Introduction
Generally, the worldwide incidence and mortality
rates of gastric cancer have decreased substantially over the last
few decades (1). However, in China,
even following curative resection, patients with advanced gastric
cancer continue to have an adverse prognosis (2). The current medical management of
patients with gastric cancer is not satisfactory, and the great
expense of the treatment places a large economic burden on the
family of the patient and on society.
Peritoneal metastasis is a common pattern of spread
that is observed in gastric cancer, with its occurrence leading to
malignant ascites, intestinal obstruction and emaciation, the major
cause of gastric cancer-associated mortality (3,4).
Preliminary research has demonstrated that tumor invasion and
metastasis to the peritoneum has a close association with gastric
cancer. Two major theories of tumor carcinogenesis have been
proposed: i) Epithelial-mesenchymal transition, which reduces
adhesion between tumor cells, results in metastasis during the
embryonic period (5); and ii) the
subsets of cancer stem cells (like stem cells), which have the
capacity to self-renew, self-differentiate and control a steady
state, contribute to cell metastasis (6). Degradation of the extracellular matrix
(ECM) is a precondition required for tumor invasion and metastasis
(7). In a study by Steeg (8), it was reported that tumor cells are
required to translocate out of the primary foci and adhere to
proteins bound to cell surface receptors in the basement membrane
and ECM; this allows the cell to invade the ECM. Peritoneal
metastasis is random and unpredictable, primarily due to the large
surface area and full disease spread over the peritoneal tissue in
the abdominal cavity (9). Despite
numerous studies providing evidence that tumor metastasis is
associated with gastric cancer, the mechanisms of gastric cancer
with peritoneal metastasis have not yet been fully discussed.
It has been reported that the urokinase-type
plasminogen activator (uPA) system is associated with the
differentiation, stages, pathology and prognosis of gastric cancer.
As a protease, uPA participates in the degradation of the ECM and
serves a crucial role in tumor metastasis (10). uPA receptor (uPAR), plasminogen
activator inhibitor-1 (PAI-1) and plasminogen (PIg) are the key
members within the uPA system (11).
It has been demonstrated that uPA is a primary factor required for
the processes of tumor invasion and metastasis. For example,
Pulukuri and Rao (12) reported that
the silent expression of uPA inhibits the internal invasion and
vessel formation of tumor cells in prostate cancer. Dass et
al (13) also demonstrated that
the silent expression of uPA in lung cancer cells inhibits the
hyperplasia of tumor cells and pulmonary metastasis (13). Kaneko et al (14) reported that uPAR and vascular
endothelial growth factor can contribute synergistically to tumor
progression in gastric cancer. Furthermore, Lee et al
(15) observed that PAI-1 induces the
invasive behavior of gastric cancer cells by upregulating the uPA
system.
Despite numerous studies demonstrating an
association between the uPA system and gastric cancer, few studies
have confirmed an association between the uPA system and peritoneal
metastasis in gastric cancer. In the present study, the expression
level and enzyme activity of the uPA system was analyzed in four
different gastric cancer cell lines, and the peritoneal
implantation capability of the four cell lines was subsequently
compared in rats. Additionally, the effect of the uPA system on the
biological behaviors (including adhesion, migration and invasion)
of peritoneal cells in gastric cancer was studied in vitro.
Overall, the present study aimed to investigate the mechanisms of
the uPA system in peritoneal metastasis and confirm the diagnostic
significance of the uPA system in gastric cancer.
Materials and methods
Cell culture
The human gastric cancer cell lines, AGS, SGC7901,
MKN45 and MKN28, with varying differentiation degrees, and the
peritoneal mesothelial cell line, HMrSV5, were donated by the
Department of Nephrology, Shanghai First People's Hospital
(Shanghai, China). Gastric cancer cells were maintained in Roswell
Park Memorial Institute (RPMI) 1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), while the peritoneal
mesothelial cells were maintained in Dulbecco's modified Eagle's
medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA). All of the media
were supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.). All of the cells were subcultured almost every
day by 1:4 or 1:5 dilutions in culture flasks at 37°C, under an
atmosphere of 5% CO2. Cells that had a cell viability
>90% in the logarithmic phase were selected for later
experimentation. The gastric cancer cells were introduced to the
culture flask at a density of 2×106/ml. When the cells
reached confluence of 70–80%, they were digested with 0.25% trypsin
(Sigma-Aldrich), washed three times with phosphate-buffered saline
(PBS; pH, 7.4) and then centrifuged at 1,200 × g for 5 min to yield
cell precipitation.
Semiquantitative reverse transcription
polymerase chain reaction (RT-PCR)
Total RNA from the cells was isolated using the
RNeasy Mini kit following the manufacturer's protocols (Qiagen,
Inc., Valencia, CA, USA). The RNA extracted from the gastric cancer
cells was reverse-transcribed in a final volume of 20 µl using AMV
Reverse Transcriptase (Promega Corporation, Madison, WI, USA)
according to the manufacturer's protocols with the following
system: 1 µg oligo(dT) primers, 2 µl 10X RT buffer, 2 µl
deoxynucleotide triphosphate (dNTPs), 4 µl MgCl2, 0.5 µl
RNasin, 1 µl Oligo(dT)18, 0.75 µl reverse transcriptase
and 1 µg RNA. The reactions were performed at 70°C for 10 min, 42°C
for 15 min, 99°C for 15 min and then stored at 4°C.
Semiquantitative RT-PCR analyses for uPA (16), uPAR (17), PAI-1 (17), β-actin (18) and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) mRNAs were performed using the Eppendorf
MasterCycler® (Eppendorf, Hamburg, Germany). Primers and
probes for the TaqMan system were designed using the Primer Premier
5.0 programme (Premier Biosoft International, Palo Alto, CA, USA)
and were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China).
The sequences of the PCR primer pairs used for each gene are
presented in Table I.
