Introduction
Acute lymphocytic leukemia (ALL) is a malignant
hematological disease, which originates from B or T lymphoid
progenitor cells (1). The central
nervous system (CNS) is a region in which direct infiltration and
involvement or relapse occurs in adults with ALL (1). If no preventative therapy is
administered, a total of 30–50% of adults with ALL eventually
present with CNS leukemia (CNSL) (2).
Following advances in chemotherapy and effective CNS prophylaxis,
the incidence of CNS relapse in cases of ALL has decreased to 5–10%
(1). Intrathecal administration of
chemotherapy, high dose chemotherapy and brain radiotherapy are the
primary measures used for the prevention of CNSL (3,4).
The central nervous system (CNS) has long been
recognized as a site, and indeed a sanctuary, for leukemic cells
(1). However, few patients (<5%)
with ALL present with overt CNSL initially (1). The clinical manifestation of CNSL ranges
from mild to severe, and infiltration of the arachnoid membrane and
dura mater is the most common, followed by the brain parenchyma and
cranial nerves; spinal cord infiltration is the most rare
presentation (1,3,4).
The most commonly used treatment for CNSL is
intrathecal (IT) administration of chemotherapy (1,3–5). However, IT chemotherapy is associated
with certain complications, which most frequently include
peripheral neuropathy, cranial neuropathies, acute encephalopathy,
acute vasculopathies, headaches and seizures (5). Transverse myelopathy is a rare
complication (5). The current study
reports the case of a patient who experienced a reversible spinal
