Introduction
Gastric cancer patients who receive a standard
gastrectomy with lymph node dissection can suffer from
post-gastrectomy syndromes (1). As
the symptoms associated with these syndromes can cause lifelong
problems, a function-preserving gastrectomy with limited
lymphadenectomy is recommended to improve post-operative quality of
life (1). A sentinel node (SN) biopsy
is the most reliable method to date for the detection of
node-negative cases, along with the application of an oncologically
safe function-preserving gastrectomy.
The SN is defined as the node that directly receives
lymphatic drainage from a primary tumor (2). Gastric cancer is one of the cancers in
which the validity of the SN concept has been well investigated by
numerous studies (3–17). Recently, a prospective multicenter
trial demonstrated the feasibility of SN biopsy in gastric cancer
(3). However, a number of unresolved
issues remain regarding the clinical use of SN biopsy for gastric
cancer, including identification of an adequate tracer for
laparoscopic gastrectomy (3,11,12). The
dual-tracer method with blue dye and radioactive colloid appears to
be a temporary standard procedure in gastric cancer. However, blue
dye deteriorates quickly, and radioactive colloid exhibits a
shine-through effect during γ probe detection of hot nodes. These
weaknesses limit their usefulness for laparoscopic SN biopsy.
One tracer candidate being evaluated for
laparoscopic gastrectomy is indocyanine green (ICG). With the
recent invention of useful devices such as the Photodynamic Eye
(PDE) and Hyper Medical Eye System, ICG fluorescence imaging for SN
biopsy has been widely used in various cancer surgeries (18–27). ICG
fluorescence imaging has unique characteristics, including an
extra-high sensitivity (26,28) and signal stability (23,26,28).
However, the optimal settings of ICG fluorescence imaging for SN
biopsy, such as ICG dose and concentration, injection timing and
biopsy technique, have not been determined. Furthermore, little is
known regarding the weaknesses of this technique.
The present study determined whether ICG
fluorescence imaging is a suitable method for SN biopsy in
laparoscopic gastrectomy. In addition, the optimal setting and
weaknesses of this method were investigated.
Patients and methods
Patients
Between October 2009 and December 2014, 72 patients
with histologically confirmed clinical type 0 (superficial-type)
adenocarcinoma of the stomach (29),
single primary lesions <5 cm in diameter, no previous history of
chemotherapy, and no distant metastasis (cM0) or evident nodal
metastasis (cN0) and out of indication of endoscopic submucosal
dissection were enrolled in the study. Clinical staging was
determined by pre-operative endoscopy and multidetector-row
computed tomography. All patients enrolled in the study were
pre-operatively registered in the data center in Kanazawa Medical
University Hospital (Ishikawa, Japan).
All patients provided written informed consent. This
study was approved by the Ethics Committee of Kanazawa Medical
University and conducted in accordance with the Good Clinical
Practice guidelines and Declaration of Helsinki.
SN mapping procedure
The PDE (Hamamatsu Photonics Co., Ltd., Hamamatsu,
Shizuoka, Japan) or PDE neo was used to detect ICG fluorescence.
PDE neo is a newer version of the PDE with similar sensor
sensitivity. A single, well-trained surgeon performed all tracer
injections, surgeries, mappings and nodal harvestings to avoid
disruption of the learning phase. During the study period,
laparoscope-assisted gastrectomy was categorized as clinical
research in the Gastric Cancer Treatment Guidelines of the Japanese
Gastric Cancer Association (30).
Therefore, the surgical approach adopted (open or
laparoscope-assisted) was based on patient decision under informed
consent.
The day prior to the surgery, ICG solution was
endoscopically injected into four quadrants of the submucosal layer
of the primary lesion using an endoscopic puncture needle. The
gastrocolic ligament was divided to visualize all possible
directions of lymphatic flow from the stomach. In open surgery
cases, the PDE or PDE neo was then used to detect ICG fluorescence.
For laparoscope-assisted surgery cases, the stomach and perigastric
lymph nodes were pulled up and exposed from a 4–6 cm median upper
abdominal incision, and ICG fluorescence in the lymphatic vessels
and lymph nodes was detected with the PDE or PDE neo in the same
manner as used for open surgery. Bright nodes were defined as
clearly fluorescent nodes. The bright nodes were believed to
contain the SNs. The number and direction of the lymphatic basins
were observed and recorded intraoperatively by the surgeon.
Lymphatic basins were defined as lymphatic zones divided by the
stream of fluorescent lymphatic canals. The proximal border was the
fatty tissue attached to the stomach wall, and the distal border
was the front of the bright node most distal from the stomach
(4,5).
SN biopsy procedures
The feasibility of ICG imaging for SN biopsy was
evaluated from the beginning of the study until December 2012 in 42
sequential patients who received standard gastrectomy with lymph
node dissection up to D1+ or D2 according to the Gastric Cancer
Treatment Guidelines (30). After
noting the state of the lymphatic basin, a standard gastrectomy
with lymph node dissection was conducted. Bright node detection was
performed post-operatively in the initial 16 cases. Subsequent to
surgery, all dissected nodes were harvested and analyzed for
fluorescence using the PDE. Intraoperative bright nodes biopsy was
performed in the other 26 cases. Following stomach removal and
nodal dissection, but prior to reconstruction, bright nodes were
detected and harvested at the surgical field and sent for
pathological diagnosis of the intraoperative frozen section.