Semiquantitative RT-PCR was performed with a final volume of 25 µl
using the following system: 1 µl complementary DNA (cDNA), sense
and anti-sense primers (each 0.25 µl), 2.5 µl 10X PCR buffer, 2 µl
25% MgCl2, 0.5 µl Taq polymerase, 1 µl dNTP and 17 µl
RNase Free H2O. The amplification conditions were as
follows: Initial denaturation at 95°C for 5 min, followed by 30
cycles of denaturation at 94°C for 1 min, annealing at 56°C for 30
sec for uPA, 60°C for 1 min for uPAR and 55°C for 1 min for PAI-1,
and extension at 72°C for 1 min. uPA and PAI-1 expression was
normalized to the reference gene GAPDH, whilst uPAR was normalized
to β-actin.
 | Table I.Primers used in the present
study. |
Table I.
Primers used in the present
study.
| Name | Primer | Sequence
(5′-3′) | Length, bp |
|---|
| uPA | Sense |
AGAATTCACCACCATCGAGA | 20 |
|
| Anti-sense |
ATCAGCTTCACAACAGTCAT | 20 |
| uPAR | Sense |
ACAGGAGCTGCCCTCGCGAC | 20 |
|
| Anti-sense |
GAGGGGGATTTCAGGTTTAGG | 21 |
| PAI-1 | Sense |
CTTTGGTGAAGGGTCTGC | 18 |
|
| Anti-sense |
CTCCACCTCTGAAAAGTCC | 19 |
| β-actin | Sense |
TTGAAGGTAGTTTCGGGAAT | 20 |
|
| Anti-sense |
GAAAATCTGGCACCACACCTT | 21 |
| GAPDH | Sense |
GAAGGTGAAGGCGGAGTC | 18 |
|
| Anti-sense |
GAAGATGGTGATGGGATTTC | 20 |
Western blot analysis
Cells were washed 3 times with ice-cold PBS and
extracted in ice-cold lysis buffer [50 mM Tris-HCl (pH, 8.0), 1%
Nonidet 40, 0.5 mM ethylenediaminetetraacetic acid, 100 µg/ml
phenylmethysulfonyl fluoride, 2 µg/ml leupeptin, 100 µm sodium
vanadate, 1 mM dithiothreitol, 1 µg/ml aprotinin and 150 mM NaCl]
for 30 min. An aliquot lysate was used to determine protein
concentration using a bicinchoninic acid assay (Pierce
Biotechnology, Inc., Rockford, IL, USA). The equal amount of
protein per lane was separated on 10% sodium dodecyl
sulfate-polyacrylamide gel and then transferred to a nitrocellulose
membrane (EMD Millipore, Billerica, MA, USA). The membrane was
blocked with 5% skimmed milk for 3 h, and was subsequently
incubated for 3 h with monoclonal mouse anti-human uPA antibody
(dilution, 1:500; catalog no., TA805243; OriGene Technologies,
Inc., Rockville, MD, USA), monoclonal mouse anti-human uPAR
antibody (dilution, 1:500; catalog no., sc-376494; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and monoclonal mouse
anti-human PAI-1 antibody (dilution, 1:500; catalog no., sc-5297;
Santa Cruz Biotechnology, Inc.), respectively. Following 3 washes
with Tris-buffered saline, the membrane was incubated with the goat
anti-mouse alkaline phosphatase-conjugated IgG secondary antibody
(dilution, 1:5,000; catalog no., sc-2008; Santa Cruz Biotechnology,
Inc.). The signal was detected using the NBT/BCIP Reagent kit
(Wuhan Huamei Biotech Co., Ltd., Wuhan, China).
Quantitative enzyme-linked
immunosorbent assay (ELISA) analysis
uPA, uPAR and PAI-1 antigen concentrations were
determined by ELISA kits according to the manufacturer's protocols
(American Diagnostica GmbH; Sekisui Diagnostics, Lexington, MA,
USA). The reaction was stopped by the addition of 50 µl
H2SO4, and the absorption was measured at 450
nm using the EL312e Automated Microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA). The values of the uPA, uPAR
and PAI-1 antigen were measured as ng/mg protein.
uPA activity assay
uPA activity in the different cell lines was
measured using the ECM600 uPA Activity assay kit (EMD Millipore,
Billerica, MA, USA). Following concentration of the cell medium, it
was mixed with the assay buffer and subsequently incubated on a
chromogenic substrate in 96-well plates at 37°C, for 2 to 24 h. The
optical density (OD) was read at 405 nm, and the activity (units)
was extrapolated from a standard curve.
Animal experiments
All procedures in the study were approved by the
Institute of Health Services Research at Shanghai East Hospital
Affiliated to Tongji University (Shanghai, China) with regard to
the protection of animals used for scientific purposes. The
manuscript was prepared according to the guidelines of Animal
Research, Reporting of In Vivo Experiments (19).
A total of 24 male BALB/C nu/nu rats (220–280 g, 4–5
weeks of age) were utilized in the study. The rats had free access
to food and water, and were housed in specific pathogen-free
conditions. The rats were randomly divided into 4 groups (n=6 per
group) and each group was injected with one type of gastric cancer
cell line (MKN28, AGS, MKN45 or SGC7901), with a concentration of
5×106 cells in the peritoneal cavity. The rats injected
with the cancer cell lines were all injected at the same sites and
were observed once a week. Within each group, 1 injected mouse was
randomly selected and sacrificed via an intraperitoneal injection
of ketamine (80 mg/kg) at day 14, while a second rat was sacrificed
at day 28 using the same method. The extent of peritoneal
metastasis was assessed macroscopically on the rat bodies.
Additionally, the survival time of the rats was recorded in each
group.
In vitro adhesion assays
In vitro adhesion assays were used to
determine the viability of the mesothelial cells, which were
cultured with serum-free conditioned media (SF-CM) from the gastric
cancer MKN45 cell line with the highest uPA activity. When they
reached a satisfactory confluence, the mesothelial cells were
digested with 0.25% trypsin (Sigma-Aldrich) and were seeded in a
24-well plate with 2×104 cells for 12 to 24 h.
