Introduction
In tumors, the formation of vascular channels, cords
and sinuses that lack an endothelial cell (EC) lining is observed
in addition to the classical angiogenesis. These structures are
formed by tumor cells and possess a PAS-positive basement membrane
(1). Once plated in vitro on
appropriate matrices, these tumor-derived cells adopt certain
EC-like properties and develop highly patterned capillary-like
structures (CLSs). This vasculogenic mimicry (VM) may be considered
as another mechanism by which tumor cells can obtain nutrients and
oxygen to survive, particularly in less vascularized tumor areas.
In accordance with this, VM has been suggested to be regulated by
hypoxia (2). The occurrence of VM is
relatively rare within tumors, but the presence of VM networks in
these tumors correlates with the increased risk of metastasis and,
therefore, a poor outcome (3).
The development and progression of tumors is the
result of evolving crosstalk between a range of cell types within
the tumor and the supporting tissue or tumor stroma. The expansion,
invasion, metastasis and angiogenesis of the tumor is hypothesized
to be modulated by mutual interactions between tumor and stromal
cells through direct contact or via paracrine action. The concept
of an instructive role for the bone marrow mesenchymal stromal
cells (MSCs) in regulating tumor cell fate was introduced at least
30 years ago and has been validated over the past decade (4). The secretion of chemokines/cytokines
from the tumor, including stromal cell-derived factor 1
(SDF-1)/chemokine (C-X-C motif) ligand 12, hepatocyte growth
factor, vascular endothelial growth factor (VEGF), tumor growth
factor, basic fibroblast growth factor (bFGF), platelet-derived
growth factor and interleukin-8, is known to promote MSC migration
from the bone marrow to solid tumors (5). Carcinoma-associated MSCs (CA-MSCs) are
non-tumorigenic, and display a normal morphological appearance and
karyotype. CA-MSCs combined with tumor cells promote in vivo
tumor growth more effectively than control MSCs (6). Moreover, upon prolonged exposure to
tumor cell conditioned medium, MSCs activation occurs, followed by
differentiation into CAFs, which become members of the tumor
microenvironment (7). According to
the study by Annabi et al, MSCs play active angiogenic roles
through the regulation of novel vessel formation, stabilization and
maturation (8).
Previous studies by Nico et al (9) and Scavelli et al (10) demonstrated that macrophages and mast
cells contribute to the formation of neovessels in the bone marrow
in active multiple myeloma through VM, and this ability proceeds in
parallel to the progression of the plasma cell tumors. The
involvement of bone marrow stromal cells in the mimicry process in
acute leukemia has been shown by Mirshahi et al (11). We hypothesized that there may also be
crosstalk between the solid tumor cells and MSCs, leading to the
formation of neovessels by the MSCs.
The present study demonstrates that aggressive
melanoma cells educate MSCs to adopt certain EC-like properties and
develop highly patterned CLSs. This evidence provides a novel
perspective into the complex interplay between stromal and vascular
components in tumors.
Materials and methods
Materials
Matrigel basement membrane matrix, Growth Factor
Reduced (GFR) Matrigel, VEGF, bFGF and pro-epidermal growth factor
(EGF) were obtained from Becton Dickinson Labware (Bedford, MA,
USA). SDF-1α was purchased from R&D Systems, Inc. (Minneapolis,
MN, USA). Anti-VEGF neutralizing antibody (anti-human mouse
monoclonal; cat. no. аb1316) was obtained from Abcam (Cambridge,
MA, USA). Roswell Park Memorial Institute (RPMI) 1640 medium and
Collagenase Type 1 were obtained from Sigma-Aldrich (St. Louis, MO,
USA). Fetal bovine serum was purchased from HyClone Laboratories,
Inc. (Logan, UT, USA).
Cell culture
The four melanoma cell lines (Mel Cher, Mel Kor, Mel
P and Mel Me) were derived from the surgical specimens of patients
with disseminated melanoma, who were treated at the Blokhin Russian
Cancer Research Center (Moscow, Russia). The derivation and
characterization of these cell lines has been described previously
(12). All experiments were performed
with 70–75% confluent cells.
