Introduction
Sinonasal organized hematoma (SNOH) was initially
described in 1917 by Tadokoro (1) as
a benign lesion characterized by an expansile nature and locally
aggressive behavior, capable of mimicking a potentially dangerous
neoplastic process (2–5). The diagnosis of SNOH is challenging,
even given the integration of multiple informative symptoms
(2). Computed tomography (CT) and
magnetic resonance imaging (MRI) are useful for the assessment of
potential cases of SNOH (2,5), and usually reveal typical bony changes
and a distinct speckled pattern that exhibits various signal
intensities, respectively. The hallmarks of SNOH are typically a
dark peripheral rim on T2-weighted imaging (T2WI), and a nodular
and patchy enhancement in post-contrast T1-weighted imaging (T1WI)
(2,5).
The successful treatment of SNOH relies on complete surgical
excision (3,5). However, one notable and unexplained
anomaly is that the disease demonstrates a high tendency for
occurrence in the maxillary sinus (3,5–7). Only one case involving the sphenoid
sinus, an intricately structured space in close vicinity to vital
structures, has been reported to date (4). To the best of our knowledge, the current
report presents the first case of an OH of the sphenoid sinus with
multiple cranial nerve involvement, which was identified following
surgery for the treatment of isolated sphenoid sinus aspergillosis
