Introduction
At present, lung cancer is the leading cause of
cancer-associated mortality worldwide (1). Therefore, treatment strategies for lung
cancer are of significant interest. The standard approach to lung
cancer treatment is multidisciplinary and includes surgery,
chemotherapy, radiotherapy and targeted therapy. Targeted therapy
is a novel and promising therapeutic modality. The Chinese
guidelines on the diagnosis and treatment of primary lung cancer
(2011 version) (2) state that
systemic therapy improves quality of life and prolongs the survival
of stage IV non-small cell lung cancer (NSCLC) patients. Targeted
treatment of NSCLC patients with epidermal growth factor receptor
(EGFR) mutations is more likely to be effective than in patients
without EGFR mutations; therefore, gefitinib or erlotinib are
recommended as the first-line treatment for stage IV NSCLC patients
with sensitive EGFR mutations (2).
In 2006, oral erlotinib (150 mg, daily) was approved
by the China Food and Drug Administration for the treatment of
NSCLC in China (3). Erlotinib, an
EGFR tyrosine kinase inhibitor, inhibits the phosphorylation of
EGFR, which is expressed on the surface of normal and tumor cells.
In non-clinical experimental models, the inhibition of EGFR
phosphorylation has been demonstrated to cause cell growth arrest
and cell death (3). The most common
adverse reactions following erlotinib administration are rash and
mild diarrhea. Additional adverse reactions include severe rash,
severe paronychia, interstitial pneumonia, myocardial ischemia and
acute hepatitis (3).
The present study reports the first case of acute
myocardial infarction occurring after one month of treatment with
erlotinib in an NSCLC patient, and highlights the importance of
careful monitoring following this treatment.
Case report
On March 25, 2013, a 63-year-old male patient was
admitted to the Provincial Hospital Affiliated to Shandong
University (Jinan, China) with chest tightness that had lasted for
14 days. The patient had smoked for 40 years (≤20 cigarettes per
day) and reported a 30-year history of hypertension. A computed
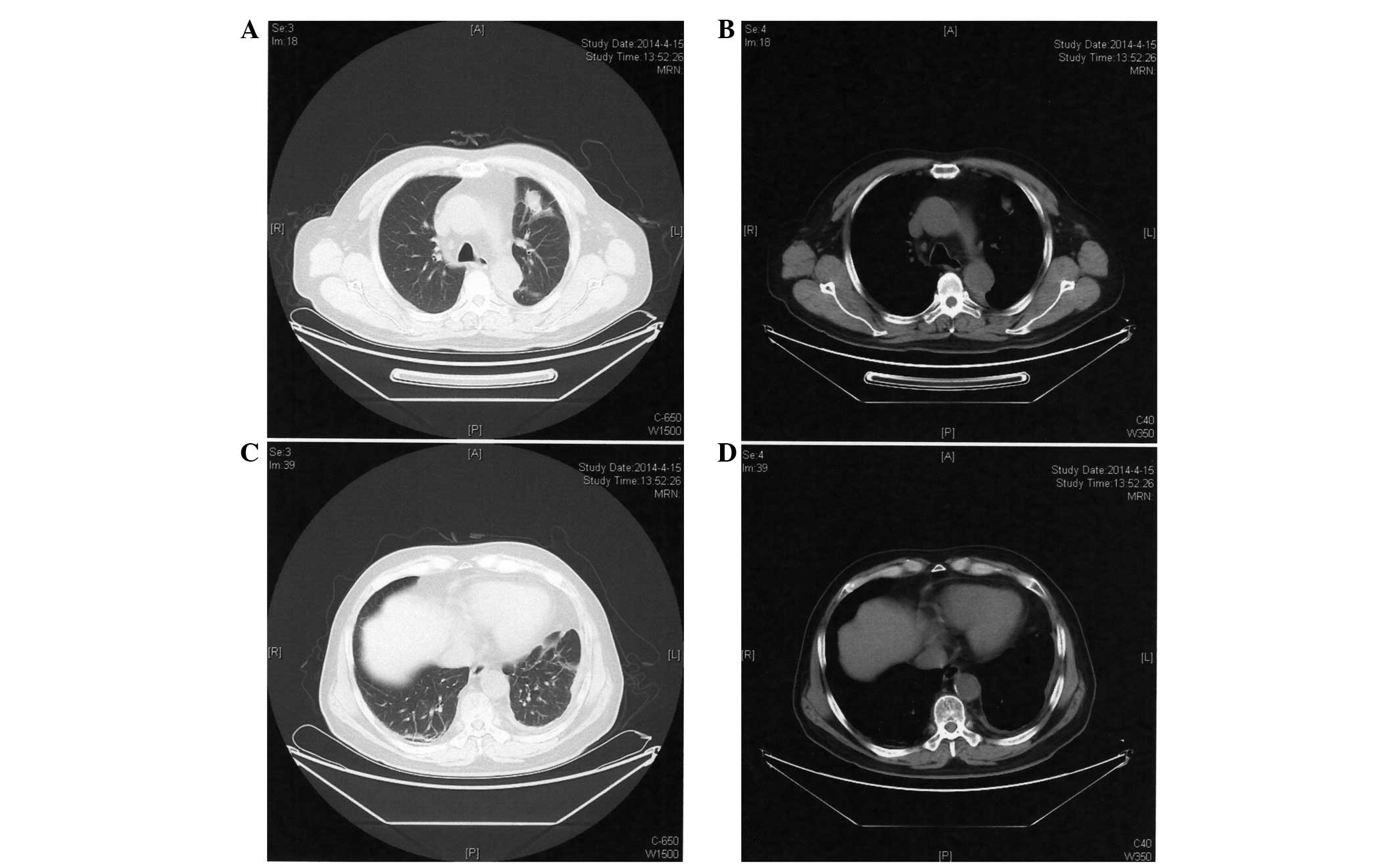
