Introduction
Nasopharyngeal carcinoma (NPC), a type of malignant
head-and-neck cancer, is a geographical predilection in Southeast
Asia, particularly in the Southern provinces of China with an
estimated incidence rate of ~20–50/100,000 (1,2). Despite
the specificity of the pathological location and the anatomical
structure, the majority of patients are unfortunately diagnosed at
advanced stage (3). Currently,
radiotherapy is the mainstay of treatment for NPC. However, a high
proportion of NPC patients, particularly patients with advanced
NPC, exhibit radioresistance and exhibit poor outcomes (4,5).
Therefore, exploring the molecular mechanisms underlying
sensitivity to radiation or resistance as well as the combination
of chemo- and radiotherapy is of crucial significance for NPC
therapy.
Curcumin (diferuloylmethane, Cur) is a non-flavonoid
polyphenol derived from turmeric plants that demonstrates great
potential in tumorigenesis and tumor progression (6,7).
Accumulating evidence has indicated that Cur can sensitize numerous
radioresistant tumor cells, including NPC (8–10). Our
previous work also demonstrated that Cur could enhance the
radiosensitivity in the CNE2 cell line at an appropriate
concentration (11,12). Although recent discoveries have
revealed the underlying mechanisms to a certain extent, the
relevance of the association between Cur and its radiosensitivity
to NPC and the function of related miRNAs are not fully
recognized.
MicroRNAs (miRNAs) are a class of small endogenous
non-coding RNA that can regulate gene expression at the
post-transcriptional level by inhibiting translation of messenger
RNA and by inducing mRNA degradation (13). Recent studies indicate that various
biological and pathological processes, including cellular
proliferation, differentiation, and apoptosis, have been caused by
the deregulation of mRNAs (14–16). Among
the mRNAs, miR-593 has been reported to be down-exposed in
esophageal and gastric cancer (17).
The aim of the present study was to investigate whether miR-593
could radiosensitize the NPC cell line CNE2 in vitro and
in vivo by regulating multidrug resistance gene 1 (MDR1),
and to identify whether MDR1 is a direct and functional target of
miR-593.
Materials and methods
Cell culture and reagents
The human NPC cell line CNE2 was obtained from Sun
Yat-sen University (Guangzhou, China) and cultured in RMPI-1640
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) in a humid atmosphere containing 5%
CO2 at 37°C. Cur (Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in 0.5% DMSO (Sigma-Aldrich) and diluted with RPMI-1640
medium to the desired concentrations.
Clonogenic survival assay
Cells were divided into three groups: control group
(CN), IR (irradiation) group (CX), and IR+Cur group (JX). Cells
were seeded in 6-well plates and routinely cultured for 24 h. The
CX and JX groups were radiated with X-rays at 6 MV using 600C/D
linear accelerator (Varian Medical Systems, Inc., Palo Alto, CA,
USA) to deliver the indicated doses (2, 4, 6 and 8 Gy), and all
cells were further cultured for another 12 days. The cells were
fixed and stained with methanol containing 1% crystal violet
(Beijing Dingguo Changsheng Biotech Co., Ltd., Beijing, China).
Colonies (≥50 cells) were counted under the microscope (U-LH100L-3;
Olympus Corporation, Tokyo, Japan) by using the following formula:
Plate clone formation efficiency = number of colonies/number of
cells inoculated (18).
Animal studies
The study was conducted in accordance with the
guideline for the Administration of Affairs Concerning Experimental
Animals, and all procedures involving animals were approved by
Animal Care and Ethics Committee of Southern Medical University.
Balb/c nude mice (Experimental Animal Center of Southern Medical
University, Weifang, China) for tumor implantation were 4–6 weeks
old with a body mass of 18–22 g. The mice were housed under
controlled laboratory conditions at an ambient temperature of
23±2°C for 2 weeks.
For the xenograft tumor assay, 1×106
cells in 200 µl of RMPI-1640 were injected subcutaneously into the
right flank of nude mice. The mice were divided into 5 groups of 6
mice when the tumor volume reached 61 mm3. Cur was
intragastrically administered at 3 different dosages [50, 100
(which was determined to be the optimal concentration of Cur,
according to the results of a preliminary test) and 150 mg/kg] once
a day for 7 days. Saline was injected as a control. After 7 days,
all groups were irradiated with 4 Gy IR every other day, 3 times.