Meanwhile, the gastric cancer MKN45 cells were resuspended in SF-CM
at a density of 1.0×104 cells/ml, followed by incubation
with uPA, uPAR and PAI-1 antibodies (each antibody was set with 3
concentrations: 0.1, 1 and 10 µg/ml) for 45 to 60 min at 37°C under
an atmosphere of 5% CO2. The mesothelial cells were
rinsed with SF-CM and were then incubated with antibody-treated
gastric cancer cells, with a dilution cell ratio of 1:1. Gastric
cancer cells without antibody treatment were used as a control.
Following 24 h of incubation, 50 µl methyl thiazolyl tetrazolium
(MTT; Sigma-Aldrich) was added and the mixture was incubated at
37°C for a further 4 h. Once the mixture had been centrifuged at
2,000 × g for 10 min, the supernatant was discarded. The pellet was
dissolved in 400 µl dimethyl-sulfoxide and oscillated for 10 min
until the crystals had completely dissolved. The OD at 570 nm of
each well was measured using a microplate reader.
In vitro migration and invasion
assays
The invasion and migration abilities of the cells
were assayed using non-coated (migration) and Matrigel-coated
filters (invasion) using modified 24-well Falcon™ Cell Culture
inserts (8-µm pore size; BD Biosciences, Franklin Lakes, NJ, USA).
For the migration assay, the digested mesothelial HMrSV5 cells were
seeded at a density of 2×104 cells in 0.5 ml DMEM in the
upper compartment of Transwell chambers. The gastric cancer MKN45
cells were treated in the same manner as those in the adhesion
assay. Following 12 to 24 h of incubation, the mesothelial HMrSV5
cells were rinsed with SF-CM and were subsequently incubated with
antibody-treated gastric cancer cells, with a cell ratio of 1:1.
Cancer cells without antibody treatment were used as a control.
RPMI-1640 medium with fibronectin and cancer cells were added into
the lower chamber as a chemoattractant. Following 72 h of
co-cultivation, MTT was added into the upper and lower compartments
for OD detection.
For the invasion assay, Matrigel was washed with
SF-CM and added to the upper compartment. The co-cultivation time
between the cancer cells and the mesothelial HMrSV5 cells was 48
h.
Statistical analysis
All data were analyzed using SAS 6.12 (SAS
Institute, Inc., Cary, NC, USA) and the results were measured as
the mean ± standard deviation. A comparison of the survival time
among different groups was performed using post-hoc pair wise
comparisons in a one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
uPA, uPAR and PAI-1 mRNA expression in
four gastric cancer cell lines
The RT-PCR amplification products for uPA, uPAR,
PAI-1, β-actin and GAPDH were 474, 1,046, 409, 591 and 230 bp,
respectively (Fig. 1). Analysis of
the uPA system mRNA expression level demonstrated that uPA, uPAR
and PAI-1 were all expressed in the four cell lines to varying
degrees, and the SGC7901 cells exhibited the highest mRNA
expression, whilst the AGS cells demonstrated the lowest mRNA
expression (Table II).
 | Figure 1.mRNA expression of uPA, uPAR and
PAI-1 in four gastric cancer cell lines. (A) uPA, (B) PAI-1 and (C)
uPAR. The upper lane in each image represents the uPA, uPAR and
PAI-1 expression, respectively, while the lower lane in each image
represents the internal reference gene expression. The internal
reference gene for uPA and PAI-1 was glyceraldehyde 3-phosphate
dehydrogenase, and for uPAR was β-actin. Lane 1, AGS cell line;
lane 2, SGC7901 cell line; lane 3, MKN45 cell line; and lane 4,
MKN28 cell line. M, DNA marker; uPA, urokinase-type plasminogen
activator; uPAR, uPA receptor; PAI-1, plasminogen activator
inhibitor-1. |
 | Table II.Relative mRNA expression level of
uPA, uPAR and PAI-1 in four gastric cancer cell lines. |
Table II.
Relative mRNA expression level of
uPA, uPAR and PAI-1 in four gastric cancer cell lines.
| uPA system | AGS | SGC7901 | MKN45 | MKN28 |
|---|
| uPA | 0.078 | 0.151 | 0.148 | 0.132 |
| uPAR | 0.263 | 0.348 | 0.325 | 0.335 |
| PAI-1 | 0.537 | 0.680 | 0.349 | 0.670 |
uPA, uPAR and PAI-1 protein expression
in four gastric cancer cell lines
From the western blotting analysis of the uPA
system, it was observed that the level of uPA protein expression
was highest in the MKN45 cells, while there was minimal uPA protein
expression in the AGS cells (Fig.
2A). There was no expression of uPAR protein in the MKN28
cells, and no difference in the expression of the other three cell
lines (Fig. 2B). PAI-1 protein was
expressed in all four cell lines, with the MKN28 cells exhibiting
the lowest expression level (Fig.
2C).
 | Figure 2.uPA, uPAR and PAI-1 protein
expression in four gastric cancer cell lines: (A) uPA (60 kDa), the
upper lane demonstrates the expression of combined uPA, and the
lower lane is the expression of single uPA (52 kDa); (B) uPAR (60
kDa); (C) PAI-1, the upper lane is the expression of combined PAI-1
(60 kDa), and the lower lane is the expression of single PAI-1 (47
kDa). (D) Protein expression levels of combined uPA, single uPA,
uPAR, combined PAI-1 and single PAI-1 the four gastric cancer cell
lines. Lane 1, AGS cell line; lane 2, SGC7901 cell line; lane 3,
MKN45 cell line; lane 4, MKN28 cell line; M, DNA marker; uPA,
urokinase-type plasminogen activator; uPAR, uPA receptor; PAI-1,
plasminogen activator inhibitor-1. |
uPAR and PAI-1 protein content in
culture supernatant and intracellularly in four gastric cancer cell
lines
An ELISA assay of the protein content demonstrated
that, in supernatant, the uPA protein content was the lowest in the
AGS cells. The PAI-1 protein content was the lowest in the MKN28
cells, and there was no detection of uPAR in this cell line.