tomography (CT; Discovery CT750 HD; GE Healthcare Life Sciences,
Shanghai, China) scan identified a mass in the upper lobe of the
left lung and left pleural effusions (Fig. 1). Cytology of pleural effusions was
clearly positive at malignant cells, with tumor marker
carcinoembryonic antigen (CEA) levels of >1,000 ng/ml (normal
range, 0–10 ng/ml). A percutaneous lung biopsy was performed in
April 2013, which confirmed the diagnosis of histologically
invasive adenocarcinoma. Subsequently, the patient received 7
cycles of chemotherapy with pemetrexed (0.8 g on day 1 every 21
days) and nedaplatin (40 mg on days 2–4 every 21 days) and
experienced significant bone marrow suppression during
chemotherapy. During the chemotherapy treatment, the patient
complained of chest discomfort. However, repeated electrocardiogram
(ECG; 9310P; Nihon Kohden, Tokyo, Japan) examinations revealed no
obvious abnormalities. An echocardiographic examination performed
on October 8, 2013 indicated that the cardiac structures were
normal (Fig. 2A) with a left
ventricular ejection fraction (LVEF) of 63%.
In April 2014, the patient was admitted to the
Provincial Hospital Affiliated to Shandong University for review.
ECG examination demonstrated sinus rhythm and no evidence of
arrhythmia or myocardial ischemia (Fig.
2B). A CT scan revealed that the mass had decreased in size
compared with that prior to the 7 cycles of chemotherapy (Fig. 3). Emission CT revealed increased
imaging agent (iodinated contrast) uptake in the 6th, 7th and 8th
left front ribs compared with the contralateral ribs. Although no
significant progression of the lesions was evident following 7
cycles of pemetrexed and nedaplatin, evidence of bone metastasis
was identified using bone imaging examination, and the patient had
experienced adverse reactions to chemotherapy; the blood cell count
of the patient was significantly reduced, demonstrating bone marrow
suppression. Due to the limited efficacy and undesirable side
effects of chemotherapy, and the presence of sensitive EGFR
mutations in the tumor, demonstrated by genetic testing of
pathological specimens, erlotinib maintenance treatment (150 mg
orally, once daily) was selected.