Following the final treatment, mice were euthanized, and the tumors
were dissected and weighed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA from cells and nude mice was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
following the manufacturer's instructions. Quantification was
performed using the Quantitect SYBR Green PCR Kit (Stratagene, San
Diego, CA, USA) with an MX3005P multiplex quantitative qPCR system
(Stratagene), according to the manufacturer's instructions. GAPDH
and U6 were used as the internal control for detecting mRNA and
miRNA, respectively. The comparative ΔΔCq method was employed as
previously described (19). The fold
changes were calculated according to 2−ΔΔCq equation.
All of the primers used are listed in Table I.
 | Table I.Primers used in this study. |
Table I.
Primers used in this study.
| Primer name | Primer sequence |
|---|
| GAPDH | F:
ATCATCAGCAATGCCTCCTG |
|
| R:
ATGGACTGTGGTCATGAGTC |
| MDR1 | F:
TCATTCGAGTAGCGGCTCTT |
|
| R:
CTTCTTTGCTCCTCCATTGC |
| U6 | F:
CTCGCTTCGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGT |
| miR-593 | F:
TGTCTCTGCTGGGGTTTCT |
|
| R:
GTGCAGGGTCCGAGGTATT |
| pGL3- | F:
gattatagaACTCTGACTGTATGAGATGT |
| MDR1 | R:
gattatagaTCACATGAAAGTTTAGT |
| si-MDR1 | S:
CAGAAAGCUUAGUACCAAAdTdT |
| Si-NC | S:
UAACGACGCGACGACGUAAdTdT |
Western blot analysis
The portion of xenograft tumors were extracted by
RIPA buffer with protease inhibitors (Cell Biolabs Inc., San Diego,
CA, USA) and quantified by the BCA method (Thermo Fisher
Scientific, Inc.). Equal amounts of total protein (30–50 µg) were
resolved by 10% SDS-PAGE (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and transferred onto the PVDF membrane (Thermo Fisher
Scientific, Inc.), which was blocked in 5% non-fat milk at room
temperature for 1 h. Thereafter, they were incubated with rabbit
monoclonal anti-MDR1 antibody (1:2,000; catalogue no. ab170904;
Abcam, Cambridge, UK) overnight at 4°C. The membranes were blotted
for 1 h at room temperature with goat anti-rabbit horseradish
peroxidase-conjugated immunoglobulin G secondary antibody (1:1,000;
catalogue no. 7074; Cell Signaling Technology, , Inc., Danvers, MA,
USA). The bands were developed using ECL Kit (Cell Signaling
Technology, Inc.) and quantified on Gel Logic 2200 PRO Imaging
System (Kodak, Rochester, NY, USA).
Luciferase assay
The entire 380-base pair fragment of MDR1 3′ UTR
that contains the putative binding site of miR-593 was amplified by
PCR and cloned downstream of the luciferase gene at the XbaI sites
in the pGL-3 plasmid (Promega Corporation, Madison, WI, USA). The
construct was designated as pGL3-MDR1 (primers are listed in
Table I). HEK293 cells were
co-transfected with 30 pmol of either miR-593 mimics or miR-593 NC
and pGL3-MDR1 using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Luciferase activity was measured using the
Promega dual-luciferase assay kit and normalized by β-galactosidase
activity. Relative protein levels were expressed as Renilla/firefly
luciferase ratios. Each experiment was repeated twice (11).
RNA interference
CNE2 cells at 20–30% confluence were transfected
with 50 nM of siRNAs using Lipofectamine 2000 following the
manufacturer's protocol. Small interfering RNA (siRNA) and
scrambled negative control siRNA (siRNA-NC) were obtained from
Invitrogen (Thermo Fisher Scientific, Inc.). The target sequence of
MDR1 is listed in Table I. A total of
36 h after transfection, cells were harvested for RT-qPCR.
Statistics
SPSS software, version 13.0 (SPSS Inc., Chicago, IL,
USA) was used for all the statistical analyses in the present
study. Quantitative data are presented as mean ± standard
deviation. χ2 test and Student's t-test were
appropriately applied for different types of data. P<0.05 was
considered to indicate a statistically significant difference.