Human (h)MSCs from adipose tissue were kindly
provided by Dr E. Solov'yova (National Research Centre Kurchatov
Institute, Moscow, Russia), and cultured under hypoxic conditions
(5% CO2 and 5% O2). The identity of hMSCs was
confirmed by fluorescence-activated cell sorting analysis using
positive surface markers, namely, cluster of differentiation
(CD)13, CD44, CD90 and CD105, and negative markers, namely, CD34
and CD45, and by differentiation along adipogenic and osteogenic
lineages. The following antibodies (all from BioLegend, San Diego,
CA, USA) were used for hMSC surface phenotyping: Anti-human mouse
monoclonal CD13, PE-conjugated (clone WM15; cat. no. 301703);
anti-human/mouse CD44 rat monoclonal, FITC-conjugated (clone IM7;
cat. no. 103005); anti-human CD90 mouse monoclonal, FITC-conjugated
(clone5E10; cat. no. 328107); anti-human CD105 mouse monoclonal,
FITC-conjugated (clone 43A3; cat. no. 323203); anti-human CD34
mouse monoclonal, FITC-conjugated (clone 4H11; cat. no. 316405);
and anti-human CD45 mouse monoclonal, FITC-conjugated (clone HI30;
cat. no. 304005). For labeling, hMSCs were resuspended at a
concentration of 2х106 cells/ml in 0.5% fetal bovine
serum and 2 mM EDTA in phosphate-buffered saline. The cells were
incubated with dye-antibody conjugates diluted according to the
manufacturer's protocols, for 40 min at 4°C, and washed twice prior
to the analysis. The surface phenotype of viable (propidium
iodide-negative) cells was analyzed using the Gallios flow
cytometer (Beckman Coulter, Indianapolis, IN, USA). MSCs from the
adipose tissues of healthy mice and mice with melanoma were
isolated by finely mincing the tissues with scissors and then
incubating them in collagenase solution (type IA; 2 mg/ml;
Sigma-Aldrich) for 30 min at 37°C. This was then filtered through a
40-µm cell strainer (BD Biosciences, Franklin Lakes, NJ, USA), and
washed twice by centrifugation at 400 × g for 10 min. The final
cell suspension was cultured under hypoxic conditions.
Non-contact co-culture system
To set up a non-contact co-culture system, a
Transwell® system (Corning Inc., Corning, NY, USA) was
used. A total of 2×104 live MSCs were seeded on the
surface of each tissue culture well in a 24-well plate and allowed
to adhere for 4–5 h. Melanoma cells, Mel Cher, Mel Kor, Mel P or
Mel Me, were seeded onto a filter (4-µm pore size), and then the
filters were inserted into the wells and co-incubated for 4–5 days
at 37°C in a humidified atmosphere containing 5% CO2.
The two compartments shared the same culture medium through a 4-µm
Transwell membrane that prevented cell migration. MSCs co-cultured
without melanoma cells were used as the control. Following the
treatment, the ability of the MSCs to engage in CLS formation on
Matrigel was tested.
CLS formation in three-dimensional
culture
Matrigel (8.7 mg/ml) was thawed at 4°C, and 100 µl
was added to each well of a 24-well plate and allowed to solidify
for 1 h at room temperature. Incubation was then performed for 30
min at 37°C in a humidified 5% CO2 incubator. Following
co-culture with aggressive or poorly aggressive melanoma cells,
2×105 MSCs were seeded in complete RPMI 1640 medium onto
the gel, and incubated at 37°C for 5–6 h. CLS formation was then
analyzed. The effect of a neutralizing anti-VEGF antibody (5 µg/ml)
on educated MSCs was also tested. The antibody was added to the
cell suspension just prior to plating on Matrigel. After 18 h of
incubation, images of the wells were captured using an inverted
light microscope (Nikon Eclipse Ti-S; Nikon Corporation, Tokyo,
Japan) at ×20 magnification. The effect of the cytokines on CLS
formation was studied on GFR Matrigel. The cytokines (all at a
10-µM final concentration) were added just prior to plating the
cells on GFR Matrigel, and CLS formation analysis was performed as
aforementioned.
