Introduction
Transforming growth factor-β1 (TGF-β1) is a
bioactive polypeptide cell factor. During tumor development, TGF-β1
signaling imbalance or change may occur; as a result of a variety
of cancer gene mutations, tumor cells secrete large amounts of
active TGF-β1, changing the micro-environment of tumor cells, and
tumor invasion and metastasis is promoted. Triple negative breast
cancer (TNBC), characterized by tumors that do not express estrogen
receptor, progesterone receptor or human epidermal growth factor
receptor 2 genes, represents a significant clinical challenge, as
this type of cancer does not respond to endocrine therapy or other
currently available targeted agents (1). The incidence of TNBC in terms of all
breast cancer subtypes is 10–15% and the 10-year survival is 74.8%
(2,3).
In general, survival rates tend to be lower with TNBC compared with
other forms of breast cancer (4).
TNBC is also more likely than other types of breast cancer to
recur, particularly during the initial few years following
treatment (1). Instead of hormone
therapy, the treatment of TNBC often involves chemotherapy,
radiation and surgery (2). The
present study used immunohistochemistry to detect TGF-β1 in TNBC
specimens, and followed up the 5 year disease-free survival (DFS)
rate, to elucidate the clinical features of TNBC with TGF-β1
expression and its association with prognosis. In addition,
MDA-MB-231 breast cancer cells were treated with 5 ng/ml TGF-β1 and
the invasion and migration ability were assessed as well as the
protein expression levels of members of certain signal transduction
pathways. The present study also aimed to provide a biological
interpretation of the effects of high TGF-β1 expression in TNBC
cells using molecular biology tehcniques.
Materials and methods
Patient selection, characteristics,
treatment and follow up
A total of 80 patients were randomly selected from
TNBC patients diagnosed at Cangzhou Central Hospital from June 2003
to June 2008. The patients were all female, 26–69 years old, the
median age was 48.2 years, all patients were free from distant
metastasis, and received parallel modified radical mastectomy for
breast cancer with post-operative chemotherapy based on
anthracycline and paclitaxel drug for 6–8 cycles. Those cases with
≥3 axillary lymph node metastasis received local radiotherapy.
Axillary lymph node metastasis was present in 49 cases, 6 cases
presented with a family history of breast cancer. A total of 40
patients with non-TNBC were selected as a control group: All the
patients in the control group had no evidence of distant
metastasis, and received parallel operation resection, chemotherapy
and radiotherapy for axillary lymph node metastasis, as above. The
time of follow up was calculated from the time treatment was
received to June 2013; the 5-year DFS rate for statistical
analysis, which was assessed using a combination of telephone
follow-up and out-patient review. The present study was approved by
the Ethics Committee of Cangzhou Central Hospital (Cangzhou, China)
and written informed consent was supplied by the participants.
Immunohistochemical experiments
Specimens were fixed with 10% formalin
(Sigma-Aldrich, St. Louis, MO, USA) and prepared into 4-µm thick
sections, and were subjected to hemotoxylin and eosin staining and
immunohistochemical staining. The sections were blocked using milk
and incubated at room temperature for 2 h, followed by incubation
for 24 h at 4°C with rabbit polyclonal TGF-β1 antibody (catalog
no., BA0290; dilution, 1:2,000; Boster Biological Technology, Ltd.,
Wuhan, China). Samples were subsequently incubated with goat
anti-rabbit biotinylated secondary immunoglobulin G antibody
(dilution, 1:25; catalog no., BA1003; Boster Biological Technology,
Ltd.) at room temperature for 1 h. Negative controls were
established following the same method but with the absence of
primary antibodies. Immunohistochemistry used the Avidin-Biotin
Complex (Boster Biological Technology, Ltd.) staining method to
visualize the staining. TGF-β1 expression predominantly appeared as
diffuse or granular cytoplasmic staining. The sections were divided
into groups based on the total proportion of positive cells: (−),
No positive cells; (+), positive cells in <25%; (++), 25–75%
positive cells; and (+++), >75% positive cells. The pathological
results were determines by two pathologists independently, where
(−) and (+) groups were defined as the low expression group, and
(++) and (+++) groups were defined as the high expression
group.
Cells derived and cultured
MDA-MB-231 cells were purchased from Boster
Biological Technology, Ltd., seeded at a density of
1×106 and cultured with L-15 culture medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C with 5%
CO2.