After the feasibility phase of the study, the
clinical application of ICG SN mapping in guiding limited surgery
was evaluated from January 2013. In this phase of the study,
patients received function-preserving gastrectomy with lymphatic
basin dissection. Function-preserving gastrectomies included
pylorus-preserving gastrectomy, proximal gastrectomy, segmental
gastrectomy and local resection of the stomach. Lymphatic basin
dissection is a selective lymphadenectomy to dissect en bloc the
lymphatic basins that contain lymph nodes and lymphatic vessels
(4,5).
Following resection, the bright nodes were detected and harvested
at the surgical field and sent for pathological diagnosis of the
intraoperative frozen section. The surgeon performed the
reconstruction while waiting for the pathology results. For bright
nodes without metastasis, the surgical team continued the
reconstruction and finished the procedure. For cases with nodal
metastasis, the surgery was converted to a standard D2 gastrectomy
(distal gastrectomy or total gastrectomy).
Post-operatively, all dissected nodes were
reexamined for fluorescence using the PDE. Therefore, there were
two types of bright nodes, nodes detected intraoperatively and
those detected post-operatively.
Pathological examination
The frozen section and permanent diagnoses of nodal
metastasis were made on a plane of the maximal dimension containing
the hilus of the node according to the Japanese Classification of
Gastric Carcinoma (29). The
intraoperative frozen section diagnosis was followed by an
additional permanent pathological diagnosis following node fixation
in formaldehyde. Nodal metastasis was defined as the presence of
tumor cells, tumor cell clusters or tumor cell nests within the
lymph node, as detected by hematoxylin-eosin staining. Size was not
included as a criterion for nodal metastasis and therefore, nodal
metastasis included micrometastases and isolated tumor cells. The
regional lymph nodes of the stomach were classified into stations
numbered according to the Japanese Classification of Gastric
Carcinoma (29).
Determination of the adequate
concentration and injection volume of ICG
To determine the adequate ICG concentration for SN
biopsy, 0.5 mlx4 points of 2.5 mg/ml, 125 µg/ml, 50 µg/ml and 5
µg/ml ICG were preliminarily examined in one or two cases. After
identifying the adequate concentration, ICG injection volumes of
0.5 mlx4 points and 0.2 mlx4 points were evaluated.
Results
Patients
A total of 72 patients were enrolled in the study.
This consisted of 42 patients for the feasibility phase and 30
patients for the clinical application phase. Patient
characteristics are shown in Table I.
A laparoscopic approach was performed in 55.6% of patients. No
serious allergic reactions were observed following tracer
injection. Intraoperative and post-operative adverse effects and
complications associated with the SN biopsy procedures were not
observed.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Feasibility phase
(n=42) | Application phase
(n=30) |
|---|
| Median Age (range),
years | 68 (47–82) | 71 (54–84) |
| Male/female, n | 26/16 | 18/12 |
| Tumor location,
n |
|
|
| Upper
third G/A/L/P | 1/0/3/1 | 1/0/8/2 |
| Middle
third G/A/L/P | 3/3/15/2 | 4/2/4/2 |
| Lower
third G/A/L/P | 6/2/6/0 | 0/2/3/2 |
| Pathological depth
of invasion |
|
|
|
T1a(M)/T1b(SM)/T2(MP)/T3 (SS),
n | 16/17/8/1 | 13/14/2/1 |
| Median
tumor size post-gastrectomy (range), mm | 28 (10–55) | 27 (8–65) |