Tumor implantation
Female C57BL/6 mice (n=12; 43 days old) were housed
for a week in a room maintained at 22±1°C with 60% relative
humidity, and were provided with free access to a non-purified
diet. The animal experimental protocols were approved by the
Committee for Ethics of Animal Experimentation of the Blokhin
Russian Cancer Research Center, and the experiments were conducted
in accordance with the Guidelines for Animal Experiments in Blokhin
Russian Cancer Research Center. Solid-type melanoma tumors were
prepared by subcutaneous transplantation of 3×106
B16/F10 melanoma cells (0.3 ml) into the backs of the female mice
on day 0. On day 15, the mice were sacrificed by cervical
dislocation, and MSCs from the adipose tissues of healthy mice and
mice with melanomas were isolated. The ability of these cells to
form CLSs on Matrigel was tested, as previously described.
Results
Highly aggressive melanoma cells
educate MSCs to form CLSs
Based on the analogy with the results of Mirshahi
et al demonstrating the generation of VM channels by MSCs in
acute leukemic bone marrow (11), we
hypothesized that there may be a similar crosstalk between the
aggressive melanoma cells and MSCs resulting in acquisition by MSCs
of features required for tumor VM. To confirm this, a Transwell
co-culture system was utilized in the present study. Normal human
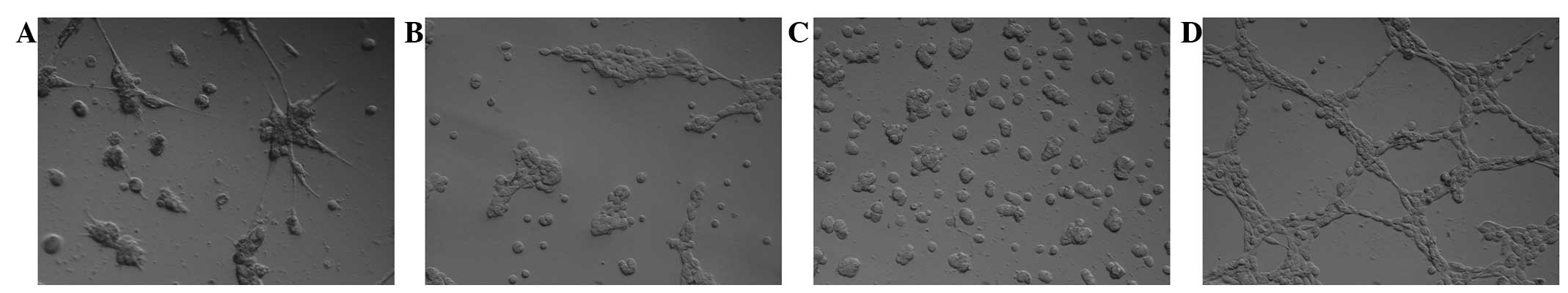
MSCs plated on Matrigel were unable to form CLSs (Fig. 1A). However, MSCs co-cultured with
highly aggressive Mel Cher melanoma cells for 5 days spontaneously
generated CLSs (Fig. 1B). The CLSs
formed by the MSCs remained stable for >3.5 days. The
penetration of CLSs into the matrix was observed (Fig. 1C). Significantly, MSCs failed to form
CLSs if grown with poorly aggressive Mel Me melanoma cells
(Fig. 1D).