Intracellularly, uPA expression was highest in the MKN45 cells,
while there was no detection of PAI-1 in the MKN28 cells.
Additionally, PAI-1 expression was highest in the SGC7901 cells
when compared with the other three cell lines (Table III).
 | Table III.Protein content of uPA, uPAR and
PAI-1 in supernatant and intracellular. |
Table III.
Protein content of uPA, uPAR and
PAI-1 in supernatant and intracellular.
|
| Supernatant,
ng/mg | Intracellular,
ng/mg |
|---|
|
|
|
|
|---|
| Cell lines | uPA | uPAR | PAI-1 | uPA | PAI-1 |
|---|
| AGS | 0.043 |
0.892 |
76.465 | 0.038 | 14.083 |
| SGC7901 | 0.195 | 11.648 | 108.067 | 0.483 | 37.289 |
| MKN45 | 0.070 |
1.765 |
87.294 | 0.582 | 10.133 |
| MKN28 | 0.059 | ND |
0.491 | 0.189 | ND |
uPA activity detection
The uPA activities of the four cell lines were 1.531
units in the AGS cells, 1.904 units in the SGC7901 cells, 4.283
units in the MKN45 cells and 1.541 units in the MKN28 cells.
Furthermore, the highest uPA activity was observed in the MKN45
cells, whilst the lowest was observed in the AGS cells (Fig. 3), and the uPA activity of SGC7901
cells was significantly higher than that of the other 3 cell lines
(P<0.05).
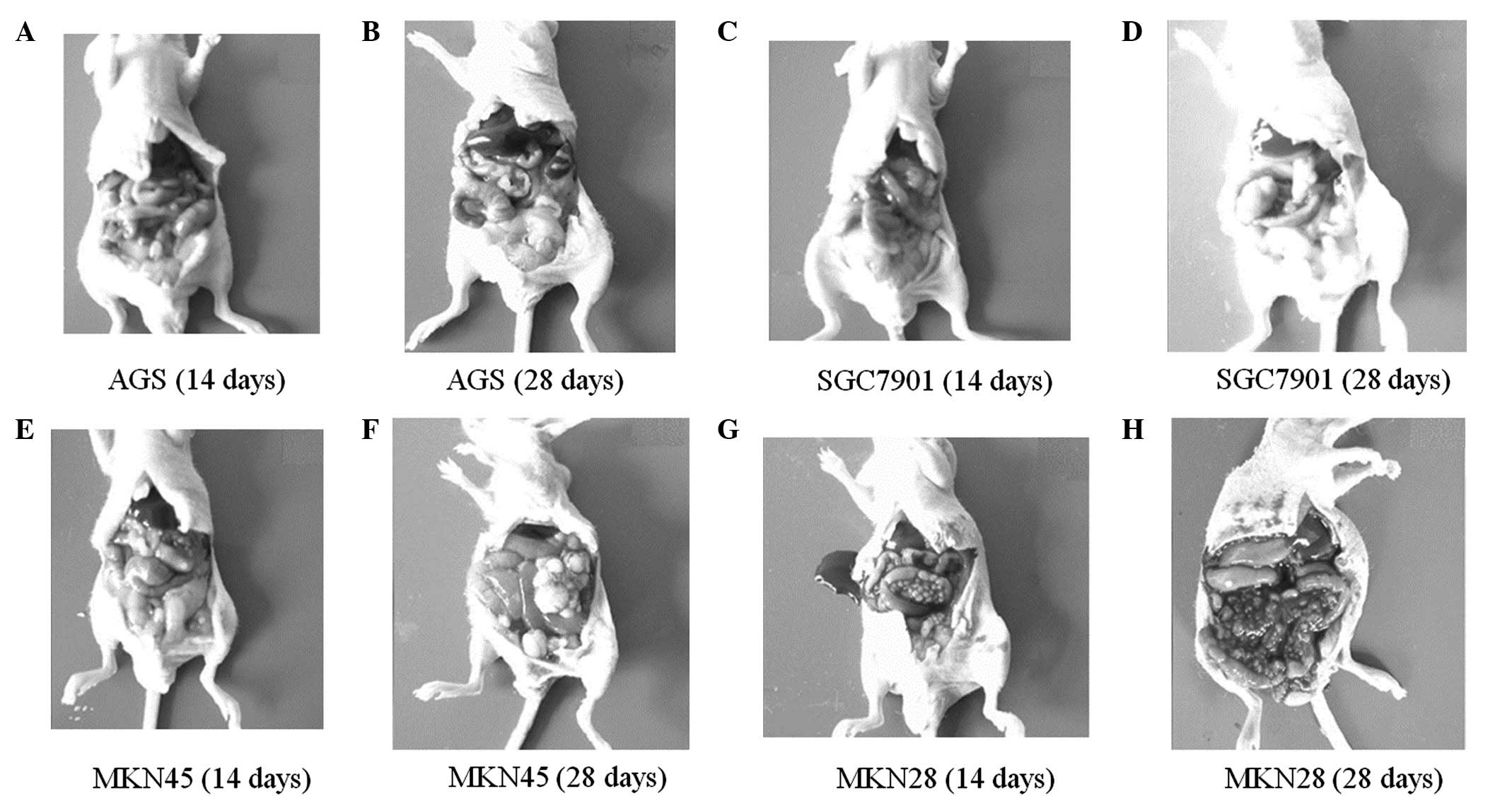
Peritoneal tumor development following
injection with different cell lines
Peritoneal tumor development was altered following
injection with the four cell lines. The time to peritoneal tumor
occurrence was different in each cell line, with tumors occurring
at ~1 week in the MKN45 cells and ~10 days in the SGC7901 and MKN28
cells, while no peritoneal tumor was identified in the AGS cells,
even at 28 days post-injection (Fig. 4A
and B). The tumor masses in the SGC7901 and MKN45 cells were
observed in similar conditions; the sizes of the masses varied and
were scattered over the whole abdominal cavity, but they were
primarily located in the omentum and pelvic cavity, and no ascites
was produced (Fig. 4C–H). Tumor
masses in the MKN28 cells were of a similar size and were primarily
distributed on the mesentery; hemorrhagic ascites also developed
during the first week subsequent to injection and progressed in a
time-dependent manner (Fig. 4G and
H).