| Macroscopic
findings, n |
|
|
|
Elevated/depressed |
9/33 | 10/20 |
| Surgical approach,
n |
|
|
|
Open/laparoscopic | 15/27 | 17/13 |
SN biopsy results
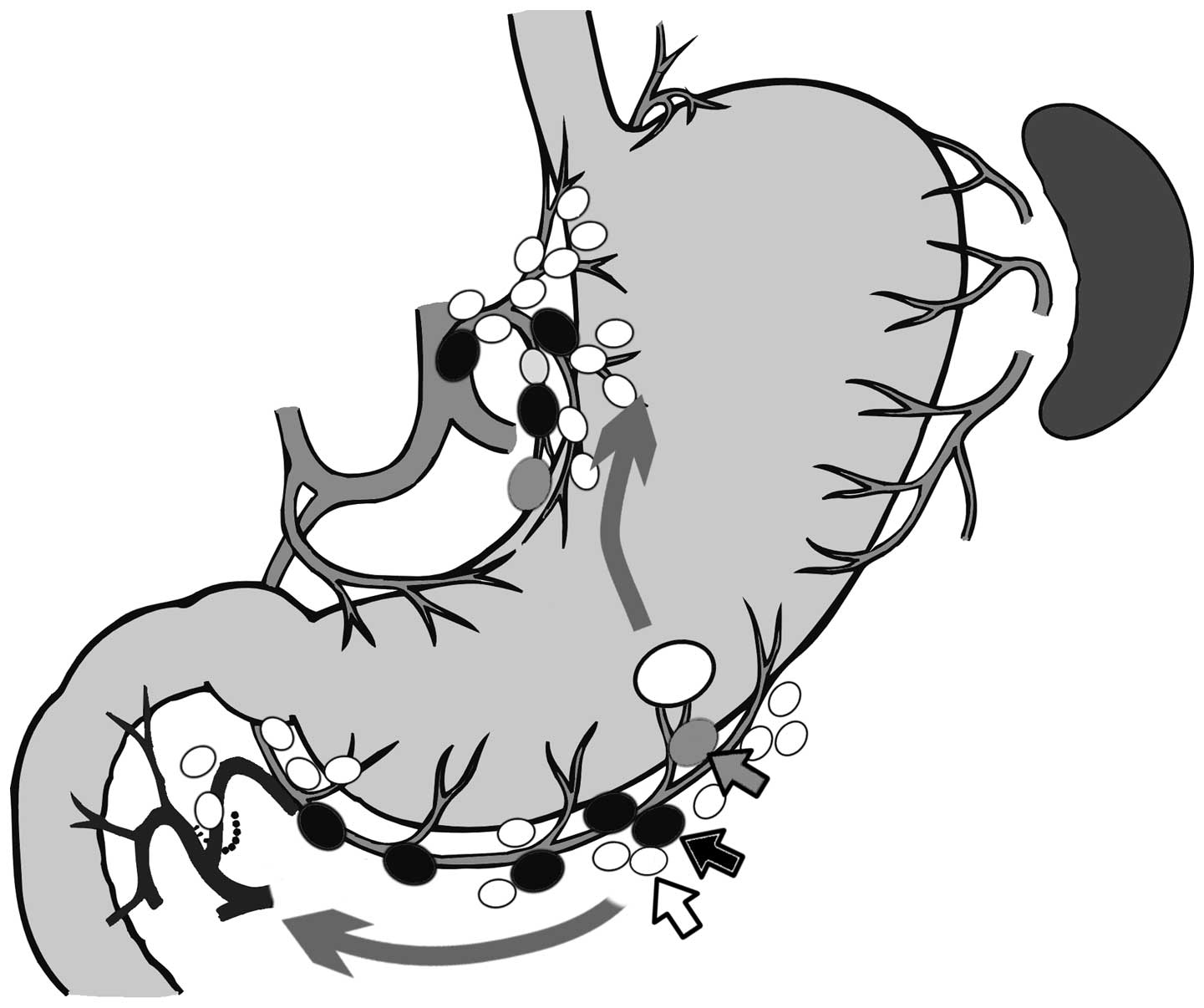
Bright nodes and lymphatic basins were detected in
all cases (Fig. 1); therefore, the
detection rate of ICG fluorescence imaging was 100.0%. The
sensitivity, specificity and accuracy of ICG fluorescence SN
mapping were 90.1, 100.0 and 98.6%, respectively (Table II).
 | Table II.Bright nodes biopsy results for the
nodal metastasis cases in the feasibility (n=42) and clinical
(n=30) phases. |
Table II.
Bright nodes biopsy results for the
nodal metastasis cases in the feasibility (n=42) and clinical
(n=30) phases.
|
| Nodal status of all
dissected nodes |
|---|
|
|
|
|---|
| Status of sentinel
node biopsy | Metastasis | No metastasis |
|---|
| Feasibility phase,
n |
|
|
| Bright
node biopsy pathological diagnosis pathological diagnosis |
|
|
|
Metastasis | 7 |
|
|
No metastasis | 0 | 35 |
| Clinical phase,
n |
| Bright
node biopsy frozen section diagnosis |
|
|
|
Metastasis | 3 |
|
|
No metastasis | 1 | 26 |
| Sensitivity %
(n/total n) | 90.1 (10/11) |
| Accuracy % (n/total
n) | 98.6 (71/72) |
In total, 11 cases presented with metastasis. In the
feasibility case series (n=42), 7 cases presented with lymph node
metastasis, and all cases were able to be diagnosed using bright
node biopsy. Of these 7 cases, 4 were diagnosed post-operatively in
the permanent pathological diagnosis and 3 were diagnosed
intraoperatively in the frozen section diagnosis. In the clinical
application series, 4 cases presented with lymph node metastasis,
and of these, 3 cases were able to be diagnosed by bright node
biopsy. Thus, 1 case with nodal metastasis failed to be diagnosed
intraoperatively.
Details of the false-negative
case
Intraoperative bright node biopsy resulted in 1
false-negative case. The false-negative result occurred in a
72-year-old female with a 20-mm IIc-type tumor with an ulcer scar
located on the greater curvature near the posterior wall of the
middle third of the stomach. The final pathological diagnosis was
non-solid type poorly-differentiated adenocarcinoma, submucosal
cancer with submucosal invasion of >500 µm from the muscularis
mucosa, marked lymphatic invasion and minimal venous invasion,
based on the Japanese Classification of Gastric Carcinoma (29). The lymphatic basins were the left
gastric artery and right gastroepiploic artery areas, and a
laparoscopic pylorus-preserving gastrectomy with lymphatic basin
dissection was performed. The station numbers of the dissected
nodes were nos. 1, 3a, 4d, 6 and 7. A total of 8 bright nodes were
detected intraoperatively and of these, 2 nodes were no. 3a, 1 node
was no. 7 and 5 nodes were no. 4d. Post-surgery, at the time of
nodal harvesting, 2 bright nodes were found at no. 3a and 4d. The
no. 3a node exhibited weak fluorescence and appeared to be a
secondary node. The no. 4d node exhibited strong fluorescence and
appeared to be a node that failed to be detected intraoperatively
due to the shine-through effect.
A total of 2 nodal metastases were present and were
not the bright nodes detected intraoperatively. The involved nodes
were both no. 4d and were located near the tumor. One of the nodes
was the bright node detected after surgery and was classified as a
micrometastasis. The other metastasis was a non-bright node located
near the bright node and was classified as isolated tumor cells.
