Introduction
T-cell acute lymphoblastic leukemia (T-ALL)
comprises aggressive hematological tumors that derive from the
malignant transformation of T-cell progenitors. T-ALL accounts for
10–15% of pediatric and 25% of adult acute lymphoblastic leukemia
(ALL) cases (1). With the
introduction of improved treatment regimens, including risk-adapted
chemotherapy, hematopoietic stem cell transplantation (HSCT) and
supportive care, the prognosis of T-ALL has gradually improved and
cure rates have reached over 85% in children and ~50% in adults
(2,3).
However, the outcome of T-ALL patients with relapsed and refractory
leukemia remains poor (1–3). Various novel therapeutic strategies have
been recently studied, including tyrosine kinase inhibitors (TKIs)
for the treatment of breakpoint cluster region-Abelson
(BCR-ABL)-positive T-ALL and γ-secretase inhibitors (GSIs) for the
treatment of T-ALLs with aberrant Notch 1 activation (4). Arsenic compounds have also been
previously used to treat T-ALL cell lines and patients (5–8). Several
studies have demonstrated that a safe and effective serum
concentration of arsenic trioxide (ATO; 2–6 µmol/l) for treating
acute promyelocytic leukemia (APL) patients could induce apoptosis
in T-cell leukemia cell lines, notably in the Molt-4 cell line, or
in leukemia cells from glucocorticoid-resistant ALL patients,
independently or in combination with other agents, such as
glucocorticoids (6–9). The combination of ATO and other common
chemotherapy drugs provides a therapeutic target that may
potentially be used to induce the remission of relapsed or
refractory T-ALL patients.
During normal T-cell development, the earliest
established T-cell lineage, immature cluster of differentiation
(CD)34+ cells, enter the thymus and subsequently
differentiate into mature T-cells, gaining a functional T-cell
receptor (TCR) that belongs to the αβ or γδ lineage (10). T-cells implicated in T-ALL are
characterized by the clonal expansion of malignant T-cells arrested
at an early stage during T-cell differentiation (1). Clonally expanded malignant T-cells
(leukemic clones) vary in certain patients due to TCR gene
rearrangement diversity (11,12). The combination of reverse
transcription (RT)-polymerase chain reaction (PCR) and the GeneScan
technique, also referred to as ‘immunoscope’, has been widely used
to analyze TCR repertoires and dynamically monitor clonal changes
in T-cells in patients with leukemia and other diseases, including
autoimmune diseases and certain types of viral infections (13,14). Using
this method, the immune status of patients could be characterized
and the evolution of malignant T-cell clones identified, which may
aid in monitoring minimal residual disease (MRD) and designing
specific therapeutic strategies for T-ALL (12,15). The
present study reports a rare case of T-ALL, whereby the patient
responded poorly to standard chemotherapy but achieved complete
remission (CR) following treatment with protocols involving
arsenic. In order to evaluate the effects of the treatment and
monitor the reconstitution of the immune system following treatment
with arsenic-combined regimens, the TCRβ, γ and δ repertoires of
the T-ALL patient were monitored at 7 time points, between the time
of diagnosis and when CR was achieved, over 4 months.