In May 2014, after 1 month of treatment with
erlotinib, the patient was admitted for follow-up, complaining of
mild nausea and occasional pain in the left chest area, without
radiating pain, vomiting, fever or expectoration. Physical
examination revealed a body temperature of 35.6°C (normal range,
36.0–37.0°C), pulse rate of 92 bpm (normal range, 60–100 bpm),
respiratory rate of 23 times/min (normal range, 16–20 times/min),
blood pressure of 115/74 mmHg (normal range, 90–130/60–90 mmHg),
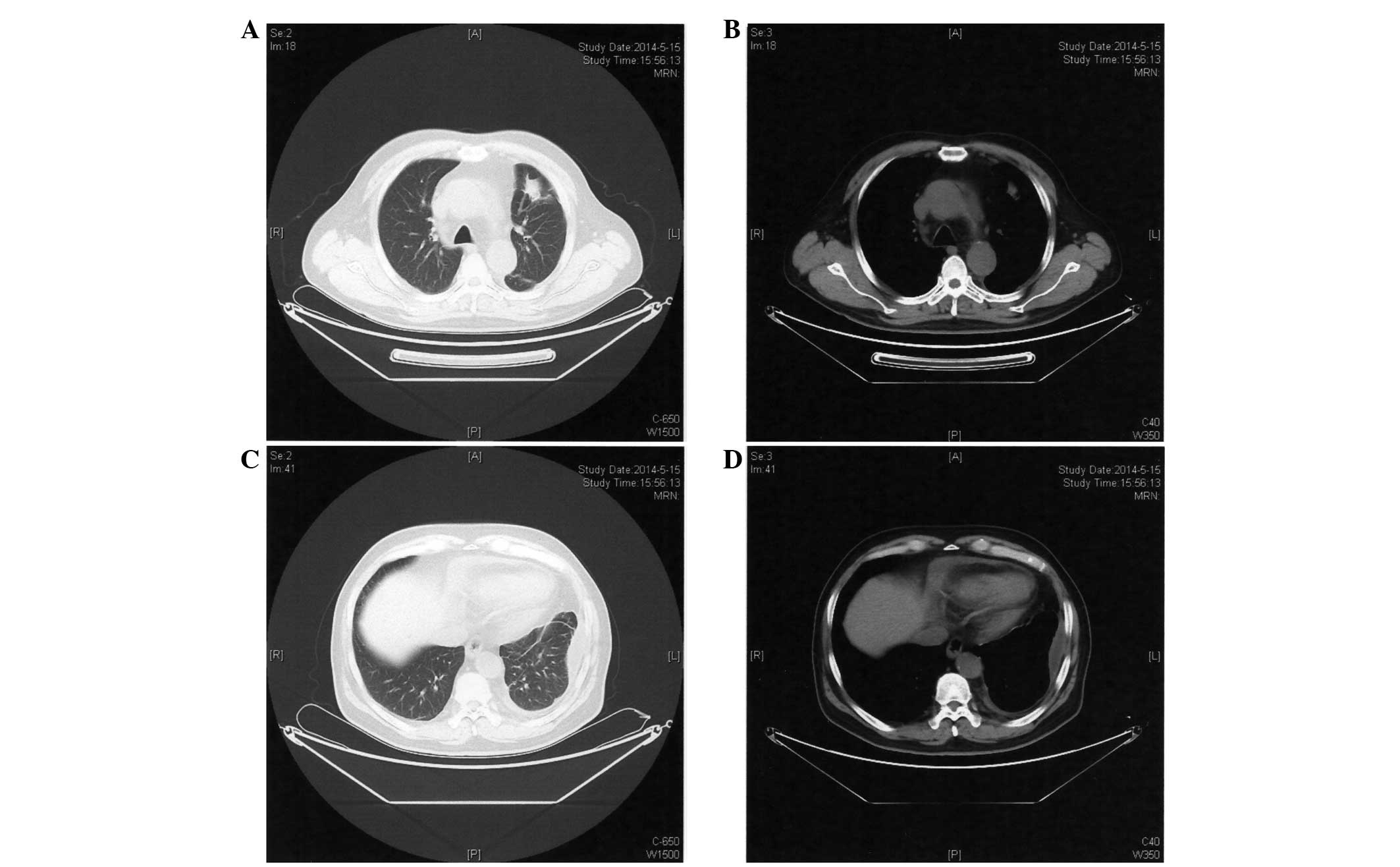
and a rash located on the face and lower jaw. A CT scan performed
on May 15, 2014 (Fig. 4) revealed no
significant differences in the mass located in the upper lobe of
the left lung compared with the previous CT scan. ECG showed that
ST-segments of V1-V5 were arched upward and inverted T waves were
present (Fig. 5A). High-sensitivity
troponin T assay revealed troponin T levels of 23.77 pg/ml (normal
range, 3–14 pg/ml) and N-terminal pro-brain natriuretic peptide
levels of 2,771 pg/ml (normal range, 0–125 pg/ml). Echocardiography
indicated extensive infarction of the anterior myocardial tissue
(Fig. 5B) and a LVEF of 54%. The
patient was considered to have experienced acute myocardial
infarction and thus erlotinib treatment was discontinued
immediately. Subsequently, the patient's blood pressure was
controlled and anticoagulant and diuretic drugs were administered
(asprin enteric-coated tablets, 0.1 g every day; clopidogrel
hydrogen sulphate tablets, 75 mg every day; furosemide tablets, 20
mg twice a day).