After the metastases were diagnosed, deep cuttings and other slices
of the paraffin blocks of the intraoperatively detected bright
nodes were made and revealed the presence of isolated tumor cells
in one of the no. 4d bright nodes (Fig.
2). Lymph node dissection up to D1 was performed, and the
dissection margin appeared to be cancer free. Therefore, this case
was considered to be a successful curative resection. The patient
has since been closely followed up and has shown no evidence of
recurrence to date.
Adequate concentration and injection
volume of ICG
The adequate concentration of ICG was determined in
the initial 8 cases, which were all node-negative. Table III shows the association between the
number of bright nodes and ICG concentration. The cases were
arranged in order of occurrence to show the decision process. At an
ICG concentration of 5 µg/ml (×1,000), bright lymphatics and bright
nodes were detected despite the weak fluorescence. Therefore, the
adequate concentration of ICG was determined to be 50 µg/ml
(×100).
 | Table III.Association between the number of
bright nodes and indocyanine green concentration. |
Table III.
Association between the number of
bright nodes and indocyanine green concentration.
| Case no. | Concentration | Volume | No. of bright
nodes |
|---|
| 1 | x2 (2.5 mg/ml) | 0.5 mlx4 | 17 |
| 2 | x40 (125
µg/ml) | 0.5 mlx4 | 8 |
| 3 | x100 (50
µg/ml) | 0.5 mlx4 | 4 |
| 4 | x100 (50
µg/ml) | 0.5 mlx4 | 6 |
| 5 | x40 (125
µg/ml) | 0.5 mlx4 | 8 |
| 6 | x100 (50
µg/ml) | 0.5 mlx4 | 5 |
| 7 | x100 (50
µg/ml) | 0.5 mlx4 | 7 |
| 8 | x1000 (5
µg/ml) | 0.5 mlx4 | 3 |
| 9–23 | x100 (50
µg/ml) | 0.5 mlx4 | 6
(3–11) |
| 24–42 | x100 (50
µg/ml) | 0.2 mlx4 | 6 (2–7) |
Next, the adequate injection volume of 50 µg/ml ICG
was determined. A total of 15 and 19 sequential patients were
injected with 0.5 mlx4 points and 0.2 mlx4 points of 50 µg/ml ICG,
respectively. The visualization of bright nodes and bright
lymphatics was quite similar between the two groups. The median
number of bright nodes was not significantly different between the
two groups, with 6 (range, 3–11) in the 0.5 ml group and 6 (range,
2–7) in the 0.2 ml group. Therefore, the adequate injection volume
of ICG solution was determined to be 0.5 mlx4 points due to its
technical reliability.
Number of bright nodes
In the present study, 68 patients were injected with
50 µg/ml ICG solution. Of these patients, bright nodes were
detected post-operatively only in 14 patients, but both
intraoperatively and post-operatively in 54 patients. The median
number of bright nodes was 5 (range, 4–11) and 6 (range, 2–9) in
the post-operative only and intraoperative cases, respectively. In
27 out of the 54 intraoperative cases (50.0%), bright nodes left
behind were also detected post-operatively. The total number of
nodes in these cases was 49, and the median number of nodes per
each case was 1 (range, 0–4) (Table
IV). Of these 49 nodes, 31 (63%) exhibited weak fluorescence
and 18 (37%) exhibited strong fluorescence. Almost all strongly
fluorescent nodes were small nodes <5 mm in diameter, located
near the serosa of the stomach, covered by fat, and were difficult
to detect intraoperatively due to the shine-through effect of the
serosal bright spots. By contrast, the 31 nodes with weak
fluorescence were located far from the tumor and appeared to be
secondary nodes.
 | Table IV.Association between the number of
bright nodes and timing of nodal detection. |
Table IV.
Association between the number of
bright nodes and timing of nodal detection.
| Detection of bright
nodes | No. of cases | Median no. of
bright nodes (range) |
|---|
| Post-operative
only | 14 | 5 (4–11) |
| Intraoperative and
post-operative | 54 |
|
|
Intraoperative |
| 6 (2–9) |
|
Post-operative (pick-up
failure) |
| 1 (0–4) |
Distribution of metastatic nodes
All dissected nodes in this study were divided into
the following three groups: Bright nodes, non-bright nodes within
the lymphatic basin and non-bright nodes outside the lymphatic
basin. A total of 11 cases (15.3%) presented with nodal metastasis.
Lymph node dissection up to D2 was performed in 10 of these cases,
while the other case was the false-negative intraoperative case.
Therefore, the distribution of metastatic nodes within and outside
(up to D2) the lymphatic basins was well examined in these cases.
The total number of metastatic nodes was 35 and of these, 22
(62.9%) were bright nodes, 12 (34.3%) were non-bright nodes within
the basin and 1 (2.9%) was a non-bright node outside the basin.