(ISSA). The disease was successfully treated using endoscopic
endonasal surgery, yielding a positive outcome for the patient.
Case report
An 81-year-old woman presented as an inpatient to
the Kaohsiung Veterans General Hospital (Kaohsiung, Taiwan) in
October 2013, with a 2-year history of an excruciating headache,
which was described as deep-seated and throbbing. The patient had
subsequently developed progressive deterioration of visual acuity
in the right eye and drooping of the right eyelid, although the
total duration of these symptoms was uncertain. The medical history
of the patient noted the use of endoscopic sinus surgery for the
treatment of ISSA 3 years previously, and a long-standing history
of chronic kidney disease. Upon examination, besides optic atrophy,
the patient's right eye exhibited complete ptosis and mydriasis
with a sluggish pupillary reaction. The eye was positioned looking
down and out, and the orbit could not be adducted. These symptoms
were compatible with a diagnosis of compressive occulomotor nerve
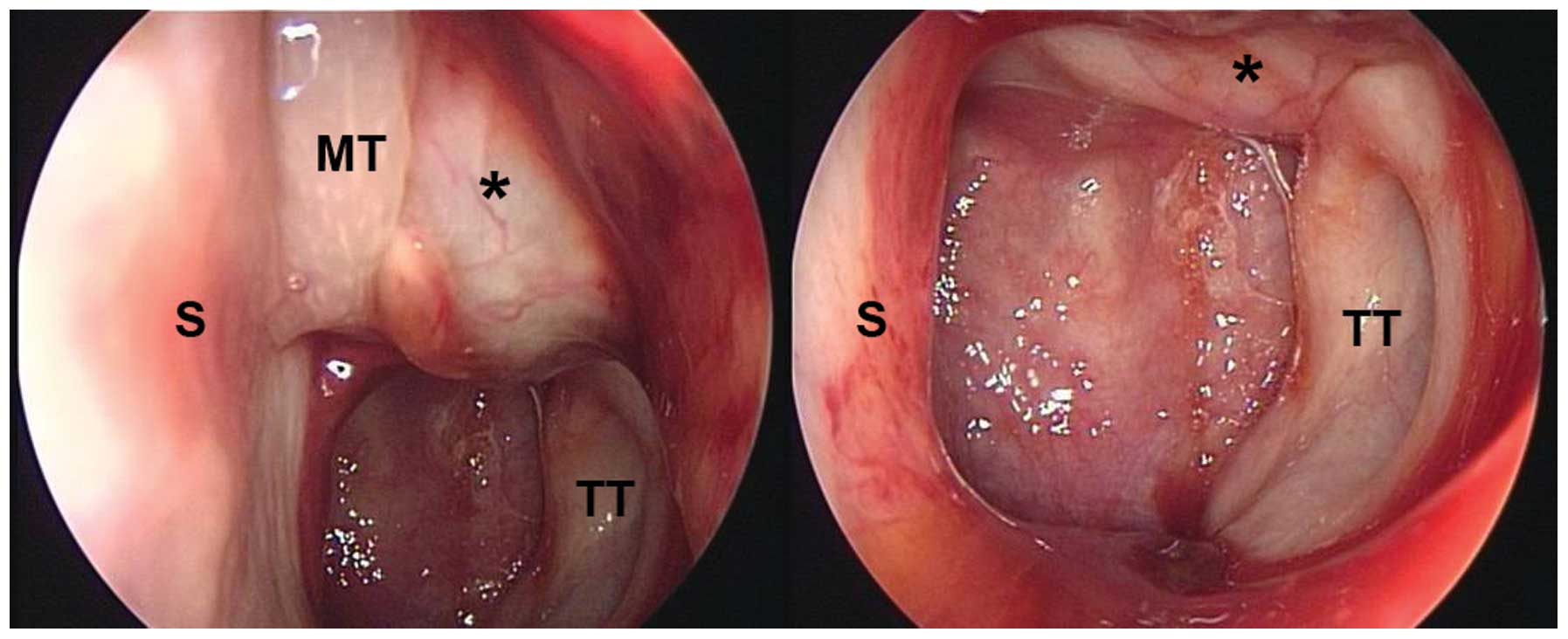
palsy. The nasal endoscopy revealed a friable mass with superficial
telangiectasia straddling the torus tubarius and extending forward
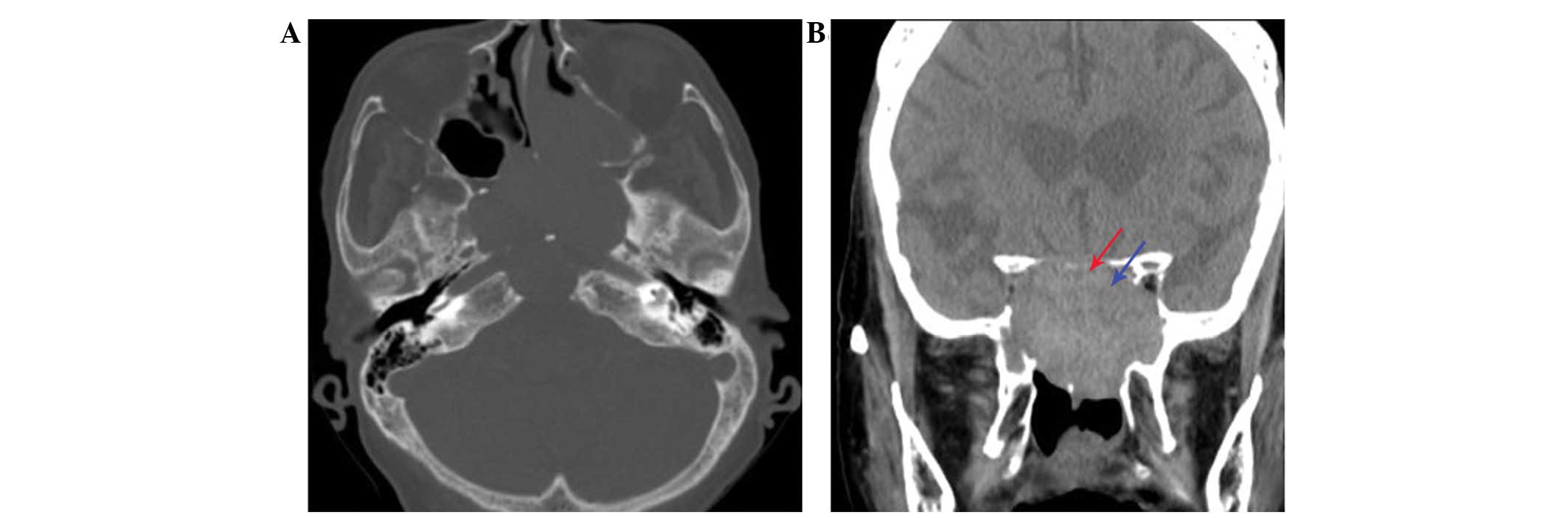
(Fig. 1). Non-contrast CT revealed an
aggressively enlarging lesion in the midline skull base, with
destructive bony structures (Fig. 2).