Survival time of rats following
injection with different cell lines
Following injection with the gastric cancer cell
lines, the rats that were injected with the AGS cells all survived.
The median survival time of rats injected with the MKN45 cells was
the shortest, at just 41 days (Table
IV). Additionally, the survival time of the SGC7901 and the
MKN28-injected rats was longer than that of the MKN45-injected
rats, and there was no significant difference between the two
groups (P=0.5970). The difference between the MKN45 and the
MKN28-injected rats was significant (P=0.0041). When compared with
the MKN45-injected rats, the survival time of all rats injected
with the SGC7901 cells was varied (range, 36–97 days; Fig. 5).
 | Table IV.Survival time (days) of rats
following injection with different cell lines. |
Table IV.
Survival time (days) of rats
following injection with different cell lines.
|
| Nude mice |
|
|---|
|
|
|
|
|---|
| Cell line | 1 | 2 | 3 | 4 | 5 | Median |
|---|
| SGC7901 | 36 | 36 | 70 | 86 | 97 | 70 |
| MKN45 | 35 | 39 | 41 | 41 | 43 | 41 |
| MKN28 | 44 | 46 | 48 | 51 | 69 | 48 |
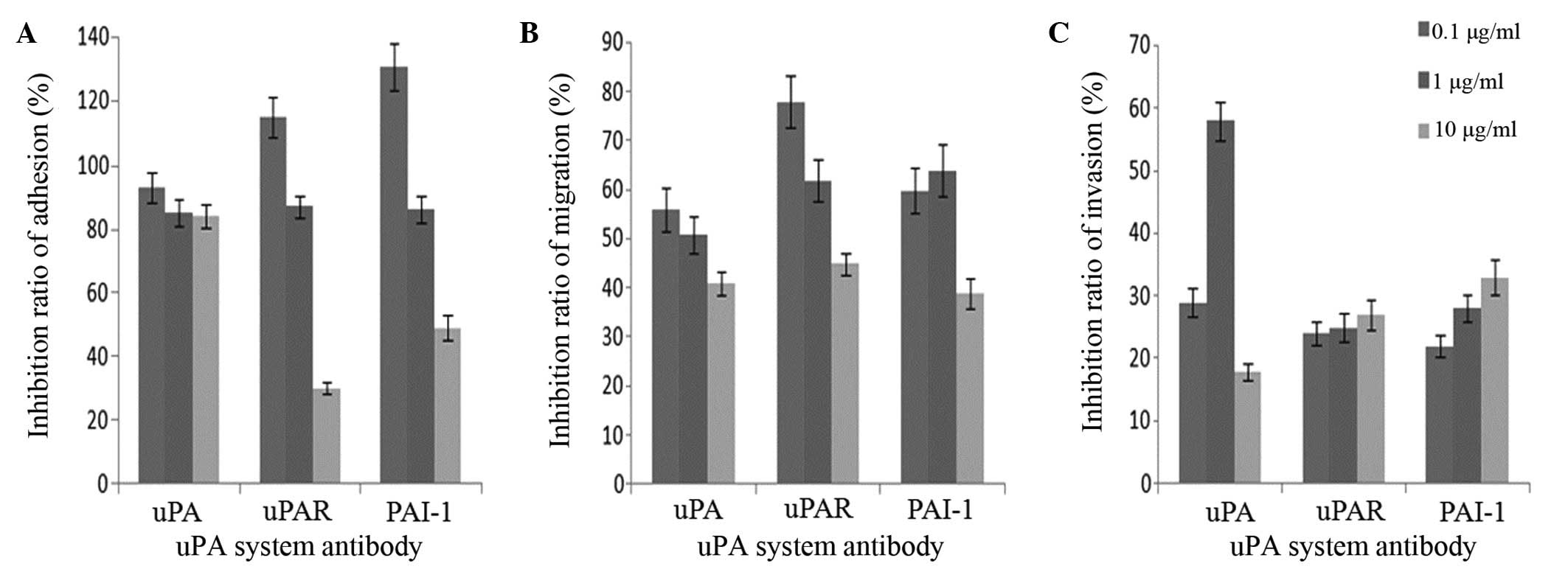
Adhesion, migration and invasion
assays in vitro
The OD of the MKN45 cells amongst the mesothelial
cells in the rats gradually declined with an increasing
concentration of uPA system antibodies (Fig. 6). MTT analysis demonstrated that the
OD value was in proportion with the live cell numbers. The results
indicate that adhesion and migration of the MKN45 cells to the
mesothelial cells decreased due to the inhibitory effect of the uPA
system antibodies (Fig. 6A and B).
When compared with the control, uPAR and PAI-1 antibodies appeared
to promote adhesion between the MKN45 cells and the mesothelial
cells (Fig. 6). Notably, the
inhibitory effects of the uPAR and PAI-1 antibodies on adhesion and
migration were more potent than that of the uPA antibody at the
same concentration (Fig. 6A and B).
Additionally, a lower concentration of the uPA system antibodies
(0.1 µg/ml) had a greater effect on the adhesion and migration of
the MKN45 cell line compared with a higher concentration (1 and 10
µg/ml). However, the uPA system antibody inhibited the migration of
the MKN45 cell line at a low concentration, and this effect was
enhanced in a concentration-dependent manner (Fig. 6A and B).
Notably, although the uPA system antibodies did
induce invasion inhibition, this effect did not appear to be
associated with the antibody concentration (Fig. 6C).
