.2×3-cm hypoechoic lesion was
detected in the pancreatic body and a 2.4×1.7-cm hypoechoic lesion
was detected in the left hepatic lobe by ultrasound (iU Elite;
Philips Healthcare, Andover, MA, USA) Fig. 1A and B, respectively), indicating the
existence of space-occupying lesions in the pancreas and liver. To
further characterize the lesions following ultrasound, the patient
underwent analysis with contrast-enhanced ultrasonography (CEUS),
using SonoVue ultrasound contrast agent (Bracco, Milan, Italy) and
color Doppler diagnostic apparatus (model LOGIQ E9; GE Healthcare,
Pittsburg, PA, USA). The lesion at the pancreatic body was highly
enhanced in the arterial phase by CEUS and a washout of this
enhancement was observed in the venous phase (Fig. 2A). The lesion at the left lobe of
liver was quickly and highly enhanced at the periphery of the
lesion (rim enhancement) in the arterial phase, but it began to
fade in the venous phase (Fig. 2B).
The washout of the enhancement in the liver lesion occurred
considerably earlier than in the surrounding hepatic parenchyma.
The CEUS results suggested that the lesion at the pancreatic body
was a primary malignant tumor, while the lesion in the left hepatic
lobe was likely a metastatic malignant tumor. Plain and enhanced
computed tomography (CT) scans were subsequently performed by
intravenous injection with the contrast agent iopromide (Bayer
Healthcare, Whippany, NJ, USA) and scanning with a dual-source
64-slice SOMATOM Definition AS (Siemens AG, Berlin, Germany). Plain
and enhanced CT scanning detected a 5.7×2.7-cm visible low-density