Case report
A 28-year-old male patient presented to Department
of Hematology, Guangdong General Hospital (Guangzhou, China) due to
dizziness in January 2013. The physical examination disclosed
lymphadenopathy, splenomegaly and hepatomegaly, and no skin lesions
were present. A complete blood count revealed a white blood cell
count of 12×1010/l (normal range,
4–10×109/l). Cytogenetic analysis showed a normal male
karyotype 46XY with 5 aneuploids. In the peripheral blood (PB) and
bone marrow (BM) aspirate smears, a high percentage of blasts (70
and 84%, respectively) were detected (normal, <0.01% in PB and
<2% in BM). For immunophenotyping analysis and MRD monitoring,
the following monoclonal antibodies were used: CD45-Percp,
CD71-FITC, CD7-FITC, CD2-PE, CD5-APC, CD10-PE, CD34-FITC,
CytoCD3-FITC, TdT-PE, CD33-PE, CD13-PE, CD56-PE, HLA-DR-PE, CD9-PE,
CD4-FITC, CD8-PE (BD Biosciences, San Jose, CA, USA). The
extracellular and intracellular staining were performed according
to the manufacturer's instructions. A total of 30,000 cells were
analyzed on a BD FACSCanto™ II flow cytometer (BD Biosciences) and
data analysis was performed with CellQuest software (BD
Biosciences). Flow cytometry (FCM) revealed that lymphoblasts
accounted for 98.3% of the 30,000 BM cells counted, the majority of
which were positive for CD71, CD7, CD2, CD5, CD10, CD34, cytoCD3
and terminal deoxynucleotidyl transferase, and a number of which
were positive for CD33, CD13, CD56, human leukocyte antigen-antigen
D related, CD9, CD38 and sCD3, while CD4 and CD8 were not expressed
(Fig. 1A; Table I). Fluorescence in situ
hybridization (FISH) analysis showed no evidence for BCR-ABL,
mixed-lineage leukemia gene (MLL) or fms related tyrosine kinase 3
(FLT3) gene rearrangements or deletions. Other laboratory tests
included a basic metabolic panel, liver test and coagulopathy
panel, and renal function, which were all unremarkable. The present
case was diagnosed with T-ALL based on cytomorphology,
immunohistochemistry and cytogenetic and molecular analysis.
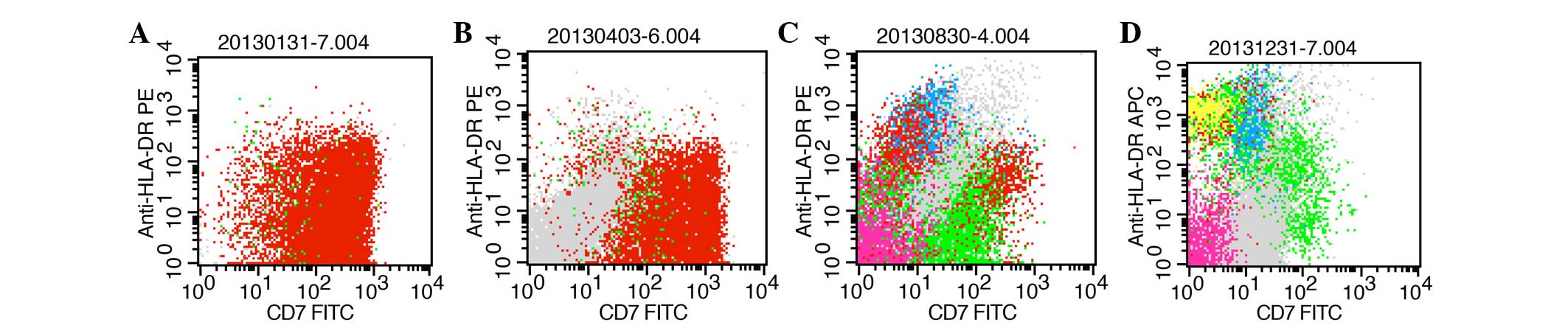 | Figure 1.FCM for CD7 and HLA-DR expression
analysis of the bone marrow at various disease states. Red scatter
points represent the lymphoblast in bone marrow. Pink, green, blue,
yellow and gray dots represent nucleated erythrocytes, mature
lymphocytes, monocytes, pre-B lymphocytes and mature granulocytes,
respectively, which gated in 2D spot figure (CD45-Percp and Side
Scatter-Height). (A) Time of new diagnosis with T-cell acute
lymphoblastic leukemia. FCM detected 98.3% blast cells, and the
majority of the blast cells were positive for CD7 and HLA-DR. (B)
Time of achieving minor remission following treatment with the
vincristine, daunorubicin, L-asparaginase, cyclophosphamide and
prednisone regimen and the cyclophosphamide, vincristine,
doxorubicin and dexamethasone regimen. FCM detected 73.3% blast
cells. (C) Time of achieving CR following treatment with ATO. FCM
detected 2.8% MRD and few cells were positive for CD7 and HLA-DR.