Cell invasion and migration
assays
MDA-MB-231 cells (1×106) were treated
with 5 ng/ml TGF-β1 or L-15 alone as a control. A BD Matrigel™
Basement Membrane Matrix (BD Biosciences, San Jose, CA, USA) was
used to detect changes in invasive ability, according to the
manufacturer's protocol. The relative number of invasive cells was
used to indicate the cell invasion ability, the experiment was
repeated three times, and the mean is presented. Migration was
assessed using a Transwell chamber assay according to the
previously described protocol (5),
similar to the invasion experiment.
Western blot analysis of signaling
pathway protein expression
MDA-MB-231 cells were treated with 5 ng/ml TGF-β1
and total protein was extracted with cell lysis buffer (Thermo
Fisher Scientific, Inc.), 25 µg protein was separated by 10% sodium
dodecyl sulfate polyacrylamide gel electrophoresis, the proteins
were transferred to film. The polyvinylidene difluoride membrane
was blocked by non-fat milk at 37°C for 24 h, and then incubated
overnight at 4°C with the following antibodies: Rabbit polyclonal
anti-Smad2 (dilution, 1:1,000; catalog no., ab192175; Abcam,
Cambridge, UK), rabbit polyclonal anti-p38 (dilution, 1:1,000;
catalog no., ab38238; Abcam), rabbit polyclonal anti p-Smad2
(dilution, 1:800; catalog no., 3101; Cell Signaling Technology,
Inc., Danvers, MA, USA) and rabbit monoclonal anti p-p38 (dilution,
1:800; catalog no., 9215; Cell Signaling Technology, Inc.). The
membrane was then incubated with horseradish peroxidase-conjugated
goat anti-mouse polyclonal secondary antibody (dilution, 1:5,000;
catalog no., ab6789; Abcam) or horseradish peroxidase-conjugated
goat anti-rabbit polyclonal secondary antibody (dilution, 1:5,000;
catalog no., ab6721; Abcam) at room temperature for 2 h. The
protein bands were visualized using DAB reagent (BD Biosciences),
and images were captured using a Vilber-Fusion chemiluminescence
system (Molecular Imaging Vilber Fusion X7; Vilber Lourmat
Deutschland, Eberhardzell, Germany) and analyzed using Image J
software version 1.48 (imagej.nih.gov/ij/index.html). The optical density
ratio comparing the expression of each target protein prior to and
following TGF-β1 treatment was recorded, and β-actin was used as a
control. The experiment was repeated 3 times.
Statistical analysis
Data was analyzed using single factor analysis of
variance using multiple indexes between the two indexes or an
unpaired students t test. P<0.05 was considered to indicate a
statistically significant difference. Kaplan-Meier curves were
generated and used to perform survival analysis. Data analysis was
performed using SPSS software, version 19.0 (IBM SPSS, Inc.,
Chicago, IL, USA).
Results
The expression levels of TGF-β1 in the
TNBC tissues
A total of 42 TNBC cases expressed TGF-β1 at a high
level (52.5%), while the TGF-β1 expression rate in non-TNBC
patients was 27.5% (t=6.759 P<0.005) (Fig. 1).
Association between TGF-β1 expression
and clinical features of TNBC patients
No significant associations were observed between
high TGF-β1 expression levels and age, menopausal status, tumor,
family history or tumor size. However, high TGF-β1 expression
levels were associated with tumor histological grade (P<0.0001)
and axillary lymph nodes (P=0.001) metastasis (Table I).
 | Table I.Expression of TGF-β1 in triple
negative breast cancer. |
Table I.
Expression of TGF-β1 in triple
negative breast cancer.
| Clinical
features | TGF-β1 (high
expression) | TGF-β1 (low
expression) | χ2 | P-value |
|---|
| Age, years |
|
| 0.001 | 1.000 |
|
>50 | 20 | 18 |
|
|
|
<50 | 22 | 20 |
|
|
| Menopausal |
|
| 3.193 | 0.111 |
| Non
menopausal | 20 | 25 |
|
|
|
Post-menopausal | 22 | 13 |
|
|
| Family history |
|
| 0.956 | 0.416 |
| Yes | 2 | 4 |
|
|
| No | 40 | 34 |
|
|
| Tumor size, cm |
|
| 0.856 | 0.437 |
|
<2 | 14 | 9 |
|
|
| 2–5 | 20 | 21 |
|
|
|
>5 | 8 | 8 |
|
|
| Lymph node |
|
| 11.178 | 0.001 |
|
Positive | 33 | 16 |
|
|
|
Negative | 9 | 22 |
|
|
| Histological
grading |
|
| 39.679 | <0.0001 |
| I–II | 8 | 34 |
|
|
| III | 34 | 4 |
|
|
DFS
The 5 year DFS rate was assessed by the Kaplan-Meier
method. The 5-year DFS was significantly higher in the TGF-β1 low
expression group compared to the high expression group (Fig. 2).