Among the 11 nodal metastasis cases, all metastatic
nodes were bright nodes in 5 cases, and metastatic nodes were
bright and non-bright nodes within the basins in 5 cases. In the
remaining case, the metastatic node was located not only within the
basin, but also outside the basin. This case involved a 62-year-old
male whose tumor was located at the greater curvature of the lower
third of the stomach. The macroscopic findings revealed a IIc tumor
measuring 40×30 mm, and the pathological findings were moderately
differentiated tubular adenocarcinoma, tumor invasion of the
muscularis propria, tumor with mild infiltrating growth and mildly
indistinct border with the surrounding tissue, and moderate
lymphatic and venous invasion, based on the Japanese Classification
of Gastric Carcinoma (29). The
lymphatic basin was the right gastroepiploic artery area, and the 5
bright nodes were no. 4d. The pre-operative diagnosis based on
helical computed tomography was cN0, even though the patient had an
evident macroscopic metastatic node at station no. 6. The patient
presented with 7 metastatic nodes consisting of 2 bright nodes, 4
non-bright nodes within the basin and 1 non-bright node outside the
basin (no. 7). This case was believed to be an out of indication
for SN biopsy due to the presence of macrometastases and the ease
of metastasis diagnosis.
Clinical application of SN biopsy with
ICG
In total, 30 cases were used to evaluate the
usefulness of ICG fluorescent SN navigation to guide limited
surgery. The specifics of the surgeries are shown in Table V. A function-preserving curative
gastrectomy with lymphatic basin dissection (limited surgeries
beyond the guideline procedures) was performed in 20 out of the 30
cases (66.7%). No evidence of recurrence has been observed in any
of these cases to date.
 | Table V.Surgeries performed in the
feasibility and clinical phases. |
Table V.
Surgeries performed in the
feasibility and clinical phases.
| A, Feasibility
phase (n=42) |
|---|
|
|---|
| Type | Procedure | No. |
|---|
| Meta(+) | DG | 2 |
|
| LADG | 4 |
|
| PG | 1 |
| Meta(−) | DG | 6 |
|
| LADG | 10 |
|
| PPG | 2 |
|
| LAPPG | 13 |
|
| PG | 3 |
|
| TG | 1 |
|
| B, Clinical phase
(n=30)a |
|
| Type | Procedure | No. |
|
| Meta(+) | LADG | 3 |
|
| LAPPG | 1 |
| Meta(−) | DG | 1 |
|
| LADG | 2 |
|
| PPG | 2 |
|
| LAPPG | 1 |
|
| Limited
DG | 1 |
|
| LA-limited
DG | 1 |
|
| SG | 2 |
|
| LASG | 1 |
|
| Limited
PG | 6 |
|
| Local
resection | 4 |
|
| LA-local
resection | 5 |
Discussion
The validity of the SN concept remains under debate
even in gastric cancer. Over past decades, the application of SN
mapping in gastric cancer, which has a relatively complicated
lymphatic flow, has been a controversial issue (31). However, more recent prospective
studies have successfully demonstrated the utility of SN biopsy in
gastric cancer (6–10). Most recently, two large-scale
nationwide multicenter prospective studies, the JCOG0302 study
(11) and the SN navigation surgery
(SNNS) study by Kitagawa et al (3), were conducted to evaluate SN mapping in
gastric cancer. The JCOG0302 study provided evidence to support the
use of this technique in gastric cancer. However, the JCOG0302
trial was terminated due to the high proportion of false-negative
results obtained in the intraoperative histological examination
(11). The JCOG0302 study results
were affected by the intraoperative SN detection technique and
intraoperative diagnosis of nodal metastasis. By contrast, the SNNS
study was specifically designed to verify the SN concept in gastric
cancer (3).
SN biopsy has been considered to exhibit two main
roles in various cancers, namely, ultrastaging and the guidance of
lymph node dissection omission. In malignant melanoma and
colorectal cancer management, SN biopsy is primarily used for
ultrastaging (2,32). By contrast, SN biopsy is mainly used
to guide lymph node dissection omission in breast cancer surgery
(33). For gastric cancer, clinical
studies have primarily focused on the application of SN biopsy in
guiding lymph node dissection omission. However, lymph node
dissection omission deserves circumspection, as standard lymph node
dissection is associated with an improved prognosis in gastric
cancer patients (34). Furthermore,
in contrast to breast cancer, reoperation for additional nodal
dissection is rarely performed in gastric cancer (5).
SN biopsy is a complex multistep surgical technique.
This technique requires a suitable limitation of the indication,
the selection of an adequate tracer, a proper tracer injection
method, the objective detection of tracer uptake by the nodes, a
reliable biopsy technique for the nodes that show tracer uptake and
the precise intraoperative detection (micrometastasis level) of
nodal metastasis. A number of studies have provided recommendations
regarding the suitable indication and tracer injection method for
SN biopsy in gastric cancer. In a meta-analysis of SN biopsy for
gastric cancer by Wang et al (12), early T stage, submucosal injection
method, combined tracers, conventional open surgery and
immunohistochemistry application were demonstrated to be associated
with a higher rate of SN identification and higher sensitivity.
The selection of an adequate tracer for SN mapping
in gastric cancer has been an important issue (3,11,12). A temporary standard is combination
mapping with technetium-99m tin colloid and isosulfan blue.
However, blue dye deteriorates quickly, and radioactive colloids
exhibit a shine-through effect during γ probe detection of hot
nodes in the surgical field. We believe that combination mapping is
not suitable for laparoscopic gastrectomy.