(D) Time of CR that was maintained for 4 months in which no
significant MRD could be detected by FCM. Scarce cells positive for
CD7 and HLA-DR were found. FCM, flow cytometry; CD7, cluster of
differentiation 7; HLA-DR, human leukocyte antigen-antigen D
related; CR, complete remission; MRD, minimal residual disease;
FITC, fluorescein isothiocyanate. |
 | Table I.Sample and clinical therapy details
for the present patient with refractory T-cell acute lymphoblastic
leukemia. |
Table I.
Sample and clinical therapy details
for the present patient with refractory T-cell acute lymphoblastic
leukemia.
| Sample
collection | Date of
collection | Date of
therapy | Type of
therapy | Smear analysis
blast cells in BM/PB, % | FCM analysis blast
cells in BM, % | Disease status at
sample acquisition |
|---|
| A | 31.01.2013 |
|
| 84/70 | 98.3 | ND |
| B | 20.02.2013 | 04.02 –
28.02.2013 | VDLCP | 79/66 | 43.3 | MR |
| C | 03.04.2013 | 04.04 –
17.04.2013 | HyperCVAD-A | 62/5 | 73.3 | MR |
| D | 27.05.2013 | 29.05 –
22.06.2013 | CAT | 92/40 |
| MR |
|
|
| 15.07 –
06.08.2013 | ATO |
|
| MR |
| E | 29.08.2013 | 29.08 –
26.10.2013 | ATO | 4/0 | 2.8 | CR+MRD |
| F | 31.10.2013 | 31.10 –
05.11.2013 | ATO+CT | 2/0 | 0.0 | CR |
| G | 30.12.2013 | 03.01 –
24.01.2014 | ATO+MP | 2/0 | 0.0 | CR |
|
|
| 03.28 –
01.04.2014 | ATO+CT | 2/0 | 0.0 | CR |
|
|
| 16.07 –
18.07.2014 |
ATO+HyperCVAD-B | 1/0 | 0.0 | CR |
|
|
| 18.07.2014 –
present | ATO |
|
| CR |
The patient was admitted in January 2013 and started
on one course of vineristine, 2 mg, once a day (days 1, 8, 15 and
22); daunorubicin, 68 mg, once a day (days 1, 2, 3, 15 and 16);
cyclophosphamide, 1,200 mg, once a day (days 1 and 15);
L-asparaginase, 10,000 u (days 11, 14, 17, 20, 23 and 26);
dexamethasone, 10 mg, once a day (days 1–14) and 5 mg, once a day
(days 15–28) (VDLCP) regimen, followed by cyclophosphamide, 500 mg,
every 12 h (days 1, 2 and 3); daunorubicin, 85 mg, once a day (day
4); vineristine, 2 mg, once a day (days 4 and 11); prednisone, 40
mg, once a day (days 1–4 and 11–14) (HyperCVAD-A) protocol, which
is a more intensive chemotherapy regimen (16,17).
However, the patient had poor response to the initial chemotherapy
consisting of one course of VDLCP and one course of HyperCAVD-A
(Fig. 1B) (13,14). One
course of cyclophosphamide, cytarabine and topotecan (CAT)
chemotherapy [cyclophosphamide, 400 mg, every 12 h (days 1–3);
cytarabine, 1,500 mg, once a day (days 2–6); topotecan, 2 mg, once
a day (days 2–6)] was then administered; however, the response
assessments unfortunately indicated minor remission. Considering
the refractory situation of the present patient, who failed to
respond to the first and second line chemotherapeutics, ATO (which
is widely used for treating APL and may be used as a saving
treatment refractory leukemia cases) was then administered as
salvage chemotherapy for the present patient. ATO (10 mg; 0.16
mg/kg/day) was administered by intravenous drip for 2–3 h once a
day, and treatment was continued for 3 weeks. On August 29, 2013,
the patient surprisingly achieved CR with MRD, with 2.8% of blast
T-cells identified in the BM by FCM (Fig.
1C; Table I). The patient was
continued on cyclophosphamide, 400 mg, every 12 h (days 1 and 2);
topotecan, 2 mg, every day (days 2–4), oral arsenic (10 mg, once a
day), methylprednisolone (MP; 100 mg, at night), and mitoxantrone
and cytarabine (HyperCVAD-B), alternated with consolidation
therapy, which enabled the patient to maintain CR without MRD
(Fig. 1D; Table I). Arsenic-related hematological
toxicity or extra-hematological toxicities were not observed during
the time of treatment. The treatment details and information
regarding the seven bone marrow samples collected at various time
points are summarized in Table I.