Effect of TGF-β1 treatment on
MDA-MB-231 invasion ability and migration
MDA-MB-231 cells were treated with 5 ng/ml TGF-β1
compared with the control group. The number of cells invading
through the membrane was increased following TGF-β1 treatment
(227.22±26.1 versus 168.11±22.53 cells; P<0.05) and migration
was also significantly increased (217.22±17.23 versus 157.78±17.23;
P<0.05) (Table II).
 | Table II.Effect of TGF-β1 on the invasion and
migration of MDA-MB-231 cells. |
Table II.
Effect of TGF-β1 on the invasion and
migration of MDA-MB-231 cells.
|
| Treatment
groupa | Control
groupa | T-value | P-value |
|---|
| Invasion assay | 227.22±26.18 | 168.11±22.53 | 5.136 | <0.05 |
| Migration assay | 217.22±17.23 | 157.78±17.23 | 8.336 | <0.05 |
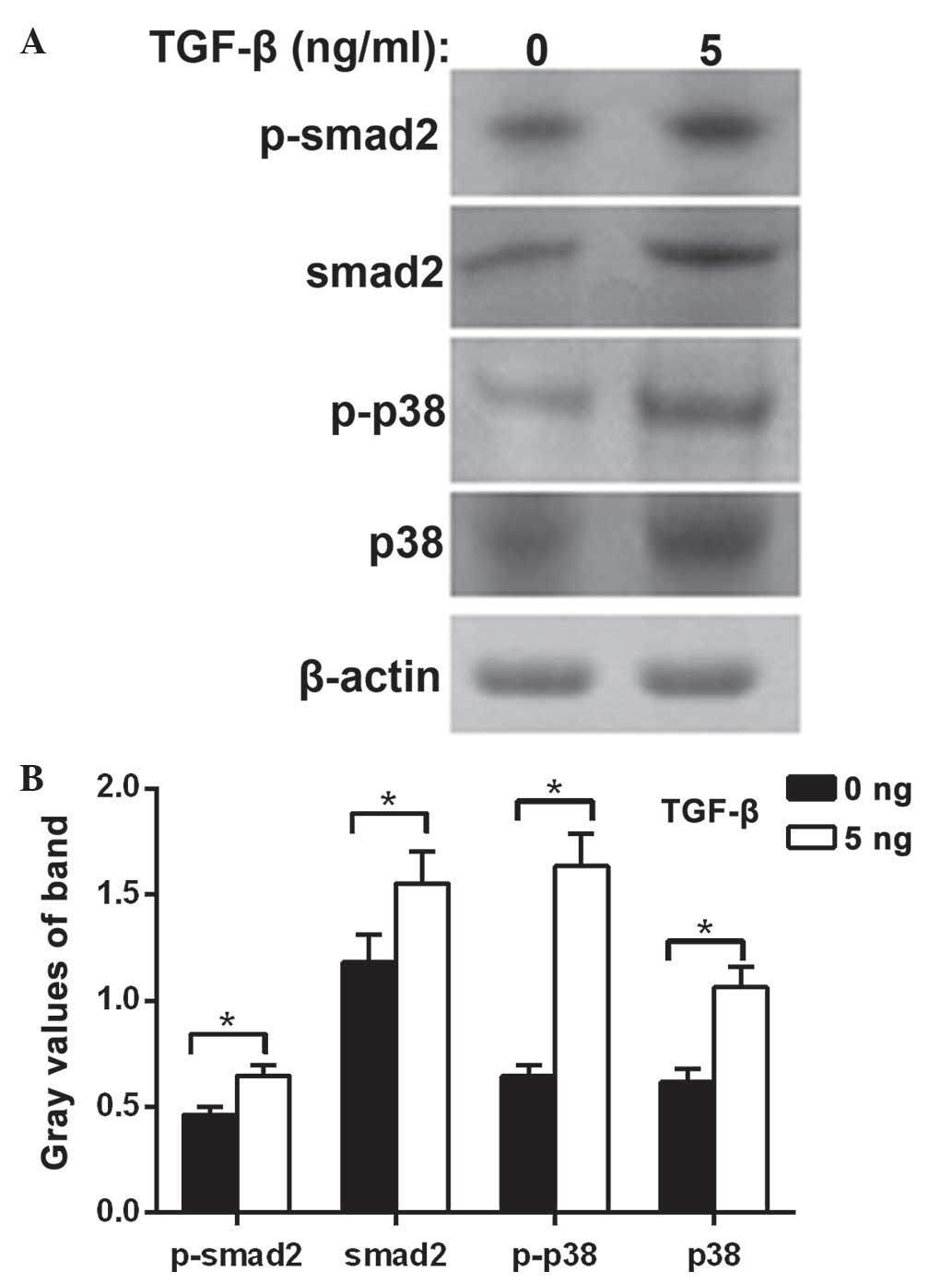
Changes in expression of signalling
proteins in MDA-MB-231 following TGF-β1 treatment
The protein expression levels of Smad2,
phosphorylated Smad2, P38 protein and phosphorylated P38 were
significantly increased in the MDA-MB-231 cells treated with TGF-β1
compared with the control group (P<0.05). These results suggest
that the cell biology effect of TGF-β1 on TNBC involves the Smad2
and P38 signal transduction pathways (Fig. 3).