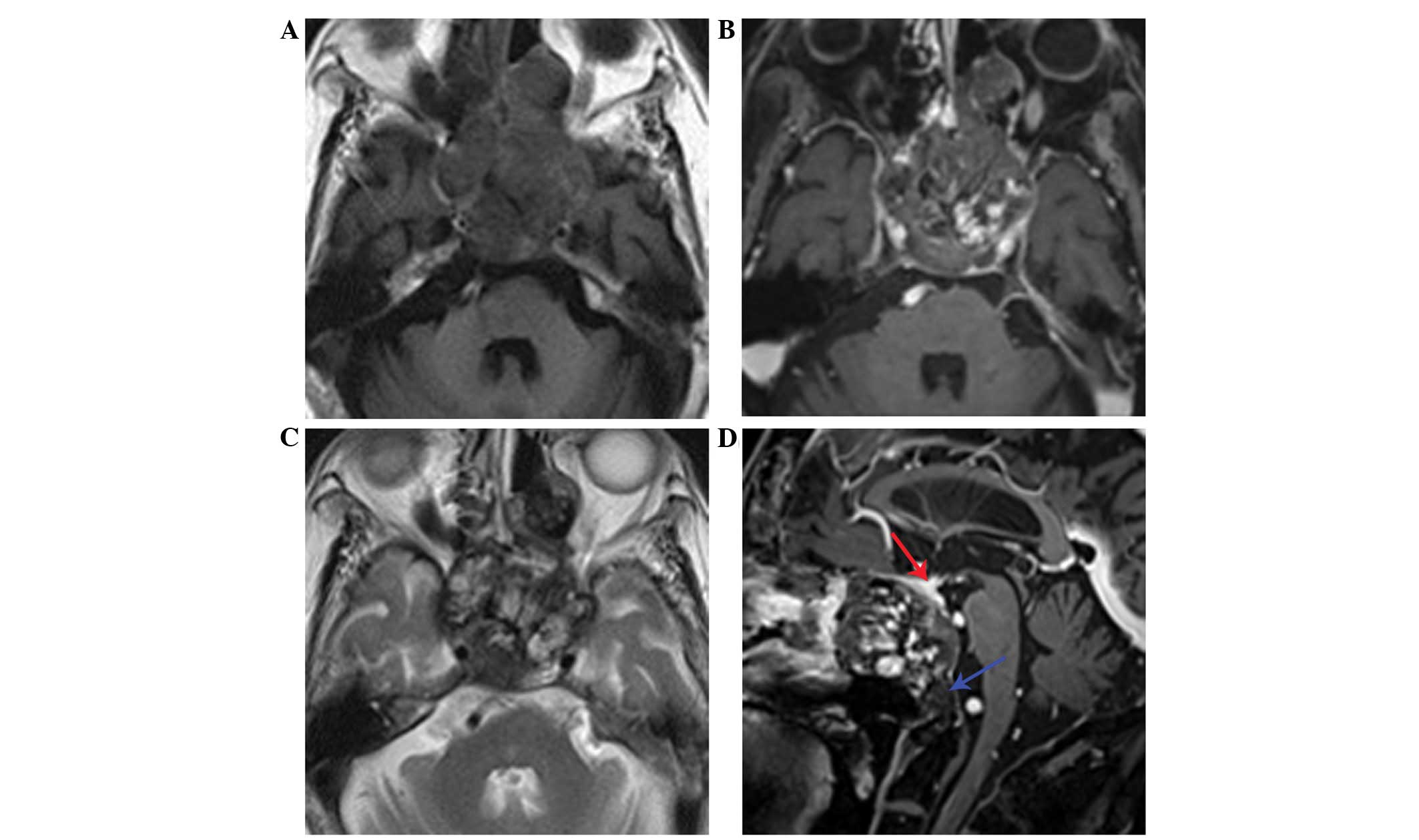
Distinctive internal heterogeneous hyperintensity and a hypointense
peripheral rim on T2WI, and a patchy enhancement pattern on
post-contrast T1W1 were observed (Fig.
3). Routine laboratory data revealed normal coagulation and
pituitary function profiles, including human growth hormone (0.23
ng/ml; normal range, 0.0–16 ng/ml), luteinizing hormone (31.35
mIU/ml; normal range, 11.3–38.7 mIU/ml), follicle stimulating
hormone (74.09 mIU/ml; normal range, 31.8–134 mIU/ml) and free
thyroxine (1.58 ng/dl; normal range, 0.8–1.9 ng/dl). Only high
sensitivity-thyroid-stimulating hormone levels were abnormal (0.245
uIU/ml; normal range, 0.4–4.0 uIU/ml).