Effect of angiogenic growth factors on
CLS formation by educated MSCs
To identify the factors that are responsible for CLS
formation by human MSCs, the effects of the addition of SDF-1α,
VEGFA, bFGF and EGF were tested. The contribution of all four
cytokines in angiogenesis has been repeatedly reported during the
last decade. On the basis of the observations showing SDF-1α to be
one of the most prominent candidates involved in the formation of
CLSs (13), the involvement of this
cytokine was expected in CLS formation in the present study.
However, in the present experiments, occasional clusters of cells
were observed, and some cells were abnormally elongated in response
to SDF-1α, but CLSs were not found in GFR Matrigel (Fig. 2A). bFGF and EGF were also inactive in
inducing the formation of CLSs by MSCs in this system (Fig. 2B and C). In contrast to the
aforementioned factors, robust CLS formation in GFR Matrigel was
induced by VEGFA (Fig. 2D).
Education of MSCs by metastatic
melanoma B16/F10 in vivo
Pilot in vitro experiments showed that
B16/F10 mouse melanoma cells can educate mouse MSCs towards a VM.
To further test the hypothesis that aggressive melanoma educates
MSCs towards a VM, in vivo experiments using a standard
B16/F10 mouse melanoma test system were performed. MSCs isolated
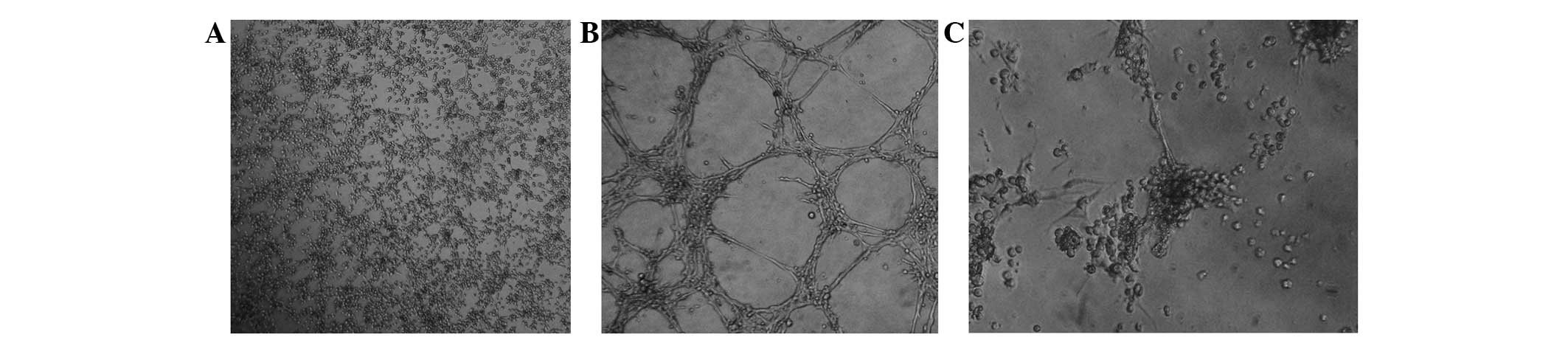
from the adipose tissues of healthy C57BL/6 mice were unable to
form CLSs on Matrigel (Fig. 3A). By
contrast, MSCs isolated from the adipose tissues of mice with
melanoma formed CLSs under similar conditions (Fig. 3B). It is worth noting that the
educated MSCs retained their ability to form CLSs after an
additional two passages.
Next, the study attempted to verify whether VEGFA
was the primary factor triggering CLS formation by educated MSCs.
When MSCs isolated from the adipose tissues of mice with melanoma
were treated with VEGF neutralizing antibody and then seeded on
Matrigel, no early signs of CLS formation were observed (Fig. 3C).
Discussion
In our previous study, it was shown that
disseminated melanoma adopted a vascular-like phenotype and engaged
in VM (14), as similarly found in
previous observations in uveal melanoma. In other previous studies,
we also identified certain molecular determinants that drive the
expression of a latent ‘vasculogenic program’ that signals CLS
formation in melanoma cells, which is a hallmark of tumor VM
(15,16). The present study demonstrated that i)
highly aggressive melanoma cells have the potential to educate MSCs
to adopt certain EC-like properties and develop highly patterned
CLSs; ii) robust CLS formation is induced by VEGFA; and iii) MSCs
isolated from the adipose tissues of mice with melanoma form
CLSs.