To monitor the T-cells in the BM of the present
patient during the burden of disease and evaluate the effects of
treatment, repertoire-specific PCR primers and the GeneScan
technique were used in combination, as previously described
(18–20). The TCR Vβ (Fig. 2), Vγ and Vδ (Fig. 3) repertoires were dynamically
characterized in BM samples collected at various times (Table I). The result showed only a subset of
the TCR Vβ family members (9–16) were detectable in each of the samples
collected at various time points (Fig.
2), which is in contrast with healthy individuals, who express
nearly all of the TCR Vβ repertoire subfamily members (18,21).
Although the expression of all of the TCR Vγ and Vδ family members
was not found in each sample (Fig.
3), the expression pattern did not appear to be significantly
different compared with the healthy individuals from a previous
study (19), particularly when the
patient achieved CR. Significantly, oligoclonally expanded T-cells
were detected in certain TCR Vβ (Vβ1, Vβ21 and Vβ24) and Vδ (Vδ3
and Vδ6) subfamilies, while the majority of TCR subfamily T-cells
displayed a polyclonal pattern. The evolution of T-cell clones was
characterized at various time points prior to and following
chemotherapy, particularly for the oligoclonally-expanded TCR
subset, to detect a factor associated with outcome and to identify
a malignant T-ALL clone. Oligoclonally-expanded TCR Vβ1 T-cells of
the same size [same complementarity determining region 3 (CDR3)
length; PCR products, 190 base pairs (bp)] were identified at the
time the patient was diagnosed with T-ALL (Fig. 4A), during VDLCP (Fig. 4B), and following HyperCAVD-A (Fig. 4D), displaying an oligoclonal trend at
the chemotherapy-free interval between VDLCP and HyperCAVD therapy
(Fig. 4C). After ATO therapy, prior
to achieving CR, FCM demonstrated 2.82% blast T-cells in the BM,
and the Vβ1 subfamily profile displayed an oligoclonal trend with
three peaks of products of various sizes, while the 190-bp T-cell
clone product remained visible (Fig.
4E). Moreover, the Vβ1 cell clone product of 190 bp appeared at
the time when the T-ALL case remained in CR without MRD. The Vβ1
clone profile distinctly showed that the major Vβ1 clone in the
sample at 2 months following CR was different compared with samples
prior to CR; the clone product was 184 bp in size, while the 190 bp
product remained present as a minor clone (Fig. 4F). Notably, the Vβ1 cell clone product
of 190 bp became the major clone again 4 months subsequent to CR
(Fig. 4G). At the time, MRD was not
detected by FCM; however, the relative fluorescence intensity of
Vβ1 was remarkably decreased compared with samples collected in the
stage without CR (data not shown). In addition to the Vβ1
subfamily, oligoclonality or an oligoclonal trend could also be
detected for the Vβ21 or Vβ24 subfamily, which had various product
sizes or clones for certain patient samples. Overall, based on the
evolution of the Vβ1 T-cell clones, the Vβ1 T-cell clone appearing
with the 190-bp product may be the malignant T-ALL clone, as the
numbers of this clone decreased following chemotherapy,
particularly ATO therapy.