Discussion
Gastric cancer is one of the leading causes of
cancer-associated mortality worldwide (1), and is responsible for large numbers of
novel cancer cases and fatalities every year (20). Although surgery and adjuvant
chemotherapy have resulted in superior periodical survival for
gastric cancer patients, relapse is particularly common (21,22).
Numerous studies have reported that the uPA system serves an
important role in various types of cancer, including breast, lung,
liver and pancreatic cancer (23–25). In
the present study, the functions of the uPA system in the
peritoneal metastasis of gastric cancer was analyzed. The
expression of the uPA system in four types of gastric cancer cells
(AGS, SGC7901, MKN45 and MKN28) appeared to vary. The highest level
of uPA system expression and uPA activity was observed in the
SGC7901 cells. Peritoneal implantation analysis demonstrated that
no tumors in the peritoneum were observed in the AGS-injected rats,
and the tumor masses were of different sizes in the SGC7901 and
MKN45-injected rats. The survival times of the rats injected with
the MKN28 and SGC7901 cells were longer than those observed in the
rats injected with the MKN45 cells. Antibodies of the uPA system
were able to inhibit the adhesion, migration and invasion to the
peritoneum in the gastric cancer MKN45 cell line, with the
strongest peritoneal implantation.
The invasion and metastasis of tumors is a complex
process, involving adhesion, alterations in tumor cell polarity,
and degradation and reconstruction of the ECM (26). The adhesion between gastric cancer
cells and mesothelial cells is crucial for the formation of
peritoneal metastasis. Mesothelial cells are the primary component
of the peritoneum (27). Following
adhesion onto peritoneal tissue, gastric cancer cells that have
interacted with proteolytic enzymes may migrate into the ECM via
mesothelial cells (28). The
hydrolysis of the ECM by a malignant tumor is a necessity for tumor
migration and invasion, which involves numerous proteolytic
enzymes. As it contains important proteolytic enzymes, the uPA
system has been demonstrated to be a key enzyme system in mediating
tumor metastasis (29,30). uPA, pro-uPA, uPAR and PAI are the
members of the uPA system (13),
which primarily participate in plasminogen activation and ECM
degradation. Overwhelming evidence from previous studies has
demonstrated that the cell surface-associated uPA/uPAR complex is
involved in the tumor invasion and metastasis of several types of
cancer, functioning through direct or indirect interactions with
integrins and growth factors (31,32).
uPA may only activate plasminogens once it has
specifically combined with uPAR existing on the surface of the
cytomembrane (13). Shimizu et
al (23) demonstrated that
downregulation of the uPA system, inhibited by Jagged-1-induced
Notch, inhibits the progression of breast cancer (23). Li et al (33) reported that the downregulation of uPA,
inhibited by microRNA193b, can prevent the invasion between
MDA-MB-231 and MDA-MB-435 cells in human breast cancer. uPA
interacts with its corresponding receptor to affect cell adhesion
and migration, and uPA allows for cross-talk between uPAR and αMβ2
integrins on peripheral blood neutrophils that express these two
receptors (34). In the present
study, the uPA activity in the MKN45 cells was higher than that
observed in the other cell lines, and uPA antibody inhibited cell
adhesion and migration, indicating that uPA serves a key role in
cell adhesion and migration in cancer. Additionally, the uPA
antibody inhibited the adhesion and migration of the MKN45 cell
line to the mesothelial cells, suggesting that uPA may contribute
to the adhesion of MKN45 cells in gastric cancer in the
peritoneum.
uPAR mediates the signaling transduction of uPA and
has an impact on cell proliferation, chemotaxis, migration and
differentiation. In tumor cell adhesion, uPAR inhibits the
combination of cells and fibrinogen by bonding with integrins
attached to the cytoskeleton; once bound, uPAR alters the
specificity of the cell adhesion matrix (35). Koochekpour et al (31) demonstrated that the upregulation of
the uPA/uPAR system, activated by saposin C, accelerated cell
growth and invasion via the p42/44 mitogen-activated protein kinase
(MAPK) signaling pathway (31). The
regulatory effect of uPAR may be exerted through the activation of
MAPK, and its overexpression can increase uPA secretion in
uPAR-expressing cells, involving its functional interaction with
integrins and fMet-Leu-Phe receptors (36). Grove et al (37) observed that uPAR ligation with the
urokinase receptor binding domain (amino-terminal fragment) results
in enhanced migration of fibroblasts on fibronectin in a
protease-independent, lipid raft-dependent manner. Nowicki et
al (38) demonstrated that the
downregulation of uPA inhibited the phosphatidylinositol 3-kinase
and Akt signaling pathways, which led to the migration of
glioblastoma cells. The interaction between uPAR and the uPA
complex affects tumor progression, invasion and migration (39). Thus, limiting the combination of uPAR
and uPA may be an important method for cancer treatment. In the
present study, the expression level of uPAR was observed to be
higher in the MKN45 cells when compared with the other cell lines,
and the uPAR antibody inhibited MKN45 cell adhesion and migration,
suggesting that uPAR may promote the adhesion and migration of
MKN45 cells to mesothelial cells.
There are three inhibitors for uPA within the uPA
system: PAI-1, PAI-2 and PAI-3. PAI-1 is typically the key
inhibitor of human plasma uPA, and it is activated during the
initial synthesis stage, but is rapidly inactivated when in serum
(40). Immunohistochemical analysis
demonstrated that PAI-1 was associated with lymphatic metastasis
and cooperated with uPA to serve a role in the invasion and
migration of tumor cells (41). The
upregulation of PAI-1 limits the accumulation of plasmin, as well
as the activation of matrix metalloproteinase (MMP)-2 and −9 in
prostate cancer (39). Huber et
al (42) observed that the
increased expression of PAI-1, activated by the proinflammatory
cytokine tumor necrosis factor α, restricted the invasion of
HTR-8/SVneo cells (42). The results
of the present study demonstrated that the PAI-1 antibody exhibited
greater inhibition of MKN45 cell invasion compared with adhesion
and migration; thus suggesting that PAI-1 primarily functions
during the invasive tumor cell stage, and may contribute to gastric
cancer adhesion and migration. Notably, the effect of the uPA
system on the invasive ability of the MKN45 cells demonstrated no
significance among antibodies with different concentrations.