Results
MDR1 is a direct target of
miR-593
A bioinformatics analysis was performed using
TargetScan version 7.0 software (http://www.targetscan.org/), which predicted that
miR-593 may target the MDR1 3′UTR region (Fig. 1A). A luciferase-based reporter was
constructed to evaluate the effect of miR-593 direct binding to the
putative target site on the 3′UTR of MDR1. To substantiate the
assumption that miR-593 can directly repress MDR1, the
reporter-construct pGL3-vector and pGL3-MDR1 were co-transfected
with miR-593 mimic, miR-NC, and miR-593 inhibitor or inhibitor-NC
to HEK293 cells. Luciferase activity was then assayed. As shown in
Fig. 1B, for pGL3-MDR1 construct,
miR-593 mimic significantly lowered luciferase activity compared
with miR-NC (P<0.05). By contrast, miR-593 inhibitor increased
luciferase activity in HEK293 cells compared with inhibitor-NC
(P<0.05). These findings support the hypothesis that miR-593
directly targets MDR1 expression.
Cur sensitizes CNE2 cells to IR
through upregulating mir-593 to reverse IR-induced MDR1
expression
The colony survival assay is considered a canonical
standard to determine radiosensitivity (20). The results confirmed that cells
pretreated with Cur were much more sensitive to IR than their
untreated counterparts. The α and β components were 0.2476/Gy and
0.01650/Gy2 for the cells treated with radiation alone,
and 0.4201/Gy and 0.02029/Gy2 for the cells treated with
the combination treatment (Fig. 2A),
respectively, leading to significantly different (P<0.001)
survival fractions, as tested with the linear regression analysis.
The data were further analyzed according to the multitarget
single-hit model. When pretreated with 10 µM Cur (IC20), the
sensitization enhancement ratio of CNE-2 reached 1.44. These data
indicate that Cur has an effective radiosensitization effect on the
CNE-2 cell line in vitro. In addition, miR-593 and MDR1
expression were detected by RT-qPCR (Fig.
2B). The result demonstrated that IR-induced miR-593
downregulation was reversed during Cur-enhanced radiosensitization
(P<0.05).
 | Figure 2.Cur sensitizes nasopharyngeal