cord injury as clinical feature, and subsequently developed
irreversible spinal cord injury following the IT administration of
methotrexate (MTX) and cytarabine (Ara-C).
Case report
A 46-year-old man was diagnosed with B-cell ALL
(Philadelphia chromosome-positive and hyperleukocytosis)
morphology, immunology, cytogenetics and molecular biology by
morphology, immunology, cytogenetics and molecular biology at The
Second Hospital of Anhui Medical University (Hefei, China) in
November 2012. Philadelphia chromosome was tested using the
G-banding technique, and a routine blood test demonstrated that the
white blood cell count was 32.93×109/l, which indicated
the presence of hyperleukocytosis. The patient underwent induction
chemotherapy consisting of DVCP (daunorubicin 80 mg, day 1, 15 and
22; vindesine 4 mg, day 1, 8, 15 and 22; cyclophosphamide 1.0 g,
day 1 and 15; and desamethasone 15 mg, days 1–28) plus imatinib
(400 mg, days 19–28) for 1 cycle. However, in January 2013, the
patient developed a sudden onset of numbness in his two lower limbs
(also known as transverse myelopathy) in addition to bladder
incontinence, shortly after achieving remission in the blood and
bone marrow following the initial course of chemotherapy. Magnetic
resonance (MR) imaging (MAGNETOM Verio 3.0T; Siemens AG, Munich,
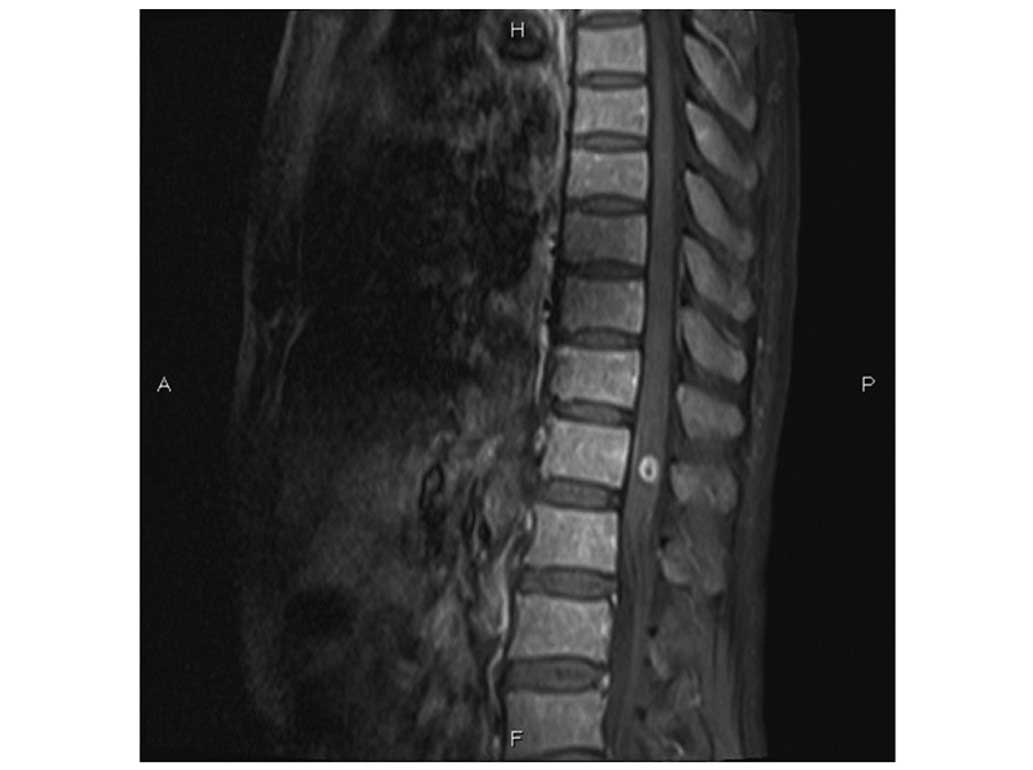
Germany) revealed lymphomatous infiltration at the T12 vertebra
(Fig. 1A). Leukemic infiltration of
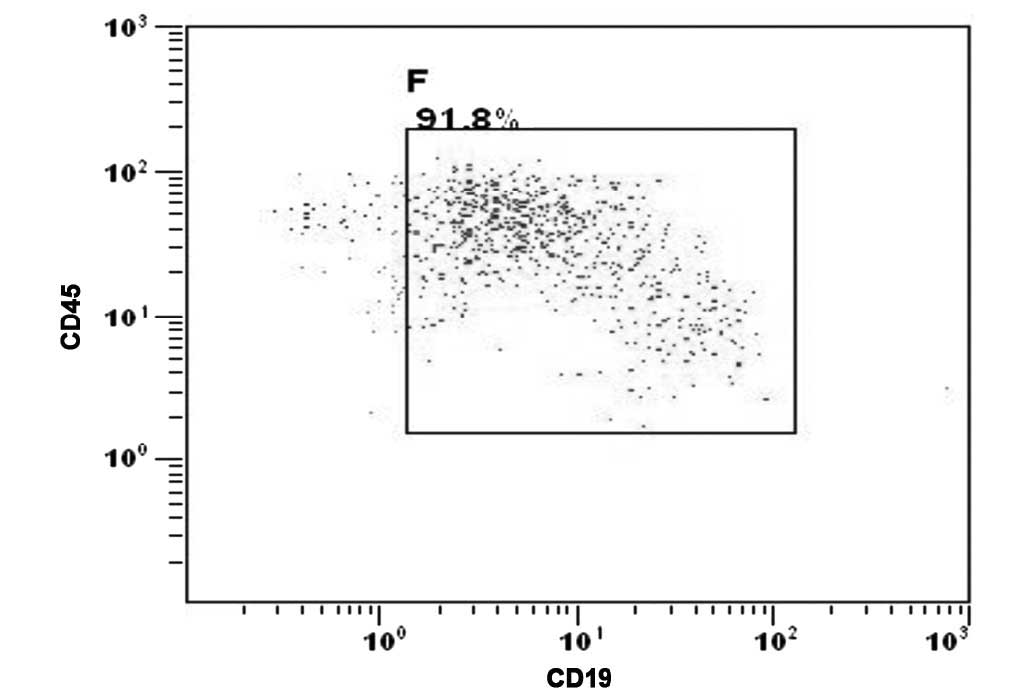
the CNS was confirmed by the presence of malignant leukemia cells
detected in the cytospin of the cerebrospinal fluid (CSF) (Fig. 2).