During follow-up, a computed tomographic angiography
of the coronary arteries identified multiple plaques in the right
and left coronary arteries with corresponding luminal stenosis.
Currently, the patient is well and his heart function is
controlled; however, pulmonary metastasis was identified in
2015.
Written informed consent was obtained from the
patient's family for the publication of this study.
Discussion
Recently, certain drugs administered for the
treatment of tumors, including target drugs such as imatinib,
sunitinib and nilotinib, have been reported to cause cardiac
toxicity (4). A cardiotoxicity study
revealed that sunitinib treatment resulted in mitochondrial damage
and myocardial apoptosis in mice and cultured rat cardiomyocytes
(5). Erlotinib has been demonstrated
as a safe and well-tolerated treatment for NSCLC (6–9), with no
cases of myocardial infarction reported to date.
The patient in the present case report had a history
of hypertension, and the patient's triglyceride, total cholesterol
and CEA levels at various time points are shown in Table I. Prior to treatment with erlotinib,
the cardiac ultrasound and repeated ECG examinations were normal.
Following treatment with erlotinib for 1 month, commencing in April
2014, the lesion in upper lobe of the left lung exhibited no
significant changes and the CEA level of the patient was lower than
before the treatment (Table I).
During targeted therapy, the patient developed a facial rash, and
subsequently experienced acute myocardial infarction following 1
month of erlotinib treatment. During this treatment period, no
other chemotherapy drugs that may cause myocardial ischemia or
myocardial infarction were administered. Based on these findings,
erlotinib was considered to be the cause of the adverse reactions
observed.
 | Table I.Triglyceride, total cholesterol and
CEA serum levels of the patient. |
Table I.
Triglyceride, total cholesterol and
CEA serum levels of the patient.
| Date | Triglycerides
(mmol/l) | Total cholesterol
(mmol/l) | CEA (ng/ml) |
|---|
| 26/03/2013 | 2.06 | 5.31 | 86.19 |
| 14/01/2014 | 2.50 | 4.75 | 61.51 |
| 18/02/2014 | 3.71 | 4.96 | – |
| 27/03/2014 | 2.96 | 4.94 | 32.86 |
| 15/04/2014 | 2.36 | 4.63 | – |
| 16/05/2014 | 2.25 | 3.61 | 14.77 |
We postulate that erlotinib may lead to myocardial
infarction; however, the mechanism remains unclear. Erlotinib
exerts anticancer effects via the inhibition of EGFR
phosphorylation. EGFR is found on the surface of cancer cells, but
is also present on various kinds of normal cells. Doherty et
al (10) investigated the
toxicity of crizotinib, sunitinib, erlotinib and nilotinib
in vitro and revealed that treatment with 10 µM erlotinib
caused human cardiomyocyte cell death. It was also demonstrated in
a phase I study that the steady-state plasma concentration of
erlotinib (150 mg, administered daily) is 1.37–1.64 µg/ml (11). Notably, Wu et al (11) reported that the plasma concentration
of erlotinib in patients with interstitial lung disease following
treatment for 6 days (150 mg, daily) was 3.62 µg/ml. Consequently,
it is possible that the in vivo concentration of erlotinib
is so high that it may trigger myocardial cell death, resulting in
myocardial infarction in addition to tumor cell apoptosis.