In order to optimize surgical planning, the patient
underwent a biopsy, which yielded a negative result. The patient
subsequently underwent endoscopic endonasal surgery to resect the
lesion; a posterior ethmoidectomy, sphenoidotomy and septoplasty
were required in order to gain complete surgical access. A
well-capsulated, russet-colored and blood clot-containing tumor was
removed in a piece-by-piece manner, leaving the periosteal layer of
dura mater that protected the intracranial structures intact. The
thinned bony components located above the right orbital apex and
right optic canal were gently flaked off. The surgical extent was
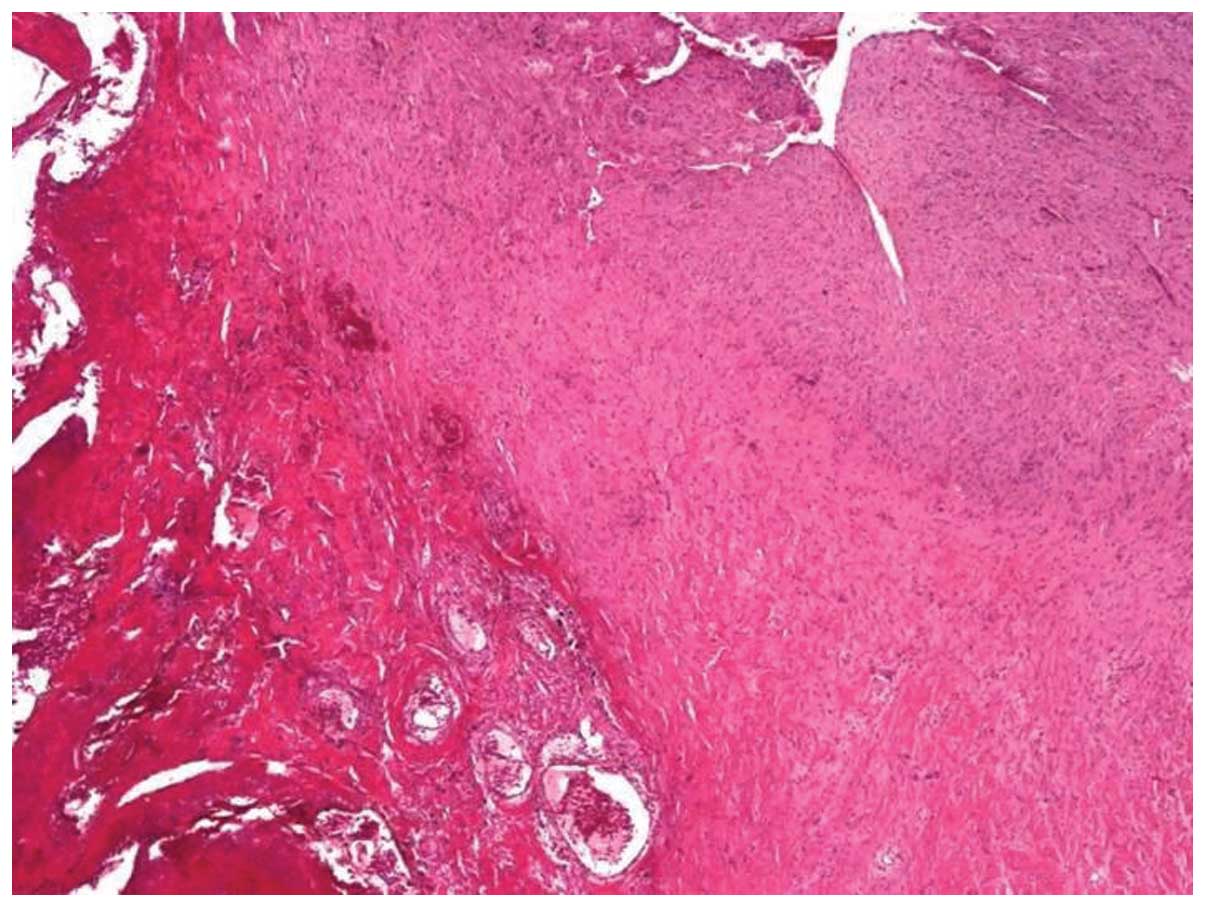
explored inferiorly to the upper clivus. A sample of the lesion
sent for pathological analysis revealed an OH without any evidence
of fungal hyphae (Fig. 4). The
immediate post-operative course was uncomplicated, with resolution
of the patient's headache. At the 2-month follow-up, the subject
had recovered fully, with the exception of the lost vision.
Written informed consent was obtained from the
patient for publication of the present case report and any
accompanying images.