EC proliferation causes new blood vessels to arise
from the preexisting vasculature. However, the recruitment of bone
marrow-derived endothelial progenitor cells also mediates tumor
vascularization. Upon histomorphological analysis, certain tumors
were shown to be vascularized by pre-existing vessels, without
significant angiogenesis; this process is termed ‘vascular
co-option’. Tumors can also be vascularized via the formation of
vascular channels with the tumor's own cells in a non-EC process
termed ‘vasculogenic mimicry’. It has been shown that mast cells or
macrophages participate in vessel wall formation in biopsy
specimens of patients with active multiple myeloma. The two cell
populations retain their lineage markers and can be regarded as
cells that do not transdifferentiate into ECs. Similar to
macrophages and mast cells in active multiple myeloma, in an
ovarian tumor, Conejo-Garsia et al found a population of
CD45-positive/vascular endothelial cadherin-positive cells, the
so-called ‘vascular leukocytes’, which displayed the morphological
and functional properties of endothelial-like cells, with the
ability to contribute functionally to vasculogenesis in vivo
(17). In the present study, it was
shown that highly aggressive melanoma Mel Cher cells may have the
capacity to educate MSCs to form CLSs through the regulation of
their angiogenic properties (Fig. 1A and
B). Our previous study showed that CLSs formed by melanoma
cells on Matrigel were stable 20–24 h after seeding the cells onto
matrix; thereafter the cell junctions characteristic of the CLSs
were lost and the spontaneous release of focal contacts occurred
(14). In the present study, the CLSs
formed by MSCs remained stable for >3.5 days. Moreover, the
penetration of CLSs into the matrix could be observed (Fig. 1C). The results found with two other
aggressive melanoma cell lines, Mel Kor and Mel P, extended the
observation that melanoma cells educate MSCs towards VM (data not
shown). In addition, the MSCs failed to form CLSs when grown with
poorly aggressive melanoma Mel Me cells (Fig. 1D), suggesting that poorly aggressive
melanoma cells lack the capacity to account for CLS formation by
MSCs.
It is now established that tumor cells secrete
chemoattractant factors that facilitate MSC homing to the tumors
(18). However, MSCs secrete factors
that stimulate tumor cell proliferation, suggesting the existence
of contact-independent interactions between MSCs and cancer cells
in vivo (19). In addition, in
a non-contact co-culture system, EC proliferation and migration is
increased by MSCs, thus promoting early angiogenic events. One of
the most prominent candidates to be involved in the formation of
CLSs by MSCs is SDF-1α. This chemokine induces an enhanced
connection between embryonic stem cells, thereby increasing the
ability of the cells to assemble in a reticular network (13). SDF-1α also enhances vasculogenesis by
recruiting vascular progenitors to the neovasculature (20). However, in the present experiments,
VEGFA was sufficient to induce CLSs on the GFR Matrigel by educated
MSCs, whereas bFGF, EGF and SDF-1α were inactive. VEGFA involvement
in the mimicry process was confirmed by adding a neutralizing
antibody against VEGF. Anti-VEGF antibody abrogated CLS formation.
This adds to the evidence that VEGF may control MSC vasculogenesis.
Our previous study (15) and studies
by other groups (21) have shown that
tumor VM is under the control of the VEGF/VEGFR1 signaling pathway,
while the VEGF/VEGFR2 signaling pathway plays a crucial role in the
survival, proliferation and differentiation of ECs and their
progenitors. MSCs have been shown to express high level of VEGFR1
and a lower level of VEGFR2 on the cell surface. Moreover, hypoxia
has been shown to increase the expression of VEGFR1 (22). These data may thus explain the
dependence of CLS formation by MSCs on VEGFA.