The patient was subsequently administered IT (via
the 3rd and 4th lumbar intervertebral space) MTX (15 mg) and Ara-C
(50 mg) immediately following a diagnostic lumbar puncture every
other day 8 times, without other therapy, from January 7 to 21,
2013. After experiencing CNSL remission, the patient was given IT
MTX (10 mg), Ara-C (50 mg) and dexamethasone (10 mg) once per week
for 4 weeks. Soon after the completion of IT injections, the
patient reported feeling that his numbness and bladder incontinence
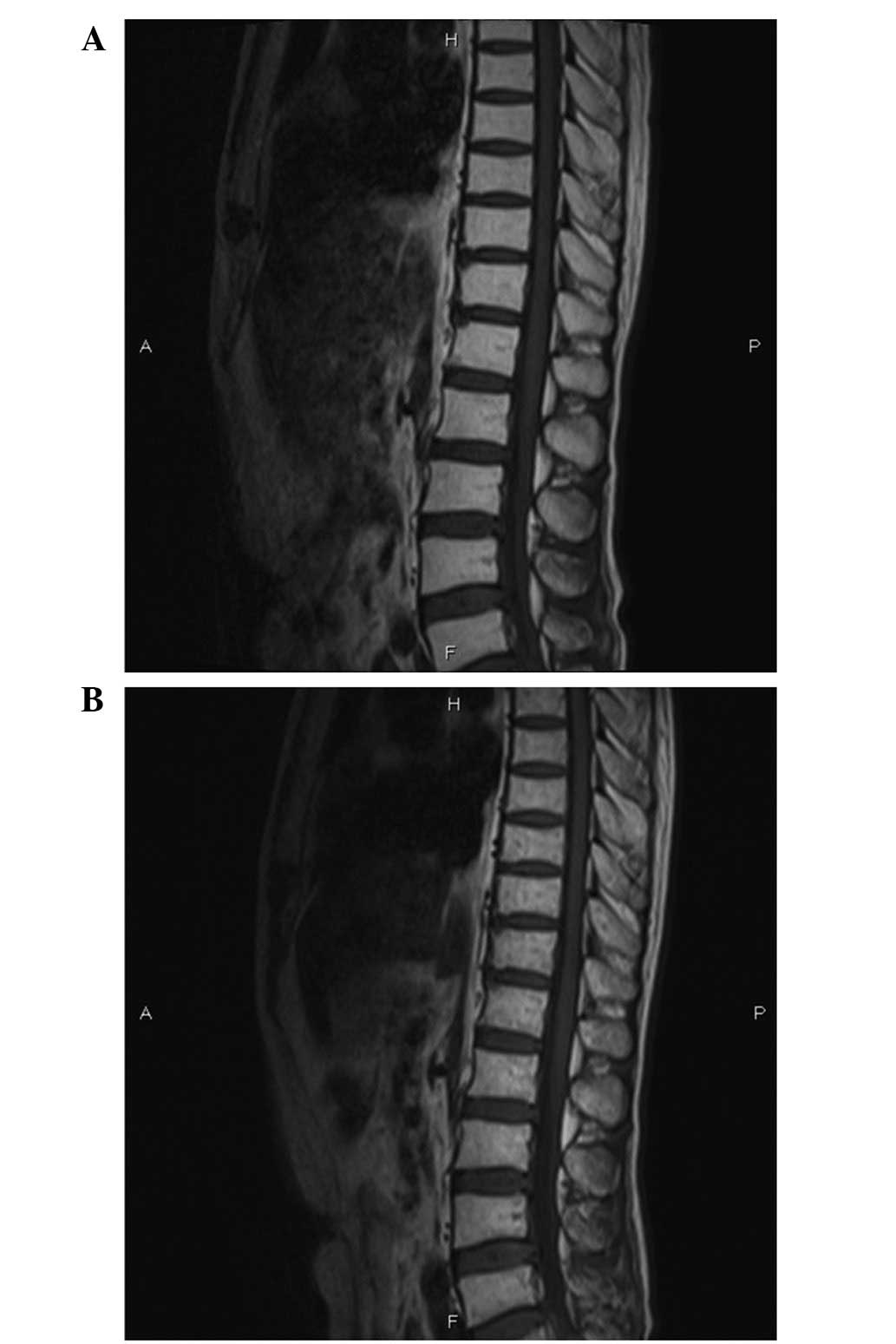
had recovered. Repeat MR imaging showed no infiltration in the
spinal cord (Fig. 3A). The patient
was subsequently administered consolidation chemotherapy once
consisting of cyclophosphamide (1.2 g; day 1), vincristine (2 mg;
day 1), Ara-C (0.2 g; days 1–5), teniposide (150 mg, days 1–4) and
dexamethasone (10 mg; days 1–7) for 1 cycle.