carcinoma cells to irradiation treatment through upregulating
mir-593 to reverse IR induced MDR1 expression. (A) CNE2 cells were
treated with 0, 2, 4, 6, or 8 Gy of IR with Cur pretreatment. The
cell survival fraction was calculated by clonogenic assay. (B)
Relative miR-593 and MDR1 expression were determined by
quantitative polymerase chain reaction. IR-induced miR-593
downregulation and MDR1 upregulation were reversed during
Cur-enhanced radiosensitization. Data are presented as mean ±
standard deviation from 3 replicate experiments. *P<0.05. CN,
control group; CX, irradiated group; IX, irradiation +Cur group;
Cur, curcumin; MDR1, multidrug resistance gene 1; IR, irradiation;
miR, micro RNA. |
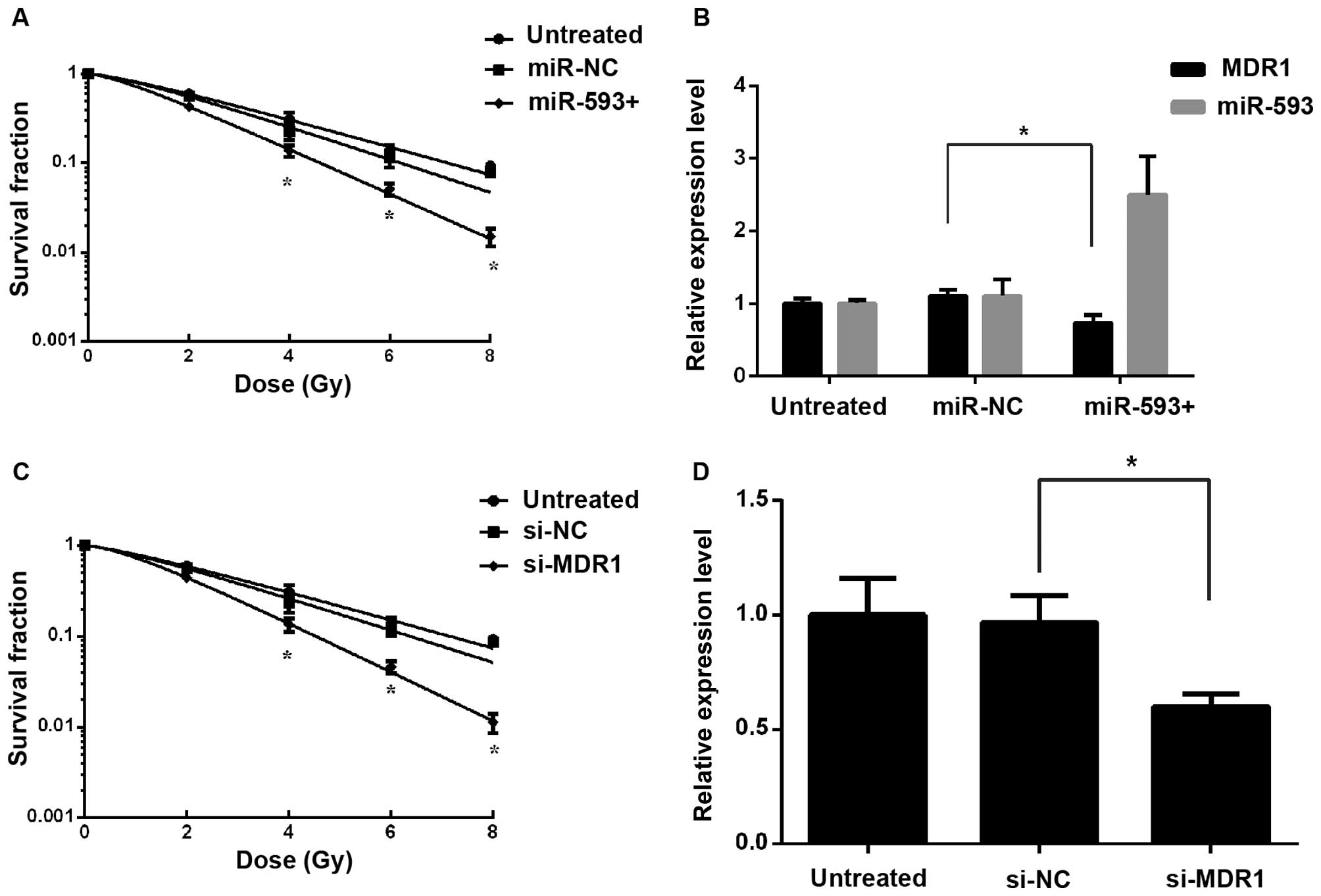
The effect of MDR1 knockdown on the
radiosensitivity of NPC cells in vitro
Cur enhances the radiosensitivity involving the
reversal of differentially expressed mir-593 and MDR1. To elucidate
whether the effect of Cur on radiosensitivity was mediated by
repression of MDR1, mir-593 mimics or si-MDR1 were transfected into
CNE2 cells. A clonogenic assay suggested that the ectopic
expression of MDR1 significantly reduced miR-593-induced
radiosensitivity (Fig. 3A and C),
which was consistent with the results of RT-qPCR (Fig. 3B and D). These observations indicated
that Cur may have sensitized cells to IR by stimulating mir-593 to
downregulate the expression of MDR1.
Radiosensitization of Cur in xenograft
tumors in vivo
The tumor weight of each mouse in each treatment
group was measured (Fig. 4A). A
single dose of 4 Gy irradiation (CX group) exhibited the expected
effect of tumor growth inhibition compared with the untreated CN
group (P<0.05, Fig. 4B). However,
the JX group (Cur 100 mg/kg and 4 Gy irradiation) had the highest
inhibition ratio (62.18%) and expressed a significant tumor growth
inhibition effect compared with the CX group (P<0.05, Fig. 4B). Mice body weights were monitored to
assess the tolerability of systemic Cur. Body weight changes over
the course of the experiment were minimal in all treatment groups,
suggesting that Cur is well tolerated (data not shown).