lesion in the pancreatic body, and the image was intensified
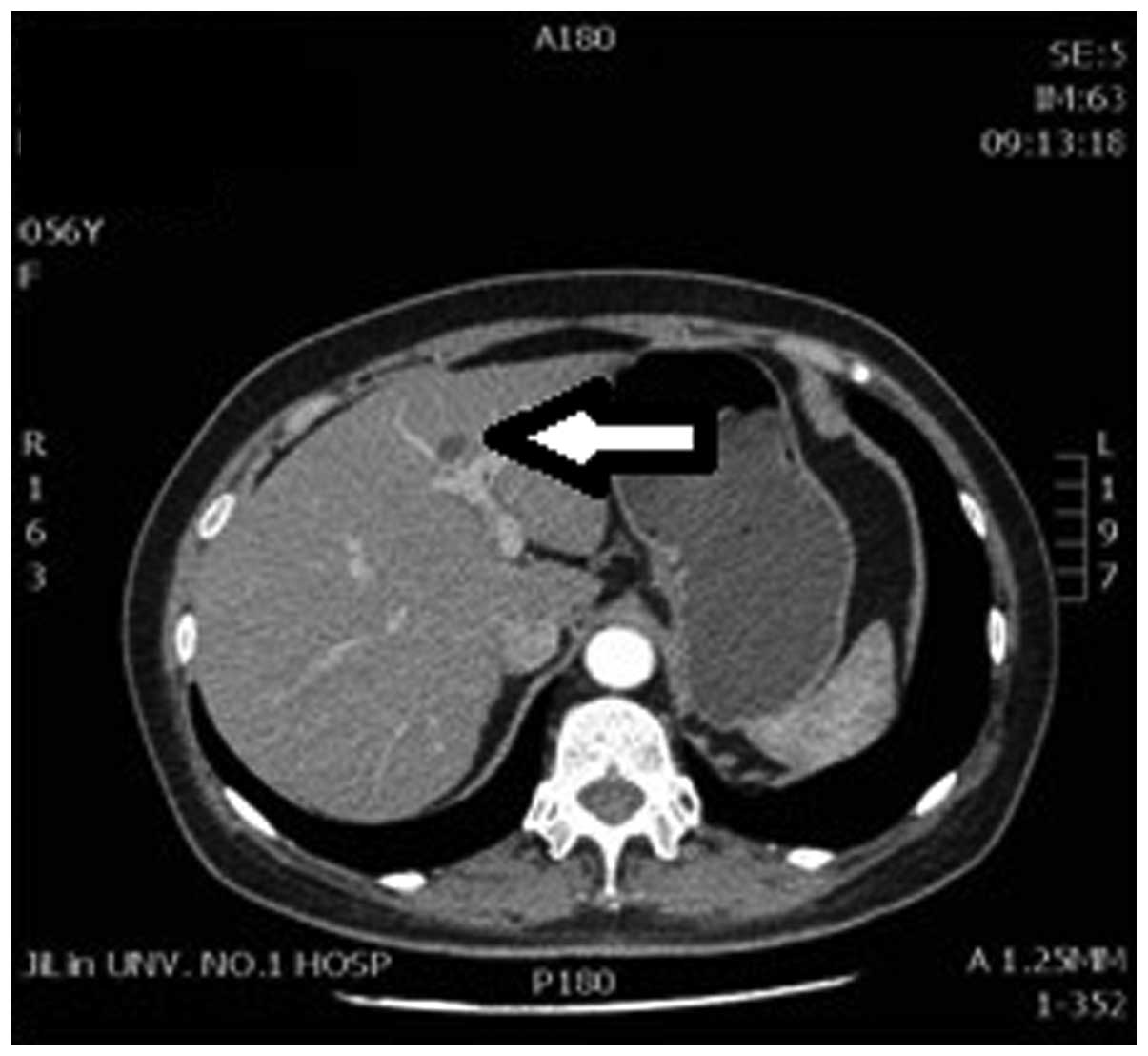
relatively poorly on the enhanced scan (Fig. 3). The plain CT also revealed a 2.2-cm
diameter low-density lesion close to the top of the left hepatic
lobe near the diaphragm, and a slight edge enhancement was observed
on the enhanced scan (Fig. 4). The CT
scan results suggested a pancreatic cancer accompanied by a
low-density lesion in the liver, and could not rule out the
possibility of hepatic metastasis. The clinical diagnosis,
therefore, was malignant pancreatic cancer with hepatic
metastases.
Exploratory laparotomy was subsequently performed.
Tumors measuring ~4.5×2.5×2.0 cm and ~2.0×1.5×1.5 cm were
identified in the pancreatic body and the left hepatic lateral
lobe, respectively, during the probing procedure. Lesion cells were
collected by fine needle aspiration and cytological examination
revealed atypical cells, with an increased cell volume and enlarged
and mitotic nuclei, suggesting the possibility of malignant
pancreatic and hepatic tumors. Following receipt of written
informed consent from the patient's family, tumor biopsy procedures
for pathological diagnosis were conducted. Biopsy tissues were
fixed in 10% neutral buffered formalin (Fuzhou Maixin Biotech Co.,
Ltd., Fuzhou, China), embedded in paraffin (Leica Microsystems,
Wetzlar, Germany) and sectioned (3–5 µm thickness; model no., 2235;
Leica Microsystems). The sections were stained with hematoxylin and
eosin (Fuzhou Maixin Biotech Co., Ltd.) and observed under a
microscope (Olympus BX51; Olympus Corporation, Tokyo, Japan).