However, the effect of PAI-1 antibody concentration on gastric
cancer cell behaviors has not been fully investigated. Thus,
further research is required to investigate the effect of antibody
concentration on gastric cancer cells.
In conclusion, the present study examined the
association between the uPA system and peritoneal metastasis in
gastric cancer. The MKN45 cell line has a high level of uPA system
expression, as well as uPA activity. Proteins in the uPA system may
inordinately promote the adhesion, migration and invasion of tumor
cells into mesothelial cell layers. The results of the current
study may provide guidelines for the use of the uPA system in
gastric cancer diagnosis, and may hopefully provide a basis for the
development of uPA therapy options to treat gastric cancer with
peritoneal metastasis.
Glossary
Abbreviations
Abbreviations:
|
uPA
|
urokinase-type plasminogen
activator
|
|
ECM
|
extracellular matrix
|
|
uPAR
|
uPA receptor
|
|
PAI-1
|
plasminogen activator inhibitor-1
|
|
PIg
|
plasminogen
|
|
SF-CM
|
serum-free media
|
|
MTT
|
methyl thiazolyl tetrazolium
|
|
MAPK
|
mitogen-activated protein kinase
|
References
|
1
|
Ferro A, Peleteiro B, Malvezzi M, Bosetti
C, Bertuccio P, Levi F, Negri E, La Vecchia C and Lunet N:
Worldwide trends in gastric cancer mortality (1980–2011), with
predictions to 2015, and incidence by subtype. Eur J Cancer.
50:1330–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanda M, Shimizu D, Nomoto S, Takami H,
Hibino S, Oya H, Hashimoto R, Suenaga M, Inokawa Y, Kobayashi D, et
al: Prognostic impact of expression and methylation status of
DENN/MADD domain-containing protein 2D in gastric cancer. Gastric
Cancer. 18:288–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fujii S, Kitayama J, Kaisaki S, Sasaki S,
Seto Y, Tominaga O, Tsuno N, Umetani N, Yokota H, Kitamura K, et
al: Carcinoembryonic antigen mRNA in abdominal cavity as a useful
predictor of peritoneal recurrence of gastric cancer with serosal
exposure. J Exp Clin Cancer Res. 21:547–553. 2002.PubMed/NCBI
|
|
4
|
Zhao P, Lu Y, Jiang X and Li X:
Clinicopathological significance and prognostic value of CD133
expression in triple-negative breast carcinoma. Cancer Sci.
102:1107–1111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thompson EW, Newgreen DF and Tarin D:
Carcinoma invasion and metastasis: A role for
epithelial-mesenchymal transition? Cancer Res. 65:5991–5995. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho RW and Clarke MF: Recent advances in
cancer stem cells. Curr Opin Genet Dev. 18:48–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
John A and Tuszynski G: The role of matrix
metalloproteinases in tumor angiogenesis and tumor metastasis.
Pathol Oncol Res. 7:14–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fenoglio-Preiser C, Carneiro F, Correa P,
Guildford P, Lambert R, Megraud F, Muñoz N, Powell SM, Rugge M,
Sasako M, et al: Gastric carcinoma. In: World Health Organisation
Classification of Tumours. Pathology and Genetics of Tumours of the
Digestive System. IARC Press. (Lyon). 35–52. 2000.
|
|
10
|
Crippa MP: Urokinase-type plasminogen
activator. Int J Biochem Cell Biol. 39:690–694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Look MP, van Putten WL, Duffy MJ, Harbeck
N, Christensen IJ, Thomssen C, Kates R, Spyratos F, Fernö M,
Eppenberger-Castori S, et al: Pooled analysis of prognostic impact
of urokinase-type plasminogen activator and its inhibitor PAI-1 in
8377 breast cancer patients. J Natl Cancer Inst. 94:116–128. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pulukuri SMK and Rao JS: Small interfering
RNA directed reversal of urokinase plasminogen activator
demethylation inhibits prostate tumor growth and metastasis. Cancer
Res. 67:6637–6646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dass K, Ahmad A, Azmi AS, Sarkar SH and
Sarkar FH: Evolving role of uPA/uPAR system in human cancers.
Cancer Treat Rev. 34:122–136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaneko T, Konno H, Baba M, Tanaka T and
Nakamura S: Urokinase-type plasminogen activator expression
correlates with tumor angiogenesis and poor outcome in gastric
cancer. Cancer Sci. 94:43–49. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee DH, Yang Y, Lee SJ, Kim KY, Koo TH,
Shin SM, Song KS, Lee YH, Kim YJ, Lee JJ, et al: Macrophage
inhibitory cytokine-1 induces the invasiveness of gastric cancer
cells by up-regulating the urokinase-type plasminogen activator
system. Cancer Res. 63:4648–4655. 2003.PubMed/NCBI
|
|
16
|
Champelovier P, Boucard N, Levacher G,
Simon A, Seigneurin D and Praloran V: Plasminogen- and
colony-stimulating factor-1-associated markers in bladder
carcinoma: Diagnostic value of urokinase plasminogen activator
receptor and plasminogen activator inhibitor type-2 using
immunocytochemical analysis. Urol Res. 30:301–309. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosanò L, Varmi M, Salani D, Di Castro V,
Spinella F, Natali PG and Bagnato A: Endothelin-1 induces tumor
proteinase activation and invasiveness of ovarian carcinoma cells.