ICG is a promising tracer candidate for laparoscopic
gastrectomy (14,15). ICG absorbs light in the near-infrared
range, with maximum absorption at the 800-nm wavelength. ICG also
emits maximal fluorescence at the 840-nm wavelength upon binding to
plasma proteins. Nimura et al (13) developed an infrared ray electronic
endoscopy system for ICG detection. Kusano et al (14) demonstrated the high sensitivity of
this ICG fluorescence imaging system for SN mapping in gastric
cancer. The advantages of ICG fluorescence imaging are the low cost
of ICG, lower equipment costs compared with radioactive tracers, no
requirement for radioactivity, a lower frequency of allergic
reactions compared with blue dye, the ability to detect bright
nodes under thick adipose tissue (14), clear visualization, easy detection of
bright nodes and lymphatic canals compared with the naked eye or
infrared ray imaging (23), an extra
high sensitivity capable of detecting minute concentrations of ICG
(26,28) and signal stability (23,26,28).
Signal stability is a unique characteristic of ICG fluorescence
imaging (15). The stability of ICG
fluorescence imaging allowed us to successfully use pre-operative
injections for SN detection. However, ICG fluorescence imaging also
has certain clear disadvantages, such as the requirement for
fluorescence detection equipment and a potential shine-through
effect. The high sensitivity of this technique is also a
disadvantage, as it can cause secondary node contamination of
bright nodes.
Based on our experience, 50 µg/ml ICG is adequate
for detection by the PDE. At this concentration, the median number
of bright nodes was 6 in the present study, which is similar to the
number obtained in our previous dye method study (4). Furthermore, good results in sensitivity
and accuracy were achieved regardless of surgical approach, i.e.,
laparoscopic or open surgery. A 50 µg/ml ICG concentration was also
used with the HyperEye Medical System in the study by Yoshida et
al (26). Nevertheless, the
proper concentration of ICG should be reexamined if other detection
devices are used.
SN biopsy for gastric cancer also requires a
reliable bright node biopsy technique and objective bright node
detection. With regard to biopsy technique, the lymphatic basin
dissection method must be used even for ICG fluorescence imaging.
Unlike other cancers, lymphatic basin dissection is a standard
technique in gastric cancer (4,5). The
lymphatic basins are believed to be the primary lymphatic drainage
area in each patient, and those individuals with gastric cancer
often exhibited two or three basins (3,4,5,16,17). Lymphatic basin dissection is superior
to the ordinary pick-up method not only in terms of preventing
missed bright nodes, but also with regard to oncological safety, as
it complements the intraoperative pathological diagnosis by serving
as a backup dissection (5,16,17). In
contrast to the biopsy technique, objective bright node detection
remains an unresolved issue of SN biopsy. In the present study, a
few bright nodes were detected post-operatively in the
intraoperative cases. These nodes were left behind in the lymphatic
basin following the intraoperative biopsy of the bright nodes and
were of two types: Strongly bright nodes that were missed in the
intraoperative biopsy due to the shine-through effect of the
primary lesion, and weakly bright nodes that were believed to be
secondary nodes. The shine-through effect could be eliminated by
using the lymphatic basin dissection method and performing bright
node detection after dissection of the basin from the gastric wall.
However, secondary node contamination is problematic, as true SNs
are difficult to distinguish from secondary nodes. Novel
fluorescence agents have already been developed that have
fluorescence and colloid particle characteristics (35–39). These
novel agents would only detect fluorescent SNs and not secondary
nodes, and therefore, would be the most potentially useful for
conducting a laparoscopic SN biopsy in gastric cancer. Furthermore,
they may potentially be used alone as a standard tracer instead of
in combination with other SN mapping tracers.
The most important issue in performing SN biopsy is
the intraoperative diagnosis of nodal metastasis. Frozen section
diagnosis with rapid hematoxylin-eosin staining can potentially
result in misdiagnosis. In the present series, only one
false-negative case occurred due to the failure of the frozen
section diagnosis. To solve this issue and establish oncological
safety, molecular methods to diagnose nodal metastasis, such as
reverse transcriptase-polymerase chain reaction and one-step
nucleic acid amplification assays, should be developed for clinical
use (40,41).
In conclusion, ICG fluorescence imaging for SN
biopsy is feasible in open and laparoscopic surgery for early
gastric cancer. The optimal tracer setting to use with the PDE is
an endoscopic submucosal injection of 0.5 ml of 50 µg/ml ICG at
four points surrounding the tumor the day prior to surgery. The
distribution of metastatic nodes in ICG fluorescence mapping is
consistent with the basin theory (3,4,5). Therefore, ICG fluorescence SN biopsy
could be used to guide lymph node dissection omission, thereby
allowing for function-preserving gastrectomy with lymphatic basin
dissection. A weakness of ICG fluorescence imaging is the
subjectivity of SN evaluation and potential secondary node
contamination. The present study findings also suggested the
requirement for the molecular diagnosis of nodal metastasis.
References
|
1
|
Eagon JC, Miedema BW and Kelly KA:
Postgastrectomy syndromes. Surg Clin North Am. 72:445–465.
1992.PubMed/NCBI
|
|
2
|
Morton DL, Wen DR, Wong JH, Economou JS,
Cagle LA, Storm FK, Foshag LJ and Cochran AJ: Technical details of
intraoperative lymphatic mapping for early stage melanoma. Arch
Surg. 127:392–399. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe
S, Terashima M, Murakami N, Fujimura T, Tsujimoto H, Hayashi H,
Yoshimizu N, et al: Sentinel node mapping for gastric cancer: A
prospective multicenter trial in Japan. J Clin Oncol. 31:3704–3710.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miwa K, Kinami S, Taniguchi K, Fushida S,
Fujimura T and Nonomura A: Mapping sentinel nodes in patients with
early-stage gastric carcinoma. Br J Surg. 90:178–182. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kinami S, Fujimura T, Ojima E, Fushida S,
Ojima T, Funaki H, Fujita H, Takamura H, Ninomiya I, Nishimura G,
et al: PTD classification: Proposal for a new classification of
gastric cancer location based on physiological lymphatic flow. Int
J Clin Oncol. 13:320–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miwa K: Sentinel node concept and its
application for cancer surgery. Nihon Geka Gakkai Zasshi.