The expression level of miR-593 was significantly
higher in the JX group compared with the CX group. To investigate
the regulation of MDR1 by miR-593, the relative expression of MDR1
was measured by western blot assay (Fig.
4C) and RT-qPCR (Fig. 4D). In
accordance with the altered expression level of miR-593, MDR1 was
significantly downregulated in the JX group. These findings also
indicate that MDR1 is a target of miR-593.
Discussion
Radiotherapy is considered one of the most effective
treatments for patients with NPC, and radioresistance is the main
risk factor that contributes to poor prognosis (21). Radioresistance occurs in primary IR
treatment, and the survived cells may be more resistant to the
second IR treatment, thereby leading to radiotherapy failure
(12,22,23). In
this regard, the exact molecules and signaling pathway involved in
radiosensitization should be determined to develop target therapy
and enhance the efficacy of radiation. In the present study, it was
observed that IR-induced downregulation of miR-593 or upregulation
of MDR1 expression was almost reversed by Cur.
Cur regulates the gene expression involved in
survival, proliferation, angiogenesis, invasion, and metastasis.
This phytochemical also modulates various mechanisms that are
associated with radioresistance, including the following:
downregulating COX-2, MRP, Bcl-2, and survivin expression;
inhibiting PI3K/AKT activation; suppressing growth factor signaling
pathways; and inhibiting STAT3 activation (24–26). The
present study demonstrated that Cur enhanced radiosensitivity in
the NPC cell line CNE2 at 10 µmol/l by MTT or clonogenic survival
test (27), although Cur exhibited
higher anti-proliferative effects when used alone at a
concentration of 20 or 40 µmol/l. Considering the cytotoxicity of
Cur and IR, a concentration of 10 µmol/l and 24 h pretreatment was
more suitable as a radioenhancer (data not shown). In addition, in
the animal studies, Cur 100 mg/kg was chosen as optimal
concentration after a preliminary test. Therefore, no significant
data were obtained by conjoint analysis with other groups although
the single Cur group (JN group) was performed (data not shown). In
the present study, the radiosensitizing effect of Cur was evaluated
in vitro and in vivo. The data indicated that the
radiosensitizing effect of Cur may be associated with mir-593 and
MDR1.
P-glycoprotein (P-gp), encoded by MDR1, has
attracted great interest because of its role in MDR in a variety of
cancers. P-gp is overexpressed in cancer cells that actively
extrude chemotherapeutic agents (28). MDR1 mediates not only chemoprotection
by drug efflux but has also been found to inhibit apoptosis induced
by chemotherapeutics, death receptor ligands, serum starvation, and
UV or ionizing irradiation. Therefore, blocked MDR1 may enhance the
efficacy of chemotherapy and reverse radioresistance in patients.
Maier et al (29) confirmed
that a protective effect of retroviral overexpression of MDR1 is
increasing the radiotolerance of haematopoietic cells and the
related apoptosis gene was downregulated. In the present study, it
was also observed that MDR1 was a radioresistant factor which could
be downregulated by mir-593.
Certain miRNAs were reported to affect the
radiosensitivity of cancer cells, such as let-7, miR-21, miR-101,
miR-421, and miR-181a (30–34). In esophageal cancer (EC), miR-593
degrades polo-like kinase 1 (PLK1) mRNA by direct binding to the
3′-UTR of PLK1 mRNA and reduced proliferation of EC cells (17). Bioinformatics analysis was used in the
present study to predict that miR-593 may also target the MDR1
3′UTR region. A luciferase-based reporter showed that miR-593
regulated MDR1 expression by directly targeting 3′- UTR,
consistently resulting in reduced expression of MDR1. Furthermore,
modulated expression tests demonstrated that radiosensitization of
Cur was triggered by miR-593 instead of directly by MDR1.
Taken together, the results demonstrated that Cur
had a radiosensitization effect on NPC cells in vivo and
in vitro; curcumin-mediated upregulation of miR-593 causes
the depression of MDR1 expression, which may promote
radiosensitivity of NPC cells.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81173616, 81202430
and 81302948) and Guangdong Science and Technology Project (grant
no. 2013A032500003), and Science and Technology Project of Haizhu
District (grant no. 2013-cg-29); the authors thank medical
personnel Mr. Jiabin Liu and Ms. Huarui Niu of NanFang Hospital for
providing X-ray radiation equipment.