To confirm the hypothesis of a novel qualitative
signature of MSCs to support tumor vascularization through
crosstalk with aggressive tumors, in vivo animal testing was
also performed in the present study. A pilot experiment using a
standard B16/F10 mouse melanoma test system showing the same
CLS-forming behavior as the human cells lines in the study was
performed. The data show for the first time that aggressive
melanoma educates MSCs towards VM.
Tumor vascularization is known to be critical for
tumor growth, since sufficient nutrients and oxygen must be
supplied to cancer cells to ensure their survival and
proliferation. VM has already been described in a number of tumors,
and it has also been shown that VM contributes to the delivery of
blood to tumors due to connection of vascular channels with regular
blood vessels. The present data indicated that MSCs may acquire the
capacity to participate in melanoma vascularization through
regulation of their angiogenic properties. The present study did
not investigate whether vascular channels formed by MSCs could
contribute to the blood supply of tumors in vivo. However,
the evidence presented highlights the importance of the stromal
microenvironment during vascularization of melanoma and may provide
clues for the development of effective strategies for MSC-based
cancer therapies.
Acknowledgements
This study was supported by a grant (no.
14-35-00107) from the Russian Science Foundation. The authors would
like to thank Dr E. Solov'yova (National Research Centre Kurchatov
Institute) who kindly provided the hMSCs used in this study, and Dr
M. Krasil'nikov (Department of Cancerogenesis, Blokhin Russian
Cancer Research Center) for valuable comments and advice. The
authors are also grateful to Mr. A. Sherbakov (Department of
Experimental Diagnosis and Biotherapy of Tumors, Blokhin Russian
Cancer Research Center) for administering technical assistance.
Glossary
Abbreviations
Abbreviations:
|
VEGFR
|
vascular endothelial growth factor
receptor
|
|
VM
|
vasculogenic mimicry
|
|
EC
|
endothelial cells
|
|
CLS
|
capillary-like structure
|
|
MSC
|
mesenchymal stromal cells
|
|
bFGF
|
basic fibroblast growth factor
|
|
SDF-1α
|
stromal cell-derived factor 1α
|
References
|
1
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun B, Zhang D, Zhang S, Zhang W, Guo H
and Zhao X: Hypoxia induces vasculogenic mimicry channel formation
and tumor invasion-related proteins expression in melanoma. Cancer
Lett. 249:188–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paulis YW, Soetekouw PM, Verheul HM,
Tjan-Heijnen VC and Griffioen AW: Signaling pathways in
vasculogenic mimicry. Biochem Biophys Acta. 1806:18–28.
2010.PubMed/NCBI
|
|
4
|
Mishra PJ, Mishra PJ, Glod JW and Banerjee
D: Mesenchymal stem cells: Flip side of the coin. Cancer Res.
69:1255–1258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Studeny M, Marini FC, Dembinski JL,
Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE and Andreeff
M: Mesenchymal stem cells: Potential precursors for tumor stroma
and targeted-delivery vehicles for anticancer agents. J Natl Cancer
Inst. 96:1593–1603. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McLean K, Gong Y, Choi Y, Deng N, Yang K,
Bai S, Cabrera L, Keller E, McCauley L, Cho KR and Buckanovich RJ:
Human ovarian carcinoma-associated mesenchymal stem cells regulate
cancer stem cells and tumorigenesis via altered BMP production. J
Clin Invest. 121:3206–3219. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barcellos-de-Souza P, Gori V, Bambi F and
Chiarugi P: Tumor microenvironment: Bone marrow-mesenchymal stem
cells as key players. Biochim Biophys Acta. 1836:321–335.