Cancer Res. 61:8340–8346. 2001.PubMed/NCBI
|
|
18
|
Yonemura Y, Fujimura T, Ninomiya I, Kim
BS, Bandou E, Sawa T, Kinoshita K, Endo Y, Sugiyama K and Sasaki T:
Prediction of peritoneal micrometastasis by peritoneal lavaged
cytology and reverse transcriptase-polymerase chain reaction for
matrix metalloproteinase-7 mRNA. Clin Cancer Res. 7:1647–1653.
2001.PubMed/NCBI
|
|
19
|
Kilkenny C, Browne W, Cuthill IC, Emerson
M and Altman DG: NC3Rs Reporting Guidelines Working Group: Animal
research Animal research: Reporting in vivo experiments: the ARRIVE
guidelines. Br J Pharmacol. 160:1577–1579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Japanese Gastric Cancer Association:
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric
Cancer. 14:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang JH, Lee SI, Lim DH, Park KW, Oh SY,
Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, et al: Salvage
chemotherapy for pretreated gastric cancer: A randomized phase III
trial comparing chemotherapy plus best supportive care with best
supportive care alone. J Clin Oncol. 30:1513–1518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shimizu M, Cohen B, Goldvasser P, Berman
H, Virtanen C and Reedijk M: Plasminogen activator uPA is a direct
transcriptional target of the JAG1-Notch receptor signaling pathway
in breast cancer. Cancer Res. 71:277–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiu X, Guo S, Wu H, Chen J and Zhou Q:
Identification of Wnt pathway, uPA, PAI-1, MT1-MMP, S100A4 and
CXCR4 associated with enhanced metastasis of human large cell lung
cancer by DNA microarray. Minerva Med. 103:151–164. 2012.PubMed/NCBI
|
|
25
|
Berasain C, Ujue Latasa M, Urtasun R, Goñi
S, Elizalde M, Garcia-Irigoyen O, Azcona M, Prieto J and Ávila MA:
Epidermal growth factor receptor (EGFR) crosstalks in liver cancer.
Cancers (Basel). 3:2444–2461. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mutsaers SE: The mesothelial cell. Int J
Biochem Cell Biol. 36:9–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Na D, Liu FN, Miao ZF, Du ZM and Xu HM:
Astragalus extract inhibits destruction of gastric cancer cells to
mesothelial cells by anti-apoptosis. World J Gastroenterol.
15:570–577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Danø K, Behrendt N, Høyer-Hansen G,
Johnsen M, Lund LR, Ploug M and Rømer J: Plasminogen activation and
cancer. Thromb Haemost. 93:676–681. 2005.PubMed/NCBI
|
|
30
|
Mekkawy AH, Morris DL and Pourgholami MH:
Urokinase plasminogen activator system as a potential target for
cancer therapy. Future Oncol. 5:1487–1499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koochekpour S, Sartor O, Hiraiwa M, Lee
TJ, Rayford W, Remmel N, Sandhoff K, Minokadeh A and Patten DY:
Saposin C stimulates growth and invasion, activates p42/44 and
SAPK/JNK signaling pathways of MAPK and upregulates uPA/uPAR
expression in prostate cancer and stromal cells. J Androl.
7:147–158. 2005.
|
|
32
|
Binder BR, Mihaly J and Prager GW:
uPAR-uPA-PAI-1 interactions and signaling: A vascular biologist's
view. Thromb Haemost. 97:336–342. 2007.PubMed/NCBI
|
|
33
|
Li XF, Yan PJ and Shao ZM: Downregulation
of miR-193b contributes to enhance urokinase-type plasminogen
activator (uPA) expression and tumor progression and invasion in
human breast cancer. Oncogene. 28:3937–3948. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pluskota E, Soloviev DA and Plow EF:
Convergence of the adhesive and fibrinolytic systems: Recognition
of urokinase by integrin alpha Mbeta 2 as well as by the urokinase
receptor regulates cell adhesion and migration. Blood.
101:1582–1590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Blasi F and Sidenius N: The urokinase
receptor: Focused cell surface proteolysis, cell adhesion and
signaling. FEBS Lett. 584:1923–1930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Montuori N, Cosimato V, Rinaldi L, Rea
VEA, Alfano D and Ragno P: uPAR regulates pericellular proteolysis
through a mechanism involving integrins and fMLF-receptors. Thromb
Haemost. 109:309–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grove LM, Southern BD, Jin TH, White KE,
Paruchuri S, Harel E, Wei Y, Rahaman SO, Gladson CL, Ding Q, et al:
Urokinase-type plasminogen activator receptor (uPAR) ligation
induces a raft-localized integrin signaling switch that mediates
the hypermotile phenotype of fibrotic fibroblasts. J Biol Chem.
289:12791–12804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nowicki TS, Zhao H, Darzynkiewicz Z,
Moscatello A, Shin E, Schantz S, Tiwari RK and Geliebter J:
Downregulation of uPAR inhibits migration, invasion, proliferation,
FAK/PI3K/Akt signaling and induces senescence in papillary thyroid
carcinoma cells. Cell Cycle. 10:100–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sheng S: The urokinase-type plasminogen
activator system in prostate cancer metastasis. Prostate Cancer:
New Horizons in Research and Treatment. Cher ML, Honn KV and Raz A:
Springer. 151–160. 2002.
|
|
40
|
Francis RM, Romeyn CL, Coughlin AM,
Nagelkirk PR, Womack CJ and Lemmer JT: Age and aerobic training
status effects on plasma and skeletal muscle tPA and PAI-1. Eur J
Appl Physiol. 114:1229–1238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Malinowsky K, Wolff C, Berg D, Schuster T,
Walch A, Bronger H, Mannsperger H, Schmidt C, Korf U, Höfler H, et
al: uPA and PAI-1-related signaling pathways differ between primary
breast cancers and lymph node metastases. Transl Oncol. 5:98–104.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huber AV, Saleh L, Bauer S, Husslein P and
Knöfler M: TNFalpha-mediated induction of PAI-1 restricts invasion
of HTR-8/SVneo trophoblast cells. Placenta. 27:127–136. 2006.
View Article : Google Scholar : PubMed/NCBI
|