However, in April 2013, the patient developed a
sudden onset of paraplegia and urinary retention again. Repeat MR
imaging of the spine and brain revealed no evidence of disease
progression, spinal cord compression or brain metastasis (Fig. 3B). A repeat CSF examination during
this neurological event was normal. Supportive treatment, which
included neuro nutrition drugs, was administered accordingly;
however, the paraplegia was irreversible. The patient gave up
further treatment due to economic factors in May 2013, and at the
last follow-up the numbness and bladder incontinence had
alleviated, but had not fully recovered.
Discussion
The CNS is a location in which direct infiltration
and involvement or relapse may occur in ALL (1). The majority of ALL relapses occur during
treatment or within the first 2 years after the completion of
treatment; however, relapses have been reported to occur even 10
years after diagnosis. The main mechanism of CNS infiltration in
leukemia is associated with blood brain barrier (7). CNSL most frequently involves
infiltration of the arachnoid membrane and dura mater, less
commonly the brain parenchyma, choroid glands and cranial nerve,
and rarely the spinal cord (5).
There are several high risk factors associated with
the occurrence of CNSL, including hyperleukocytosis at diagnosis,
the presence of extramedullary infiltration, certain types of acute
leukemia [including acute myelomonocytic leukemia and acute
monocytic leukemia (M5a)], T-cell immunophenotype, Burkitt's
lymphoma (mature), relapsed acute promyelocytic leukemia, and
high-risk genetic abnormalities [such as t(4;11) and the
Philadelphia chromosome] (8). The
current patient, who developed the symptom of paraplegia, had two
high risk factors for CNSL: Hyperleukocytosis at diagnosis and the
Philadelphia chromosome.
There are numerous methods for the treatment of
CNSL, including IT chemotherapy, systemic chemotherapy and
radiation therapy (9). The role of IT
chemotherapy has been emphasized in modern clinical usage; however,
it is associated with various possible side effects. Transverse
myelopathy, which is defined as the development of isolated spinal
cord dysfunction over hours or days in the absence of a compressive
lesion, is an unusual complication of IT MTX/Ara-C chemotherapy
(10–12). The most important MTX-associated risk
factors for the development transverse myelopathy are high dose IT
MTX, systemic MTX, repeated injection with an interval of <1
week, concurrent use with other medication or cranial radiotherapy,
and active CNS disease (13,14). The symptoms usually develop between
several minutes and 2 weeks after treatment (13,14);
however, the current patient developed the neurological symptoms 3
months after the first administration of IT MTX and Ara-C.
Although the incidence of transverse myelopathy is
low (~3% of all patients who undergo intrathecal injection) and its
occurrence is unpredictable, doctors must be aware of this
complication and attempt to avoid the aforementioned high risk
factors (15). Once the complication
has occurred, administration of IT MTX or Ara-C must be
discontinued and the patient should be reassured (15).
In conclusion, the most vital difference between the
current case and other cases in which transverse myelopathy
developed was that the neurological symptoms of the current patient
were identical when he developed CNSL and when the complication of
the subsequent IT chemotherapy occurred. To distinguish the two is
crucial as the appropriate therapies for each are completely
opposite (5). It is advisable to
monitor the CSF by light microscopy and flow cytometry. Repeated MR
imaging must also be conducted.