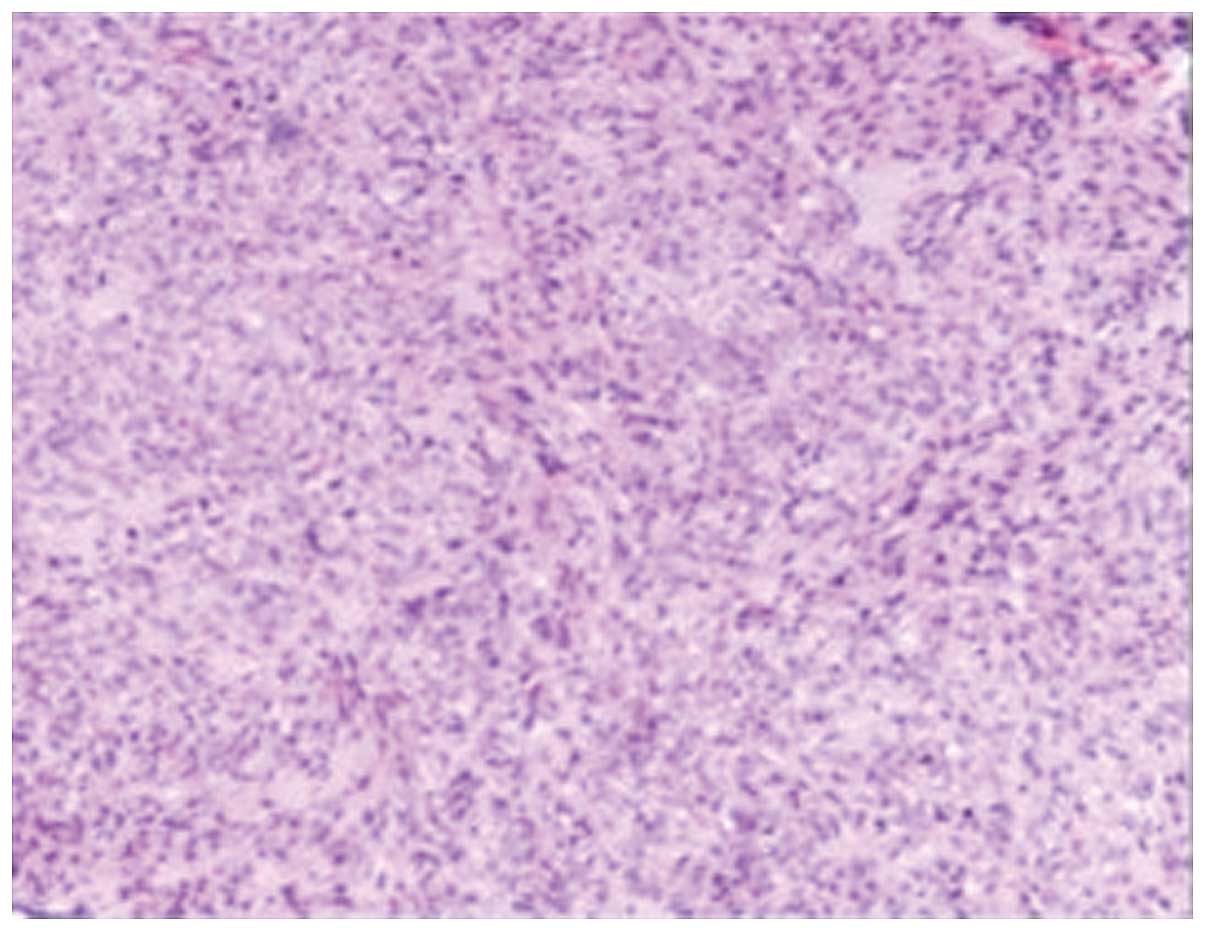
Pathological findings in the hepatic and pancreatic biopsy tissues
indicated that the tumors were mitotic spindle cell tumors with
mitotic activity of 1–2 per 50 high-power fields (HPF; Fig. 5). The stromal tumor diagnosis was
further supported by the following immunohistochemical staining
results in liver and pancreas tissues: Vimentin (+), CD117 (+),
discovered on GIST-1 (+), Ki-67 (60% +), S-100 (−), CD34 (−),
cytokeratin (−), smooth muscle actin (−), Des (−) and epithelial
membrane antigen (−). Each tumor was treated separately. On July 9,
2014, 42 125Iodine radioactive particles (half life, 2
months; energy/particle, 28.37 keV; Shanghai Xinke Pharmaceutical
Co., Ltd., Shanghai, China) were implanted in the pancreatic cancer
lesion and a microwave ablation therapy was used to target the
hepatic lesion. Following these therapeutic techniques, on July 13,
2014, oral Gleevec (400 mg; Novartis, Basel, Switzerland) was
administered daily for the following 3 years. The combination of
these adjuvant therapies is a novel strategy. The patient is
currently undergoing close follow-up via telephone interview to
determine the efficacy of this novel treatment strategy, and at
each follow-up is advised to continue taking Gleevec. Follow-up
telephone interviews were conducted in October 2014, January 2015
and June 2015. The most recent follow-up was in December 2015, and
the patient reported that she had presented at another hosptial
with abdominal metastasis. Currently, the patient is alive. Written
informed consent was obtained from the patient for the publication
of the present study.