References
|
1
|
Loong HH, Ma BB and Chan AT: Update on the
management and therapeutic monitoring of advanced nasopharyngeal
cancer. Hematol Oncol Clin North Am. 22:1267–1278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan AT, Teo PM and Johnson PJ:
Nasopharyngeal carcinoma. Ann Oncol. 13:1007–1015. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanguineti G, Geara FB, Garden AS, Tucker
SL, Ang KK, Morrison WH and Peters LJ: Carcinoma of the nasopharynx
treated by radiotherapy alone: Determinants of local and regional
control. Int J Radiat Oncol Biol Phys. 37:985–996. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji JL, Huang XF and Zhu HL: Curcumin and
its formulations: Potential anti-cancer agents. Anticancer Agents
Med Chem. 12:210–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rahmani AH, Al Zohairy MA, Aly SM and Khan
MA: Curcumin: A potential candidate in prevention of cancer via
modulation of molecular pathways. Biomed Res Int. 2014:7616082014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
López-Jornet P, Camacho-Alonso F and
Gómez-Garcia F: Effect of curcumin and irradiation in PE/CA-PJ15
oral squamous cell carcinoma. Acta Odontol Scand. 69:269–273. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang YJ, Huang CY, Hung CS, Chen WY and
Wei PL: GRP78 mediates the therapeutic efficacy of curcumin on
colon cancer. Tumour Biol. 36:633–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie YQ, Wu XB and Tang SQ: Curcumin
treatment alters ERK-1/2 signaling in vitro and inhibits
nasopharyngeal carcinoma proliferation in mouse xenografts. Int J
Clin Exp Med. 7:108–114. 2014.PubMed/NCBI
|
|
11
|
Liu Y, Cai H, Liu J, Fan H, Wang Z, Wang
Q, Shao M, Sun X, Diao J, Liu Y, et al: A miR-151 binding site
polymorphism in the 3′-untranslated region of the cyclin E1 gene
associated with nasopharyngeal carcinoma. Biochem Biophys Res
Commun. 432:660–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Q, Fan H, Liu Y, Yin Z, Cai H, Liu J,
Wang Z, Shao M, Sun X, Diao J, et al: Curcumin enhances the
radiosensitivity in nasopharyngeal carcinoma cells involving the
reversal of differentially expressed long non-coding RNAs. Int J
Oncol. 44:858–864. 2014.PubMed/NCBI
|
|
13
|
Djuranovic S, Nahvi A and Green R: A
parsimonious model for gene regulation by miRNAs. Science.
331:550–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang B, Jing C, Wang J, Guo X, Chen Y, Xu
R, Peng L, Liu J and Li L: Identification of microRNAs associated
with lymphangiogenesis in human gastric cancer. Clin Transl Oncol.
16:374–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang T, Sun Q, Liu T, Chen J, Du S, Ren
C, Liao G and Yuan Y: MiR-451 increases radiosensitivity of
nasopharyngeal carcinoma cells by targeting ras-related protein 14
(RAB14). Tumour Biol. 35:12593–12599. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nygren MK, Tekle C, Ingebrigtsen VA,
Mäkelä R, Krohn M, Aure MR, Nunes-Xavier CE, Perälä M, Tramm T,
Alsner J, et al: Identifying microRNAs regulating B7-H3 in breast
cancer: The clinical impact of microRNA-29c. Br J Cancer.