Discussion
TGF-β1 demonstrates a dual role in the malignant
tumor development process (6,7). During the early stages of
carcinogenesis, TGF-β1 exhibits a predominantly inhibitory effect
on growth, and serves as a tumor suppressor. However, with the
development of malignancy, TGF-β1 promotes tumor cell invasion and
metastasis (8,9). Previous studies have demonstrated that
high expression levels of TGF-β1 have a close association with
gastric cancer (10), lung cancer
(11), colon cancer (10,12) and
other malignant tumors. However, the association between TGF-β1
expression and TNBC has not been established.
TNBC typically has an early age of onset and the
risk of recurrence is high (1). TNBC
tumor cells do not express the estrogen and progesterone receptor
or Her-2 gene; as such, there is no clinically specific endocrine
therapy or targeted drug therapy available for TNBC (2). In the present study, the expression of
TGF-β1 was significantly higher in TNBC tissues compared to that of
non-TNBC tissues. In vitro, Transwell invasion and migration
assays demonstrated that migration and invasion were increased in
the TNBC cell line MDA-MB-231 cells when the cells were treated
with 5 ng/ml TGF-β1 compared with the control group. Therefore,
high expression levels of TGF-β1, may serve an important role in
promoting TNBC development; it may also contribute to the high
malignancy and high rate of metastasis and recurrence of TNBC.
Bao et al (13)
demonstrated that increased TGF-β1 expression level in breast
cancer, were associated with increased axillary lymph node
metastasis, and the average survival time was reduced. Similarly,
in the present study, 80 samples from TNBC patients were analyzed
and it was demonstrated that high expression levels of TGF-β1 were
associated with worse histological grade and increased axillary
lymph node metastasis. A study by Lang et al (14) also confirmed this point. In the
present study, survival curves were produced using the Kaplan-Meier
method and demonstrated that the 5-year DFS rate was significantly
lower in patients with high TGF-β1 expression levels compared to
patients with low expression levels, suggesting that the TGF-β1
expression content of TNBC tissue may be a potential prognostic
biomarker. Although the evidence in the present study and other
previous studies indicates that the content and expression levels
of TGF-β1 in TNBC tissues may be involved in the occurrence and
development of TNBC, the mechanism by which TGF-β1 expression is
upregulated in tumor invasion and metastasis remains unclear.
It is generally considered that TGF-β1 expression in
normal cells depends on the Smad-TGF-β1 signaling pathway and the
independent Smad independent pathways in equilibrium; both can
regulate TGF-β1 expression mutually, and in tumor cells this
balance is disrupted (15,16). Xue et al (17) confirmed that the TGF-β1-mediated Smad
signaling pathway is involved in tumor recurrence and metastasis.
Lang et al (14) demonstrated
that TGF-β1 participates in the recurrence and metastasis of breast
cancer via urokinase type plasminogen activator (uPA) and
plasminogen activator inhibitor (PAI-1) activation. The P38 protein
is an important member of the mitogen activated protein kinase
(MAPK) protein family, it is a member of the Smad-independent
signaling pathway that activates TGF-β1 (18); uPA and PAI-1 P38 protein is involved
in the recurrence and metastasis of breast cancer (19). Following treatment of MDA-MB-231 cells
with TGF-β1, the protein expression levels of P38 and Smad2 were
increased as well as their corresponding phosphorylated proteins,
suggesting that the P38 pathway and Smad2 pathway may serve
important roles in the effects of TGF-β1 on promoting cell invasion
and migration. The effects of TGF-β1 on promoting aggressive
behavior in TNBC cells may involve the interaction of multiple
genes and numerous pathways, and therefore the role of TGF-β1
signaling requires further study.