Discussion
A conclusive diagnosis of EGISTs relies on
pathological examination of the tumor specimen (4). The finding in the present study that
both tumor tissues consisted of spindle cells provided direct
evidence for the diagnosis of stromal tumors in the pancreas and
the liver. Additional evidence was obtained from immunophenotyping
of tumor cells by immunohistochemical staining, which revealed
positive CD117 expression in the tumor cells. CD117, a c-Kit
receptor tyrosine kinase, is the fingerprint marker that
differentiates GISTs from true smooth muscle tumors and it is
expressed in 95% of GISTs (12,32–34). Thus,
the final diagnosis in the present case of two extra-GI tumors of
the pancreas and liver is reliable and valid. However, the next
challenge was determining whether the hepatic EGIST was primary or
metastatic. Accurate differentiation of in situ tumors from
metastatic tumors is important during the selection of treatment
strategies, prediction of prognosis and provision of psychological
patient care, as the actions taken in all these cases fundamentally
depend on the extent of tumor malignancy. When two different sizes
of stromal tumors were identified in the pancreas and liver, there
were two possibilities: i) Both tumors were primary or ii) one
tumor was metastatic. If one of the tumors was not primary, the
likelihood of liver metastasis was high, as the hepatic tumor was
half the size of the pancreatic tumor. If the hepatic EGIST was
considered metastatic, the pancreatic EGIST had to be highly
malignant. When all data was re-evaluated during the writing of the
current report, uncertainty regarding the malignancy of the
pancreatic tumor was raised.
The following are evidence and reasoning supported a
diagnosis of primary hepatic EGIST in the present study: i) The
most widely accepted criteria used to predict stromal tumor
behavior are tumor size and mitotic rate (4), and the pancreatic tumor size
(4.5×2.5×2.0 cm) and mitotic activity (1–2/50 HPF) in the current
case suggested a low risk of malignancy. Thus, the low risk of
malignancy indicated that the probability of metastasis was also
low. ii) Furthermore, considering the low risk of malignancy, it is
unlikely that the pancreatic tumor began metastasizing when the
tumor was 2.5 cm, which is the estimated size of the tumor assuming
that the primary and metastatic tumors are growing at a similar
rate. For example, in a previous patient with a pancreatic EGIST of
6×5 cm and 12–15 mitoses per 50 HPF, no metastasis in the form of a
liver space-occupying lesion was observed until 2 years later
(25). iii) Finally, there was a
possibility that a mural GIST experienced extensive extramural
growth, resulting in an eventual loss of connection with the gut
wall (9). The lost tumor cells may
have first successfully seeded in the pancreas, before later
seeding in the liver, as primary EGIST of liver has been previously
reported (29–31).
An important consideration is that when a
non-stromal tumor is detected in the liver subsequent to the
detection of a malignant tumor in other organs, such as the
colon/rectum, lung or breast, it can generally be safely assumed
that the tumor detected in the liver is metastatic. However, two
possibilities exist when the same stromal tumor cell types are
identified at simultaneously in two different locations: i) The
first possibility is that both tumors are primary and ii) the
second is that one of the tumors is metastatic. If the tumor is at
a low risk of malignancy, the hepatic EGIST may have been deposited
from the same origin that seeded the pancreatic EGIST; however, if
the malignancy of the tumor is high, the hepatic tumor may have
originated from the pancreatic EGIST. The existence of these two
possibilities highlights the challenge of differentiating between
primary and metastatic EGIST. If the two EGISTs were simultaneously
identified in two different organs and if both EGISTs were at low
risk of malignancy then the possibility of a primary tumor must be
considered.
CEUS demonstrated rim enhancement at the periphery
of the lesion in the left lobe of the liver in the arterial phase
that began to fade in the venous phase. The washout of the
enhancement occurred considerably earlier in the lesion than in the
surrounding hepatic parenchyma. However, these findings only
suggest the presence of a likely hepatic tumor and, furthermore,
could not distinguish between a primary and a metastatic tumor, as
the two tumor types may exhibit similar rim enhancements at their
periphery by CEUS (35,36).