Discussion
Sphenoid disease is primarily a result of the
extension of disease anteriorly from the ethmoid complex or
posteriorly from the adjacent anatomical sites, including the sella
turcica and the surrounding skull base structures (8). Isolated sphenoid sinus disease is rarely
encountered and the associated symptoms are generally non-specific
(8). Sphenoid disease frequently
leads to nerve involvement, and the effects of masses causing
compression, destruction or invasion lead to varying levels of
impact on the extent and degree of neurological deficits (8). Etiologically, non-neoplastic lesions
constitute the majority of cases, while fungal processes are the
third most common cause (8). ISSA is
a type of fungal sinus infection that is distinct from other
categories of fungal rhinosinusitis, and is characterized by higher
levels of occurrence in older women (9). A sphenoid sinusotomy is regarded to be
the primary therapy (8,9). To the best of our knowledge, the
occurrence of an OH of the sphenoid sinus following ISSA surgery
has not been previously reported (2–6,8,9).
SNOH is rarely encountered in clinical practice
(2–6).
When it does occur, it is usually locally invasive and demonstrates
a high tendency to occur in the maxillary sinus (3,5,6,10). The
incidence of SNOH appears to be most frequent in the population of
East Asia (3,5,6,10). The exact underlying etiological
mechanisms remain to be elucidated (4,5,6). Ozaki et al (11) advanced what is now the most
widely-accepted hypothesis, the negative spiral theory (3,6). The
negative spiral theory assumes that an OH results from persistent
negative intraluminal pressure following an initial episodic
hemorrhage into a semi-closed cavity, leading to repeated rupturing
of fragile mucosal vessels. Subsequent formation of a superficial
fibrotic capsule prevents further reabsorption of the hematoma.
Under the succession of biological healing processes, an OH emerges
(3,6).
The progressive expansion of the OH results in augmentation of the
sinus antrum, and therefore results in demineralization of the
adjacent skeletal structures (3,5).
Macroscopically, SNOH is described as a
slow-growing, well-circumscribed and friable brownish mass. The
histopathological findings reveal a mixture of neovascularization,
fibrosis, hemorrhaging and hemosiderin deposition (5). A number of studies have attempted to
identify an association between SNOH and sinonasal angiomatous
polyps, a rare subtype of inflammatory sinonasal polyp, using
radiology and histopathology (10,12).
However, this association remains to be fully elucidated. Returning
to the present case, the causative etiology may be attributed to an
obstructed sinus cavity with equivalent volume to the maxillary
sinus, and repetitive post-operative hemorrhaging due to an
inflammatory vascular injury. To the best of our knowledge, such a
case was previously unknown, and has not been reported in the
relevant literature (5). It may be
postulated that the incidence of post-operative SNOH is
underestimated. Long-term follow-up for patients undergoing
endoscopic sinus surgery is necessary to provide an answer to this
hypothesis.
CT possesses an advantage over MRI in terms of its
ability to analyze the integrity of bone in detail (5). The density in unenhanced CT scans of OH
is frequently hyperattenuated compared with masticator muscles. The
hallmarks of OH are mucoperiosteal thickening, occasional
calcification, convex bowing of natural skeletal architecture,
cortical thinning or direct extension to adjacent structures
sparing frank osseous destruction (5,6). However,
CT alone does not provide enough information to allow for the
differentiation of OH from locally aggressive neoplasms. MRI
exhibits marked superiority over CT in terms of its ability to
determine the margin and true extent of tumor expansion. In
addition, MRI possesses an advantage with regard to distinguishing
adjacent secondary inflammation and nasal secretions from a tumor
mass, and has the ability to display the corresponding pathological
components within the lesion (2,5).
Typically, SNOH exhibits a mosaic of varying signal intensities in
T1WI and T2WI, and heterogeneous enhancement in a patchy pattern
following contrast administration (2,5,10). For SNOH, the most conclusive
diagnostic finding is a hypointense zone surrounding the lesion on
T2WI, indicating the pathological feature of a fibrous capsule
(2,5,10).
Therefore, obtaining knowledge of the distinctive characteristic
observations acquired using CT and MRI (2,5,10) offers clinicians valuable anatomical
and diagnostic informative clues that may allow for the achievement
of a correct diagnosis of SNOH.