101:307–310. 2000.(In Japanese). PubMed/NCBI
|
|
7
|
Hiratsuka M, Miyashiro I, Ishikawa O,
Furukawa H, Motomura K, Ohigashi H, Kameyama M, Sasaki Y, Kabuto T,
Ishiguro S, et al: Application of sentinel node biopsy to gastric
cancer surgery. Surgery. 129:335–340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carlini M, Carboni F, Petric M, Santoro R,
Guadagni F, Marandino F, Castelli M and Santoro E: Sentinel node in
gastric cancer surgery. J Exp Clin Cancer Res. 21:469–473.
2002.PubMed/NCBI
|
|
9
|
Ichikura T, Morita D, Uchida T, Okura E,
Majima T, Ogawa T and Mochizuki H: Sentinel node concept in gastric
carcinoma. World J Surg. 26:318–322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kitagawa Y, Fujii H, Mukai M, Kubota T,
Otani Y and Kitajima M: Radio-guided sentinel node detection for
gastric cancer. Br J Surg. 89:604–608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyashiro I, Hiratsuka M, Sasako M, Sano
T, Mizusawa J, Nakamura K, Nashimoto A, Tsuburaya A and Fukushima
N: Gastric Cancer Surgical Study Group (GCSSG) in the Japan
Clinical Oncology Group (JCOG): High false-negative proportion of
intraoperative histological examination as a serious problem for
clinical application of sentinel node biopsy for early gastric
cancer: Final results of the Japan Clinical Oncology Group
multicenter trial JCOG0302. Gastric Cancer. 17:316–323. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z, Dong ZY, Chen JQ and Liu JL:
Diagnostic value of sentinel lymph node biopsy in gastric cancer: A
meta-analysis. Ann Surg Oncol. 19:1541–1550. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nimura H, Narimiya N, Mitsumori N,
Yamazaki Y, Yanaga K and Urashima M: Infrared ray electronic
endoscopy combined with indocyanine green injection for detection
of sentinel nodes of patients with gastric cancer. Br J Surg.
91:575–579. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kusano M, Tajima Y, Yamazaki K, Kato M,
Watanabe M and Miwa M: Sentinel node mapping guided by indocyanine
green fluorescence imaging: A new method for sentinel node
navigation surgery in gastrointestinal cancer. Dig Surg.
25:103–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tajima Y, Murakami M, Yamazaki K, Masuda
Y, Kato M, Sato A, Goto S, Otsuka K, Kato T and Kusano M: Sentinel
node mapping guided by indocyanine green fluorescence imaging
during laparoscopic surgery in gastric cancer. Ann Surg Oncol.
17:1787–1793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kelder W, Nimura H, Takahashi N, Mitsumori
N, van Dam GM and Yanaga K: Sentinel node mapping with indocyanine
green (ICG) and infrared ray detection in early gastric cancer: An
accurate method that enables a limited lymphadenectomy. Eur J Surg
Oncol. 36:552–558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee YJ, Ha WS, Park ST, Choi SK, Hong SC
and Park JW: Which biopsy method is more suitable between a basin
dissection and pick-up biopsy for sentinel nodes in laparoscopic
sentinel-node navigation surgery (LSNNS) for gastric cancer? J
Laparoendosc Adv Surg Tech A. 18:357–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aoyama K, Kamio T, Ohchi T, Nishizawa M
and Kameoka S: Sentinel lymph node biopsy for breast cancer
patients using fluorescence navigation with indocyanine green.
World J Surg Oncol. 9:1572011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crane LM, Themelis G, Arts HJ, Buddingh
KT, Brouwers AH, Ntziachristos V, van Dam GM and van der Zee AG:
Intraoperative near-infrared fluorescence imaging for sentinel
lymph node detection in vulvar cancer: First clinical results.
Gynecol Oncol. 120:291–295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujisawa Y, Nakamura Y, Kawachi Y and
Otsuka F: Indocyanine green fluorescence-navigated sentinel node
biopsy showed higher sensitivity than the radioisotope or blue dye
method, which may help to reduce false-negative cases in skin
cancer. J Surg Oncol. 106:41–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Imai K, Minamiya Y, Saito H, Nakagawa T,
Ito M, Ono T, Motoyama S, Sato Y, Konno H and Ogawa J: Detection of
pleural lymph flow using indocyanine green fluorescence imaging in
non-small cell lung cancer surgery: A preliminary study. Surg
Today. 43:249–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishizuka M, Nagata H, Takagi K, Iwasaki Y
and Kubota K: Fluorescence imaging visualizes three sets of
regional lymph nodes in patients with lower rectal cancer.