CEUS has been increasingly used for the early
diagnosis of metastatic tumors in the liver following the
confirmation of primary malignant cancer in the colon/rectum, lung,
GI tract, pancreas or breast, as malignant tumors in those organs
are associated with a high frequency of liver metastasis. The
typical CEUS findings in nearly all hypovascular metastasis include
varying degrees of contrast enhancement in the arterial phase,
particularly in the periphery (rim enhancement) (35). However, an irregular rim enhancement
in the periphery of the lesion is also frequently observed in
primary liver cancer (36);
therefore, this feature is not a specific enough to distinguish
between a metastatic and primary lesion. Thus, CEUS findings can
only serve as an indicator of the presence or absence of a
space-occupying lesion in the liver. The suggestion of metastasis
by CEUS is predominantly based on the presence of malignant tumors
previously confirmed in other organs. Therefore, as established in
the current case, the simultaneous identification of low malignancy
stromal tumors in the pancreas and the liver make it impossible for
CEUS findings alone to provide definitive evidence of
metastasis.
The origin of EGISTs remains controversial, however,
at least two hypotheses appear reasonable. One hypothesis assumes
that interstitial cells of Cajal (ICCs) are the likely source of
the genesis of GISTs (7). ICCs are
pacemaker cells that are located throughout the wall of the GI
tract, as their function is to regulate the movement of the GI
track. If ICCs are the origin cells of EGISTs, the extensive
presence of ICCs along the GI track presents an anatomical and
pathophysiological opportunity for the occurrence of GISTs in any
part of GI track. Additionally, the distribution of ICCs also
enables a scenario in which ICC-derived tumor cells may escape and
deposit outside of the GI track. For example, the extensive
extramural growth of mural GISTs may result in complete loss of
contact with the muscularis propria (9), leading to tumor cells being scattered
outside of the GI tract. Not all tumor cells deposited in different
organs or locations would survive; however, the survival, growth
and proliferation of just one or more cells may result in the
generation of one or more primary EGISTs in different organs or
locations (37). This theory provides
a reasonable explanation for the emergence of primary EGIST in the
pancreas and liver in the present study. The molecular fingerprint
of ICC is the expression of the c-Kit receptor tyrosine kinase
(CD117 antigen). The positive detection of CD117 in the pancreatic
and hepatic EGIST cells in the current case is in agreement with
this hypothesis. The alternate hypothesis is that GISTs actually
arise from a common precursor cell of ICCs and smooth muscle cells,
which accounts for their ability to grow inside and outside of the
GI tract (37). Molecular
investigations have confirmed the presence of Cajal-like
interstitial cells within extra-digestive organs and vessels. For
example, the existence of ICCs has recently been demonstrated in
the human exocrine pancreas, and these cells have a phenotype
similar to that of enteric ICCs (38). This theory indicates that primary
EGISTs may simultaneously occur in different organs of the same
patient when the conditions for facilitating the growth of
Cajal-like cells are triggered in these locations. Although each
hypothesis postulates a different origin, both describe a scenario
where it is possible for more than one primary EGIST to
simultaneously emerge in different organs or locations.
Surgery represents the first choice for treating
resectable pancreatic and hepatic EGISTs (33). For unresectable patients, the tyrosine
kinase inhibitor imatinib mesylate (Gleevec) is the primary drug
available for treatment (39). In the
present case, although unable to perform a complete surgical
resection, radioactive particles were implanted into the pancreatic
tumor and a microwave therapy technique to target the hepatic EGIST
was performed. Following these therapeutic techniques, oral Gleevec
was regularly administered. The combination of these adjuvant
therapies is a novel strategy. The patient is currently undergoing
close follow-up to determine the efficacy of this novel treatment
strategy, and at each follow-up appointment is advised to continue
taking Gleevec. The most recent follow-up appointment was in
November 2015, and the patient presented with abdominal
metastasis.
In summary, the present study reports a case with
EGIST diagnosed in the pancreas and liver. To the best of our
knowledge, this report is the first to provide evidence that
primary EGIST can emerge independently in two different organs of
the same patient. The present study highlights the underappreciated
clinical challenge of differentiating between primary and
metastatic stromal tumors when both tumors have a low malignancy
risk and are detected simultaneously. The possible mechanism by
which two primary EGISTs may arise in two different organs is also
discussed.
References
|
1
|
Joensuu H, Fletcher C, Dimitrijevic S,
Silberman S, Roberts P and Demetri G: Management of malignant
gastrointestinal stromal tumours. Lancet Oncol. 3:655–664. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Agaimy A and Wünsch PH: Gastrointestinal
stromal tumours: A regular origin in the muscularis propria, but an
extremely diverse gross presentation. A review of 200 cases to
critically re-evaluate the concept of so-called
extra-gastrointestinal stromal tumours. Langenbecks Arch Surg.