Acknowledgements
This study was supported by the Foundation of Anhui
Medical University (grant no. 2015xkj018) and the National Natural
Science Foundation of China (grant no. 81401293).
References
|
1.
|
Sung SH and Jang IS: Isolated central
nervous system relapse of acute lymphoblastic leukemia. Brain Tumor
Res Treat. 2:114–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Mahmoud HH, Rivera GK, Hancock ML, Krance
RA, Kun LE, Behm FG, Ribeiro RC, Sandlund JT, Crist WM and Pui CH:
Low leukocyte counts with blast cells in cerebrospinal fluid of
children with newly diagnosed acute lymphoblastic leukemia. N Engl
J Med. 329:314–319. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Pui CH, Sandlund JT, Pei D, Campana D,
Rivera GK, Ribeiro RC, Rubnitz JE, Razzouk BI, Howard SC, Hudson
MM, et al: Total Therapy Study XIIIB at St Jude Children's Research
Hospital: Improved outcome for children with acute lymphoblastic
leukemia: Result of Total Therapy Study XIIIB at St Jude Children's
Research Hospital. Blood. 104:2690–2696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lange B, Bostrom B, Cherlow JM, Sensel MG,
La MK, Rackoff W, Heerema NA, Wimmer RS, Trigg ME and Sather HN:
Children's Cancer Group: Double-delayed intensification improves
event free survival for children with intermediate-risk acute
lymphoblastic leukemia: A report from the Children's Cancer Group.
Blood. 99:825–833. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Del Principe MI, Maurillo L, Buccisano F,
Sconocchia G, Cefalo M, De Santis G, Di Veroli A, Ditto C, Nasso D,
Postorino M, et al: Central nervous system involvement in adult
acute lymphoblastic leukemia: Diagnostic tools, prophylaxis, and
therapy. Mediterr J Hematol Infect Dis. 6:e20140752014. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Blasberg RG, Patlak C and Fenstermacher
JD: Intrathecal chemotherapy: Brain tissue profiles after
ventriculocisternal perfusion. J Pharmacol Exp Ther. 195:73–83.
1975.PubMed/NCBI
|
|
7.
|
Zhou W and Li JM: Mechanism and early
evaluation of CNS infiltration in acute lymphocytic
leukemia-review. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 21:1361–1364.
2013.(In Chinese). PubMed/NCBI
|
|
8.
|
Pui CH and Howard SC: Current management
and challenges of malignant disease in the CNS in paediatric
leukaemia. Lancet Oncol. 9:257–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sherman PM, Belden CJ and Nelson DA:
Magnetic resonance imaging findings in a case of cytarabine-induced
myelopathy. Mil Med. 167:157–160. 2002.PubMed/NCBI
|
|
10.
|
Gagliano RG and Costanzi JJ: Paraplegia
following intrathecal methotrexate: Report of a case and review of
the literature. Cancer. 37:1663–1668. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Dunton SF, Nitschke R, Spruce WE,
Bodensteiner J and Krous HF: Progressive ascending paralysis
following administration of intrathecal and intravenous cytosine
arabinoside. A pediatric oncology group study. Cancer.
57:1083–1088. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Werner RA: Paraplegia and quadriplegia
after intrathecal chemotherapy. Arch Phys Med Rehabil.
69:1054–1056. 1988.PubMed/NCBI
|
|
13.
|
Bleyer WA and Dedrick RL: Clinical
pharmacology of intrathecal methotrexate. I. Pharmacokinetics in
nontoxic patients after lumbar injection. Cancer Treat Rep.
61:703–708. 1977.PubMed/NCBI
|
|
14.
|
Miller KT and Wilkinson DS:
Pharmacokinetics of methotrexate in the cerebrospinal fluid after
intracerebroventricular administration in patients with meningeal
carcinomatosis and altered cerebrospinal fluid flow dynamics. Ther
Drug Monit. 11:231–237. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Teh HS, Fadilah SAW and Leong CF:
Transverse myelopathy following intrathecal administration of
chemotherapy. Singapore Med J. 48:e46–e49. 2007.PubMed/NCBI
|