To the best of our knowledge, the present study
reports the first case of an OH of the sphenoid sinus in a patient
who previously underwent ISSA surgery. The case highlights the
requirement for physicians to include SNOH in the differential
diagnosis for expansile sphenoid sinus disease, regardless of its
low prevalence. Advances in techniques mean that the performance of
endoscopic endonasal skull base surgery is clinically feasible and
reliable for the treatment of patients exhibiting SNOH (7), particularly for cases in which patients
exhibit compressive neuropathy, and timely intervention is required
in order to avoid permanent sequelae (13). The present case additionally suggests
that a preexisting inflammatory process and performance of previous
surgery may have a role in multifaceted SNOH pathogenesis. However,
increased experience with regard to the diagnosis and treatment of
sphenoid SNOH is required in order to fully elucidate the
underlying mechanisms of the disease.
In conclusion, although the pathogenesis and
epidemiology of SNOH are not fully understood, the application of
CT and MRI, combined with the involvement of a diagnosing physician
who possesses familiarity with the classical imaging findings,
allows for an accurate pre-operative diagnosis of SNOH. Care should
be exercised when interpreting the abnormal imaging results
acquired from a sinus that has been operated on, in order to ensure
that SNOH is not misidentified as recurrence or residual disease.
Once a diagnosis has been reached, following consultation of the CT
and MRI scans, the current understanding is that a complete
surgical excision is the optimal treatment for SNOH, yielding
positive patient outcomes and rarely resulting in recurrence later
in life.
Glossary
Abbreviations
Abbreviations:
|
SNOH
|
sinonasal organized hematoma
|
|
OH
|
organized hematoma
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
T1WI
|
T1-weighted imaging
|
|
T2WI
|
T2-weighted imaging
|
|
ISSA
|
isolated sphenoid sinus
aspergilloma
|
References
|
1
|
Tadokoro K: Jogakudo ketsuryu ni tsuite.
Dainichijibi. 23:359–360. 1917.(In Japanese).
|
|
2
|
Wu AW, Ting JY, Borgie RC, Busaba NY,
Sadow PM, Juliano AF, Gray ST and Holbrook EH: Diagnostic
characteristics of sinonasal organizing hematomas: Avoiding
misdiagnosis. Int Forum Allergy Rhinol. 3:598–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Omura G, Watanabe K, Fujishiro Y, Ebihara
Y, Nakao K and Asakage T: Organized hematoma in the paranasal sinus
and nasal cavity - imaging diagnosis and pathological findings.
Auris Nasus Larynx. 37:173–177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakagawa T, Kawai Y, Sakamoto T and Ito J:
Organised haematoma of the sphenoid sinus mimicking a pituitary
tumour. J Laryngol Otol. 124:83–85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim EY, Kim HJ, Chung SK, Dhong HJ, Kim
HY, Yim YJ, Kim ST, Jeon P and Ko YH: Sinonasal organized hematoma:
CT and MR imaging findings. AJNR Am J Neuroradiol. 29:1204–1208.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee PK, Wu JK and Ludemann JP: Hemorrhagic
pseudotumour of the maxillary sinus. J Otolaryngol. 33:206–208.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee LA, Huang CC and Lee TJ: Prolonged
visual disturbance secondary to isolated sphenoid sinus disease.
Laryngoscope. 114:986–990. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ng YH and Sethi DS: Isolated sphenoid
sinus disease: Differential diagnosis and management. Curr Opin
Otolaryngol Head Neck Surg. 19:16–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chakrabarti A, Denning DW, Ferguson BJ,
Ponikau J, Buzina W, Kita H, Marple B, Panda N, Vlaminck S,
Kauffmann-Lacroix C, et al: Fungal rhinosinusitis: A categorization
and definitional schema addressing current controversies.
Laryngoscope. 119:1809–1818. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang YZ, Yang BT, Wang ZC, Song L and Xian
JF: MR evaluation of sinonasal angiomatous polyp. AJNR Am J
Neuroradiol. 33:767–772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ozaki M, Sakai S and Ikeda H: Hemangioma
of the nasal cavity and sinuses - a report of twenty five cases.
Otolaryngol Head Neck Surg (Tokyo). 49:53–58. 1977.
|
|
12
|
Yfantis HG, Drachenberg CB, Gray W and
Papadimitriou JC: Angiectatic nasal polyps that clinically simulate
a malignant process: report of 2 cases and review of the
literature. Arch Pathol Lab Med. 124:406–410. 2000.PubMed/NCBI
|
|
13
|
Castelnuovo P, Dallan I, Battaglia P and
Bignami M: Endoscopic endonasal skull base surgery: Past, present
and future. Eur Arch Otorhinolaryngol. 267:649–663. 2010.
View Article : Google Scholar : PubMed/NCBI
|