391:322–329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirota S, Isozaki K, Moriyama Y, Hashimoto
K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M,
et al: Gain-of-function mutations of c-kit in human
gastrointestinal stromal tumors. Science. 279:577–580. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sarlomo-Rikala M, Kovatich AJ,
Barusevicius A and Miettinen M: CD117: A sensitive marker for
gastrointestinal stromal tumors that is more specific than CD34.
Mod Pathol. 11:728–734. 1998.PubMed/NCBI
|
|
5
|
Joensuu H, Roberts PJ, Sarlomo-Rikala M,
Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville
R, Dimitrijevic S, Druker B and Demetri GD: Effect of the tyrosine
kinase inhibitor STI571 in a patient with a metastatic
gastrointestinal stromal tumor. N Engl J Med. 344:1052–1056. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fletcher CD, Berman JJ, Corless C,
Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti
H, Rubin BP, et al: Diagnosis of gastrointestinal stromal tumors: A
consensus approach. Hum Pathol. 33:459–465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miettinen M, Monihan JM, Sarlomo-Rikala M,
Kovatich AJ, Carr NJ, Emory TS and Sobin LH: Gastrointestinal
stromal tumors/smooth muscle tumors (GISTs) primary in the omentum
and mesentery: Clinicopathologic and immunohistochemical study of
26 cases. Am J Surg Pathol. 23:1109–1118. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reith JD, Goldblum JR, Lyles RH and Weiss
SW: Extragastrointestinal (soft tissue) stromal tumors: An analysis
of 48 cases with emphasis on histologic predictors of outcome. Mod
Pathol. 13:577–585. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: Pathology and prognosis at different sites. Semin
Diagn Pathol. 23:70–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Terada T: Gastrointestinal stromal tumor
of the uterus: A case report with genetic analyses of c-kit and
PDGFRA genes. Int J Gynecol Pathol. 28:29–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neto MR, Machuca TN, Pinho RV, Yuasa LD
and Bleggi-Torres LF: Gastrointestinal stromal tumor: Report of two
unusual cases. Virchows Arch. 444:594–596. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamaura K, Kato K, Miyazawa M, Haba Y,
Muramatsu A, Miyata K and Koide N: Stromal tumor of the pancreas
with expression of c-kit protein: Report of a case. J Gastroenterol
Hepatol. 19:467–470. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krska Z, Pesková M, Povýsil C, Horejs J,
Sedlácková E and Kudrnová Z: GIST of pancreas. Prague Med Rep.
106:201–208. 2005.PubMed/NCBI
|
|
14
|
Daum O, Klecka J, Ferda J, Treska V,
Vanecek T, Sima R, Mukensnabl P and Michal M: Gastrointestinal
stromal tumor of the pancreas: Case report with documentation of
KIT gene mutation. Virchows Arch. 446:470–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Showalter SL, Lloyd JM, Glassman DT and
Berger AC: Extra-gastrointestinal stromal tumor of the pancreas:
Case report and a review of the literature. Arch Surg. 143:305–308.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan BM, Pai RK and Van Dam J: Diagnosis of
pancreatic gastrointestinal stromal tumor by EUS guided FNA. JOP.
9:192–196. 2008.PubMed/NCBI
|
|
17
|
Yang F, Jin C, Fu D and Ni Q:
Extra-gastrointestinal stromal tumor of the pancreas: Clinical
characteristics, diagnosis, treatment, and outcome. J Surg Oncol.
103:739–740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harindhanavudhi T, Tanawuttiwat T, Pyle J
and Silva R: Extra-gastrointestinal stromal tumor presenting as
hemorrhagic pancreatic cyst diagnosed by EUS-FNA. JOP. 10:189–191.