Additionally, Doherty et al (10) demonstrated that erlotinib marginally
reduced lipid deposition of human cardiac cells. Notably, coronary
plaque deposition was observed in the patient of the present study;
thus, we hypothesize that erlotinib may cause plaque instability,
subsequently leading to acute myocardial infarction.
Furthermore, erlotinib may partially inhibit a
certain type of potassium channel (10), causing prolongation of QT intervals
and leading to arrhythmia, thus inducing myocardial ischemia or
myocardial infarction.
Although this hypothesis requires further study for
validation, cardiac toxicity caused by the administration of
erlotinib requires serious attention. When administering targeted
drugs, such as erlotinib, to patients with advanced NSCLC,
particularly elderly patients or those with a history of coronary
heart disease, clinicians must be cautious and a comprehensive
assessment of the general condition of patients should be
performed. ECG, echocardiography and myocardial enzymes testing
must be performed regularly so that serious adverse reactions may
be identified early and treated quickly.
References
|
1.
|
Gaga M, Powell CA, Schraufnagel DE,
Schönfeld N, Rabe K, Hill NS and Sculier JP: ATS/ERS Task Force on
the Role of the Pulmonologist in the Management of Lung Cancer: An
official American Thoracic Society/European Respiratory Society
statement: The role of the pulmonologist in the diagnosis and
management of lung cancer. Am J Respir Crit Care Med. 188:503–507.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Zhi X, Wu Y, Ma S, Wang T, Wang C, Wang J,
Shi Y, Lu Y, Liu L, Liu D, et al: Chinese guidelines on the
diagnosis and treatment of primary lung cancer (2011 version).
Zhongguo Fei Ai Za Zhi. 15:677–688. 2012.(In Chinese). PubMed/NCBI
|
|
3.
|
Li HR and Sun F: Erlotinib serious adverse
reactions and preventive measures. Chin J Pharmacoepidemiol.
19:232–233. 2010.(In Chinese).
|
|
4.
|
Orphanos GS, Ioannidis GN and Ardavanis
AG: Cardiotoxicity induced by tyrosine kinase inhibitors. Acta
Oncol. 48:964–970. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Chu TF, Rupnick MA, Kerkela R, Dallabrida
SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai
J, et al: Cardiotoxicity associated with tyrosine kinase inhibitor
sunitinib. Lancet. 370:2011–2019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Reck M, Mok T, Wolf J, Heigener D and Wu
YL: Reviewing the safety of erlotinib in non-small cell lung
cancer. Expert Opin Drug Saf. 10:147–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Merimsky O, Cheng CK, Au JS, von Pawel J
and Reck M: Efficacy and safety of first-line erlotinib in elderly
patients with advanced non-small cell lung cancer. Oncol Rep.
28:721–727. 2012.PubMed/NCBI
|
|
8.
|
Reck M, van Zandwijk N, Gridelli C, Baliko
Z, Rischin D, Allan S, Krzakowski M and Heigener D: Erlotinib in
advanced non-small cell lung cancer: Efficacy and safety findings
of the global phase IV Tarceva Lung Cancer Survival Treatment
study. J Thorac Oncol. 5:1616–1622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wu YL, Kim JH, Park K, Zaatar A,
Klingelschmitt G and Ng C: Efficacy and safety of maintenance
erlotinib in Asian patients with advanced non-small-cell lung
cancer: A subanalysis of the phase III, randomized SATURN study.
Lung cancer. 77:339–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Doherty KR, Wappel RL, Talbert DR, Trusk
PB, Moran DM, Kramer JW, Brown AM, Shell SA and Bacus S:
Multi-parameter in vitro toxicity testing of crizotinib, sunitinib,
erlotinib and nilotinib in human cardiomyocytes. Toxicol Appl
Pharmacol. 272:245–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wu X, Gao G, Ren S and Zhou C: Four cases
of interstitial lung disease induced by erlotinib and a review of
the literatures. Zhongguo Fei Ai Za Zhi. 15:494–498. 2012.(In
Chinese). PubMed/NCBI
|