Acknowledgements
The authors thank Miss Ke SU, Mr Guo-zhong Cui, Mr
Meng Yang, Miss Yanqing Liu, Mr Dianlu Dai for advice and
discussion; Mr Wen-hua Yang, Mr Liang Chen for critical reading of
the manuscript; and Mr Meng Yang, Miss Yanqing Liu for excellent
technical assistance.
References
|
1
|
Kumar P and Aggarwal R: An overview of
triple-negative breast cancer. Arch Gynecol Obstet. 293:247–269.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cleator S, Heller W and Coombes RC:
Triple-negative breast cancer: Therapeutic options. Lancet Oncol.
8:235–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bao P, Peng P, Gu K, Wu C, Huang Z, Gong
Y, Zhang M and Zheng Y: Long-term survival analysis of different
breast cancer molecular subtypes: Shanghai Breast Cancer Survival
Study. Zhonghua Wai Ke Za Zhi. 53:928–934. 2015.(In Chinese).
PubMed/NCBI
|
|
4
|
Lund MJ, Trivers KF, Porter PL, Coates RJ,
Leyland-Jones B, Brawley OW, Flagg EW, O'Reagan RM, Gabram SG and
Eley JW: Race and triple negative threats to breast cancer
survival: A population-based study in Atlanta, GA. Breast Cancer
Res Treat. 113:357–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marshall J: Transwell(®) invasion assays.
Methods Mol Biol. 769:97–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bierie B and Moses HL: Transforming growth
factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth
Factor Rev. 21:49–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Padua D and Massagué J: Roles of TGF-beta
in metastasis. Cell Res. 19:89–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bierie B and Moses HL: Gain or loss of
TGFbeta signaling in mammary carcinoma cells can promote
metastasis. Cell Cycle. 8:3319–3327. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagaraj NS and Datta PK: Targeting the
transforming growth factor-beta signaling pathway in human cancer.
Expert Opin Investig Drugs. 19:77–91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coban S, Yüksel O, Koçkar MC, Köklü S,
Basar O, Tutkak H and Ormeci N: The significance of serum
transforming growth factor beta 1 in detecting of gastric and colon
cancers. Hepatogastroenterology. 54:1472–1476. 2007.PubMed/NCBI
|
|
11
|
Minamiya Y, Miura M, Hinai Y, Saito H, Ito
M, Ono T, Toda H, Motoyama S and Ogawa J: Transforming growth
factor-β1 29T>C genetic polymorphism is associated with lymph
node metastasis in patients with adenocarcinoma of the lung. Tumour
Biol. 31:437–441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bellam N and Pasche B: Tgf-beta signaling
alterations and colon cancer. Cancer Treat Res. 155:85–103. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao J, Wu ZS, Qi Y, Wu Q and Yang F:
Expression of TGF-beta1 and the mechanism of invasiveness and
metastasis induced by TGF-beta1 breast cancer. Zhonghua Zhong Liu
Za Zhi. 31:679–682. 2009.(In Chinese). PubMed/NCBI
|
|
14
|
Lang DS, Marwitz S, Heilenkötter U, Schumm
W, Behrens O, Simon R, Reck M, Vollmer E and Goldmann T:
Transforming growth factor-beta signaling leads to uPA/PAI-1
activation and metastasis: A study on human breast cancer tissues.
Pathol Oncol Res. 20:727–732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kajdaniuk D, Marek B, Borgiel-Marek H and
Kos-Kudła B: Transforming growth factor-β1 (TGFβ1) in physiology
and pathology. Endokrynol Pol. 64:384–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heldin CH, Landström M and Moustakas A:
Mechanism of TGF-beta signaling to growth arrest, apoptosis and
epithelialmesenchymal transition. Curr Opin Cell Biol. 21:166–176.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xue J, Lin X, Chiu WT, Chen YH, Yu G, Liu
M, Feng XH, Sawaya R, Medema RH, Hung MC and Huang S: Sustained
activation of SMAD3/SMAD4 by FOXM1 promotes TGF-β-dependent cancer
metastasis. J Clin Invest. 124:564–579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taylor MA, Parvani JG and Schiemann WP:
The pathophysiology of epithelial-mesenchymal transition induced by
transforming growth factor-beta in normal and malignant mammary
epithelial cells. J Mammary Gland Biol Neoplasia. 15:169–190. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shin BA, Yoo HG, Kim HS, Kim MH, Hwang YS,
Chay KO, Lee KY, Ahn BW and Jung YD: P38 MAPK pathway is involved
in the urokinase plasminogen activator expression in human gastric
SNU-638 cells. Oncol Rep. 10:1467–1471. 2003.PubMed/NCBI
|