110:2072–2080. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ito T, Sato F, Kan T, Cheng Y, David S,
Agarwal R, Paun BC, Jin Z, Olaru AV, Hamilton JP, et al: Polo-like
kinase 1 regulates cell proliferation and is targeted by miR-593*
in esophageal cancer. Int J Cancer. 129:2134–2146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Zheng L, Huang J, Gao F, Lin X,
He L, Li D, Li Z, Ding Y and Chen L: MiR-124 Radiosensitizes human
colorectal cancer cells by targeting PRRX1. PLoS One. 9:e939172014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-∆∆CT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yaromina A, Krause M, Thames H, Rosner A,
Krause M, Hessel F, Grenman R, Zips D and Baumann M: Pre-treatment
number of clonogenic cells and their radiosensitivity are major
determinants of local tumour control after fractionated
irradiation. Radiother Oncol. 83:304–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu ZX, Ma XQ, Yang LF, Wang ZL, Zeng L, Li
ZJ, Li XN, Tang M, Yi W, Gong JP, et al: DNAzymes targeted to
EBV-encoded latent membrane protein-1 induce apoptosis and enhance
radiosensitivity in nasopharyngeal carcinoma. Cancer Lett.
265:226–238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pearce AG, Segura TM, Rintala AC,
Rintala-Maki ND and Lee H: The generation and characterization of a
radiation-resistant model system to study radioresistance in human
breast cancer cells. Radiat Res. 156:739–750. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-kappaB. J
Clin Invest. 107:241–246. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kunnumakkara AB, Diagaradjane P, Guha S,
Deorukhkar A, Shentu S, Aggarwal BB and Krishnan S: Curcumin
sensitizes human colorectal cancer xenografts in nude mice to
gamma-radiation by targeting nuclear factor-kappaB-regulated gene
products. Clin Cancer Res. 14:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Javvadi P, Segan AT, Tuttle SW and
Koumenis C: The chemopreventive agent curcumin is a potent
radiosensitizer of human cervical tumor cells via increased
reactive oxygen species production and over activation of the
mitogen-activated protein kinase pathway. Mol Pharmacol.
73:1491–1501. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Narang H and Krishna M: Inhibition of
radiation induced nitration by curcumin and nicotinamide in mouse
macrophages. Mol Cell Biochem. 276:7–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hannoun-Levi JM, Chand-Fouche ME, Dejean C
and Courdi A: Dose gradient impact on equivalent dose at 2 Gy for
high dose rate interstitial brachytherapy. J Contemp Brachytherapy.
4:14–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Montazami N, Aghapour M, Farajnia S and
Baradaran B: New insights into the mechanisms of multidrug
resistance in cancers. Cell Mol Biol (Noisy-le-grand). 61:70–80.
2015.PubMed/NCBI
|
|
29
|
Maier P, Herskind C, Fleckenstein K, Spier
I, Laufs S, Zeller WJ, Fruehauf S and Wenz F: MDR1 gene transfer
using a lentiviral SIN vector confers radioprotection to human
CD34+ hematopoietic progenitor cells. Radiat Res. 169:301–310.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oh JS, Kim JJ, Byun JY and Kim IA:
Lin28-let7 modulates radiosensitivity of human cancer cells with
activation of K-Ras. Int J Radiat Oncol Biol Phys. 76:5–8. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van Jaarsveld MT, Wouters MD, Boersma AW,
Smid M, van Ijcken WF, Mathijssen RH, Hoeijmakers JH, Martens JW,
van Laere S, Wiemer EA and Pothof J: DNA damage responsive
microRNAs misexpressed in human cancer modulate therapy
sensitivity. Mol Oncol. 8:458–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun Q, Liu T, Zhang T, Du S, Xie GX, Lin
X, Chen L and Yuan Y: MiR-101 sensitizes human nasopharyngeal
carcinoma cells to radiation by targeting stathmin 1. Mol Med Rep.
11:3330–3336. 2015.PubMed/NCBI
|
|
33
|
Mansour WY, Bogdanova NV, Kasten-Pisula U,
Rieckmann T, Köcher S, Borgmann K, Baumann M, Krause M, Petersen C,
Hu H, et al: Aberrant overexpression of miR-421 downregulates ATM
and leads to a pronounced DSB repair defect and clinical
hypersensitivity in SKX squamous cell carcinoma. Radiother Oncol.
106:147–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ke G, Liang L, Yang JM, Huang X, Han D,
Huang S, Zhao Y, Zha R, He X and Wu X: MiR-181a confers resistance
of cervical cancer to radiation therapy through targeting the
pro-apoptotic PRKCD gene. Oncogene. 32:3019–3027. 2013. View Article : Google Scholar : PubMed/NCBI
|