2009.PubMed/NCBI
|
|
19
|
Trifan A, Târcoveanu E, Danciu M, Hutanasu
C, Cojocariu C and Stanciu C: Gastric heterotopic pancreas: An
unusual case and review of the literature. J Gastrointestin Liver
Dis. 21:209–212. 2012.PubMed/NCBI
|
|
20
|
Goh BK, Kesavan SM and Wong WK: An unusual
cause of a pancreatic head tumor. Gastroenterology. 137:e5–e6.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Padhi S, Kongara R, Uppin SG, Uppin MS,
Prayaga AK, Challa S, Nagari B and Regulagadda SA:
Extragastrointestinal stromal tumor arising in the pancreas: A case
report with a review of the literature. JOP. 11:244–248.
2010.PubMed/NCBI
|
|
22
|
Saif MW, Hotchkiss S and Kaley K:
Gastrointestinal stromal tumors of the pancreas. JOP. 11:405–406.
2010.PubMed/NCBI
|
|
23
|
Rao RN, Vij M, Singla N and Kumar A:
Malignant pancreatic extra-gastrointestinal stromal tumor diagnosed
by ultrasound guided fine needle aspiration cytology. A case report
with a review of the literature. JOP. 12:283–286. 2011.PubMed/NCBI
|
|
24
|
Čečka F, Jon B, Ferko A, Šubrt Z, Nikolov
DH and Tyčová V: Long-term survival of a patient after resection of
a gastrointestinal stromal tumor arising from the pancreas.
Hepatobiliary Pancreat Dis Int. 10:330–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vij M, Agrawal V and Pandey R: Malignant
extra-gastrointestinal stromal tumor of the pancreas. A case report
and review of literature. JOP. 12:200–204. 2011.PubMed/NCBI
|
|
26
|
Soufi M, Bouziane M, Massrouri R and Chad
B: Pancreatic GIST with pancreas divisum: A new entity. Int J Surg
Case Rep. 4:68–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim HH, Koh YS, Park EK, Seoung JS, Hur
YH, Kim JC, Cho CK and Kim HJ: Primary extragastrointestinal
stromal tumor arising in the pancreas: Report of a case. Surg
Today. 42:386–390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beltrame V, Gruppo M, Pastorelli D, Pizzi
S, Merigliano S and Sperti C: Extra-gastrointestinal stromal tumor
of the pancreas: Case report and review of the literature. World J
Surg Oncol. 12:1052014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu X, Forster J and Damjanov I: Primary
malignant gastrointestinal stromal tumor of the liver. Arch Pathol
Lab Med. 127:1606–1608. 2003.PubMed/NCBI
|
|
30
|
Chen J, Du YJ, Song JTELN and Liu BR:
Primary malignant liver mesenchymal tumor: A case report. World J
Gastroenterol. 16:5263–5266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim HO, Kim JE, Bae KS, Choi BH, Jeong CY
and Lee JS: Imaging findings of primary malignant gastrointestinal
stromal tumor of the liver. Jpn J Radiol. 32:365–370. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van der Zwan SM and De Matteo RP:
Gastrointestinal stromal tumor: 5 years later. Cancer.
104:1781–1788. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shinomura Y, Kinoshita K, Tsutsui S and
Hirota S: Pathophysiology, diagnosis, and treatment of
gastrointestinal stromal tumors. J Gastroenterol. 40:775–780. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rubin BP: Gastrointestinal stromal
tumours: An update. Histopathology. 48:83–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Larsen LP: Role of contrast enhanced
ultrasonography in the assessment of hepatic metastases: A review.
World J Hepatol. 2:8–15. 2010.PubMed/NCBI
|
|
36
|
Li R, Yuan MX, Ma KS, Li XW, Tang CL,
Zhang XH, Guo DY and Yan XC: Detailed analysis of temporal features
on contrast enhanced ultrasound may help differentiate intrahepatic
cholangiocarcinoma from hepatocellular carcinoma in cirrhosis. PLoS
One. 9:e986122014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Welch DR: Do we need to redefine a cancer
metastasis and staging definitions? Breast Dis. 26:3–12.
2006.PubMed/NCBI
|
|
38
|
Popescu LM, Hinescu ME, Ionescu N, Ciontea
SM, Cretoiu D and Ardelean C: Interstitial cells of Cajal in
pancreas. J Cell Mol Med. 9:169–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arora A and Scholar EM: Role of tyrosine
kinase inhibitors in cancer therapy. J Pharmacol Exp Ther.
315:971–979. 2005. View Article : Google Scholar : PubMed/NCBI
|