Hepatogastroenterology. 59:1381–1384. 2012.PubMed/NCBI
|
|
23
|
Miyashiro I, Miyoshi N, Hiratsuka M, Kishi
K, Yamada T, Ohue M, Ohigashi H, Yano M, Ishikawa O and Imaoka S:
Detection of sentinel node in gastric cancer surgery by indocyanine
green fluorescence imaging: Comparison with infrared imaging. Ann
Surg Oncol. 15:1640–1643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rossi EC, Ivanova A and Boggess JF:
Robotically assisted fluorescence-guided lymph node mapping with
ICG for gynecologic malignancies: A feasibility study. Gynecol
Oncol. 124:78–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schaafsma BE, van der Vorst JR,
Gaarenstroom KN, Peters AA, Verbeek FP, de Kroon CD, Trimbos JB,
van Poelgeest MI, Frangioni JV, van de Velde CJ and Vahrmeijer AL:
Randomized comparison of near-infrared fluorescence lymphatic
tracers for sentinel lymph node mapping of cervical cancer. Gynecol
Oncol. 127:126–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshida M, Kubota K, Kuroda J, Ohta K,
Nakamura T, Saito J, Kobayashi M, Sato T, Beck Y, Kitagawa Y and
Kitajima M: Indocyanine green injection for detecting sentinel
nodes using color fluorescence camera in the laparoscopy-assisted
gastrectomy. J Gastroenterol Hepatol. 27(Suppl 3): S29–S33. 2012.
View Article : Google Scholar
|
|
27
|
Kubota K, Yoshida M, Kuroda J, Okada A,
Ohta K and Kitajima M: Application of the HyperEye medical system
for esophageal cancer surgery: A preliminary report. Surg Today.
43:215–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tajima Y, Yamazaki K, Masuda Y, Kato M,
Yasuda D, Aoki T, Kato T, Murakami M, Miwa M and Kusano M: Sentinel
node mapping guided by indocyanine green fluorescence imaging in
gastric cancer. Ann Surg. 249:58–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Japanese Gastric Cancer Association.
Japanese classification of gastric carcinoma: III English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Japanese Gastric Cancer Association.
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric
Cancer. 14:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maruyama K, Sasako M, Kinoshita T, Sano T
and Katai H: Can sentinel node biopsy indicate rational extent of
lymphadenectomy in gastric cancer surgery? Fundamental and new
information on lymph-node dissection. Langenbecks Arch Surg.
384:149–157. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sirop S, Kanaan M, Korant A, Wiese D,
Eilender D, Nagpal S, Arora M, Singh T and Saha S: Detection and
prognostic impact of micrometastasis in colorectal cancer. J Surg
Oncol. 103:534–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Giuliano AE, Haigh PI, Brennan MB, Hansen
NM, Kelley MC, Ye W, Glass EC and Turner RR: Prospective
observational study of sentinel lymphadenectomy without further
axillary dissection in patients with sentinel node-negative breast
cancer. J Clin Oncol. 18:2553–2559. 2000.PubMed/NCBI
|
|
34
|
Songun I, Putter H, Kranenbarg EM, Sasako
M and van de Velde CJ: Surgical treatment of gastric cancer:
15-year follow-up results of the randomized nationwide Dutch D1D2
trial. Lancet Oncol. 11:439–449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brouwer OR, Buckle T, Vermeeren L, et al:
Comparing the hybrid fluorescent-radioactive tracer indocyanine
green-99mTc-nanocolloid with 99mTc-nanocolloid for sentinel node
identification: A validation study using lymphoscintigraphy and
SPECT/CT. J Nucl Med. 53:1034–1040. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Frontado LM, Brouwer OR, van den Berg NS,
Mathéron HM, Vidal-Sicart S, van Leeuwen FW and Valdés Olmos RA:
Added value of the hybrid tracer indocyanine
green-99mTc-nanocolloid for sentinel node biopsy in a series of
patients with different lymphatic drainage patterns. Rev Esp Med
Nucl Imagen Mol. 32:227–233. 2013.PubMed/NCBI
|
|
37
|
Heuveling DA, Visser GW, de Groot M, de
Boer JF, Baclayon M, Roos WH, Wuite GJ, Leemans CR, de Bree R and
van Dongen GA: Nanocolloidal albumin-IRDye 800CW: A near-infrared
fluorescent tracer with optimal retention in the sentinel lymph
node. Eur J Nucl Med Mol Imaging. 39:1161–1168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kong SH, Noh YW, Suh YS, Park HS, Lee HJ,
Kang KW, Kim HC, Lim YT and Yang HK: Evaluation of the novel
near-infrared fluorescence tracers pullulan polymer nanogel and
indocyanine green/γ-glutamic acid complex for sentinel lymph node
navigation surgery in large animal models. Gastric Cancer.
18:55–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Toyota T, Fujito H, Suganami A, Ouchi T,
Ooishi A, Aoki A, Onoue K, Muraki Y, Madono T, Fujinami M, et al:
Near-infrared-fluorescence imaging of lymph nodes by using
liposomally formulated indocyanine green derivatives. Bioorg Med
Chem. 22:721–727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takeuchi H, Ueda M, Oyama T, Shimizu Y and
Kitagawa Y: Molecular diagnosis and translymphatic chemotherapy
targeting sentinel lymph nodes of patients with early
gastrointestinal cancers. Digestion. 82:187–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yaguchi Y, Sugasawa H, Tsujimoto H, Takata
H, Nakabayashi K, Ichikura T, Ono S, Hiraki S, Sakamoto N, Horio T,
et al: One-step nucleic acid amplification (OSNA) for the
application of sentinel node concept in gastric cancer. Ann Surg
Oncol. 18:2289–2296. 2011. View Article : Google Scholar : PubMed/NCBI
|