2013.PubMed/NCBI
|
|
8
|
Annabi B, Lee YT, Turcotte S, Naud E,
Desrosiers RR, Champagne M, Eliopoulos N, Galipeau J and Béliveau
R: Hypoxia promotes murine bone-marrow-derived stromal cell
migration and tube formation. Stem Cells. 21:337–347. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nico B, Mangieri D, Crivellato E, Vacca A
and Ribatti D: Mast cells contribute to vasculogenic mimicry in
multiple myeloma. Stem Cell Dev. 17:19–22. 2008. View Article : Google Scholar
|
|
10
|
Scavelli C, Nico B, Cirulli T, Ria R, Di
Pietro G, Mangieri D, Bacigalupo A, Mangialardi G, Coluccia AM,
Caravita T, et al: Vasculogenic mimicry by bone marrow macrophages
in patients with multiple myeloma. Oncogene. 27:663–674. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mirshahi P, Rafii A, Vincent L, Berthaut
A, Varin R, Kalantar G, Marzac C, Calandini OA, Marie JP, Soria C,
et al: Vasculogenic mimicry of acute leukemic bone marrow stromal
cells. Leukemia. 23:1039–1048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mikhailova IN, Lukashina MI, Baryshnikov
AY, Morozova LF, Burova OS, Palkina TN, Kozlov AM, Golubeva VA,
Cheremushkin EA, Doroshenko MB, et al: Melanoma cell lines as the
basis for antitumor vaccine preparation. Vestn Ross Akad Med Nauk.
37–40. 2005.(In Russian). PubMed/NCBI
|
|
13
|
Chen T, Bai H, Shao Y, Arzigian M, Janzen
V, Attar E, Xie Y, Scadden DT and Wang ZZ: Stromal cell-derived
factor-1/CXCR4 signaling modifies the capillary-like organization
of human embryonic stem cell-derived endothelium in vitro. Stem
Cells. 25:392–401. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vartanian A, Burova O, Stepanova E,
Baryshnikov A and Lichinitser MR: The involvement of apoptosis in
melanoma vasculogenic mimicry. Melanoma Res. 17:1–8. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vartanian A, Stepanova E, Grigorieva I,
Solomko E, Baryshnikov A and Lichinitser M: VEGFR1 and PKCα
signaling control melanoma vasculogenic mimicry in a VEGFR2
kinase-independent manner. Melanoma Res. 21:91–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vartanian A, Gatsina G, Grigorieva I,
Solomko E, Dombrovsky V, Baryshnikov A and Stepanova E: The
involvement of Notch signaling in melanoma vasculogenic mimicry.
Clin Exp Med. 13:201–219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Conejo-Garcia JR, Buckanovich RJ, Benencia
F, Courreges MC, Rubin SC, Carroll RG and Coucos G: Vascular
leukocytes contribute to tumor vascularization. Blood. 105:679–681.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reagan MR and Kaplan DL: Concise review:
Mesenchymal stem cell tumor-homing: Detection methods in disease
model systems. Stem Cells. 29:920–927. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou L, Wang X, Zhou Y, Ma H, Wang Z, He J,
Hu H, Guan W and Ma Y: Inhibitory effect and mechanism of
mesenchymal stem cells on liver cancer cells. Tumour Biol.
35:1239–1250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aghi M, Cohen KS, Klein RJ, Scadden DT and
Chiocca EA: Tumor stromal-derived factor-1 recruits vascular
progenitors to mitotic neovasculature, where microenvironment
influences their differentiated phenotypes. Cancer Res.
66:9054–9064. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang JY, Sun T, Zhao XL, Zhang SW, Zhang
DF, Gu Q, Wang XH, Zhao N, Qie S and Sun BC: Functional
significance of VEGF-a in human ovarian carcinoma: Role in
vasculogenic mimicry. Cancer Biol Ther. 7:758–766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okuyama H, Krishnamachary B, Zhou YF,
Nagasawa H, Bosch-Marce M and Semenza GL: Expression of vascular
endothelial growth factor receptor 1 in bone marrow-derived
mesenchymal cells is dependent on hypoxia-inducible factor 1. J
Biol Chem. 281:15554–15563. 2006. View Article : Google Scholar : PubMed/NCBI
|