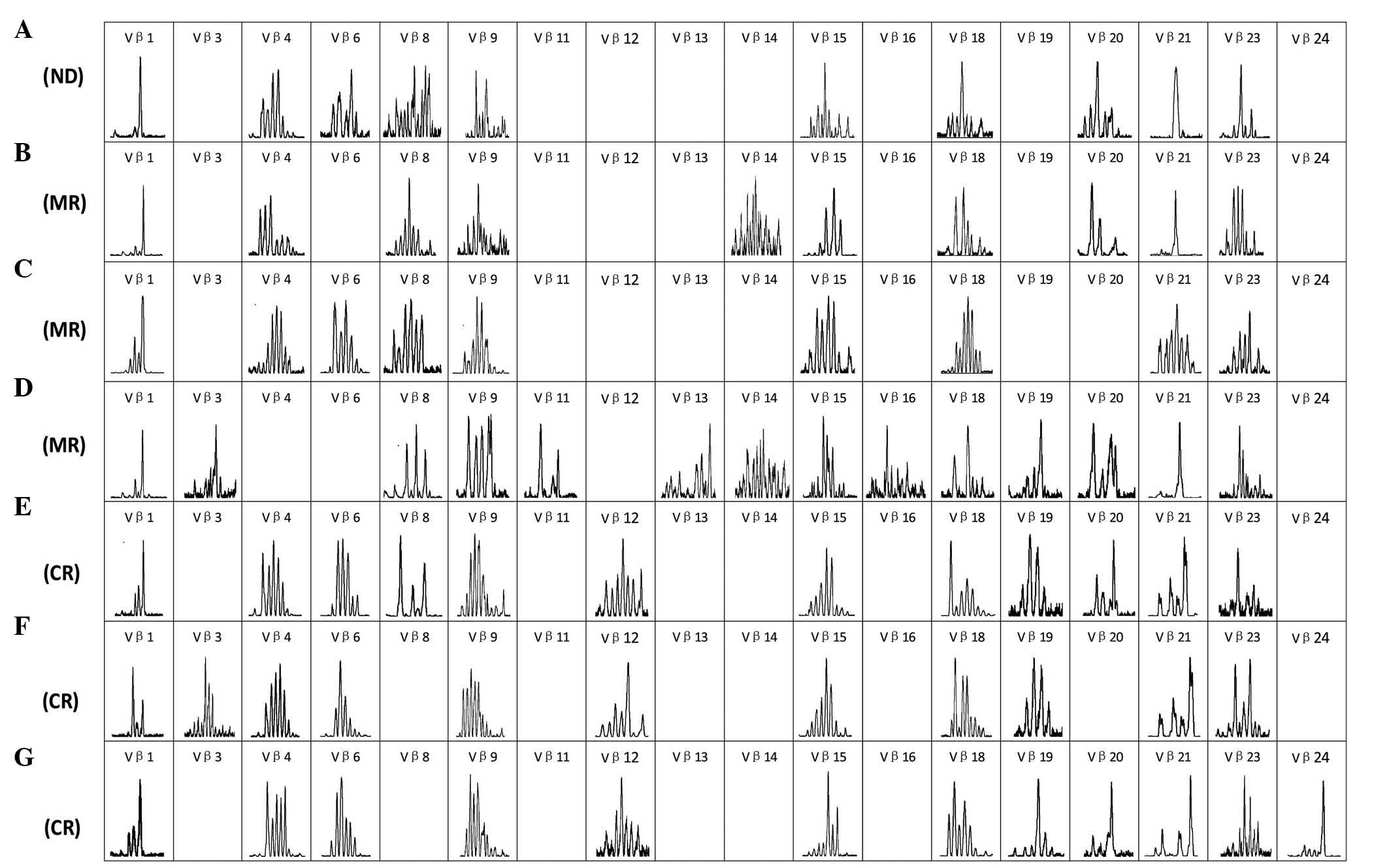 | Figure 2.Complementarity determining region 3
spectratyping of the T-cell receptor Vβ subfamily on T-cells in the
bone marrow at 7 time points. (A) Time of diagnosis with T-cell
acute lymphoblastic leukemia. (B) Time of achieving MR following
treatment with vincristine, daunorubicin, L-asparaginase,
cyclophosphamide and prednisone regimen. (C) Time of achieving MR
prior to treatment with the HyperCVAD-A regimen. (D) Time of
achieving MR following treatment with the HyperCVAD-A regimen. (E)
Time of achieving CR but with MRD following treatment with
cyclophosphamide, cytarabine and topotecan and ATO. (F) Time of
achieving CR with no MRD detected following treatment with a second
course of ATO. (G) Time of CR that was maintained for 4 months in
which no significant MRD could be detected by flow cytometry. ND,
newly diagnosed; MR, minor remission; CR, complete remission; MRD,
minimal residual disease; HyperCVAD-A, cyclophosphamide,
vincristine, doxorubicin and dexamethasone; ATO, ATO, arsenic
trioxide. |
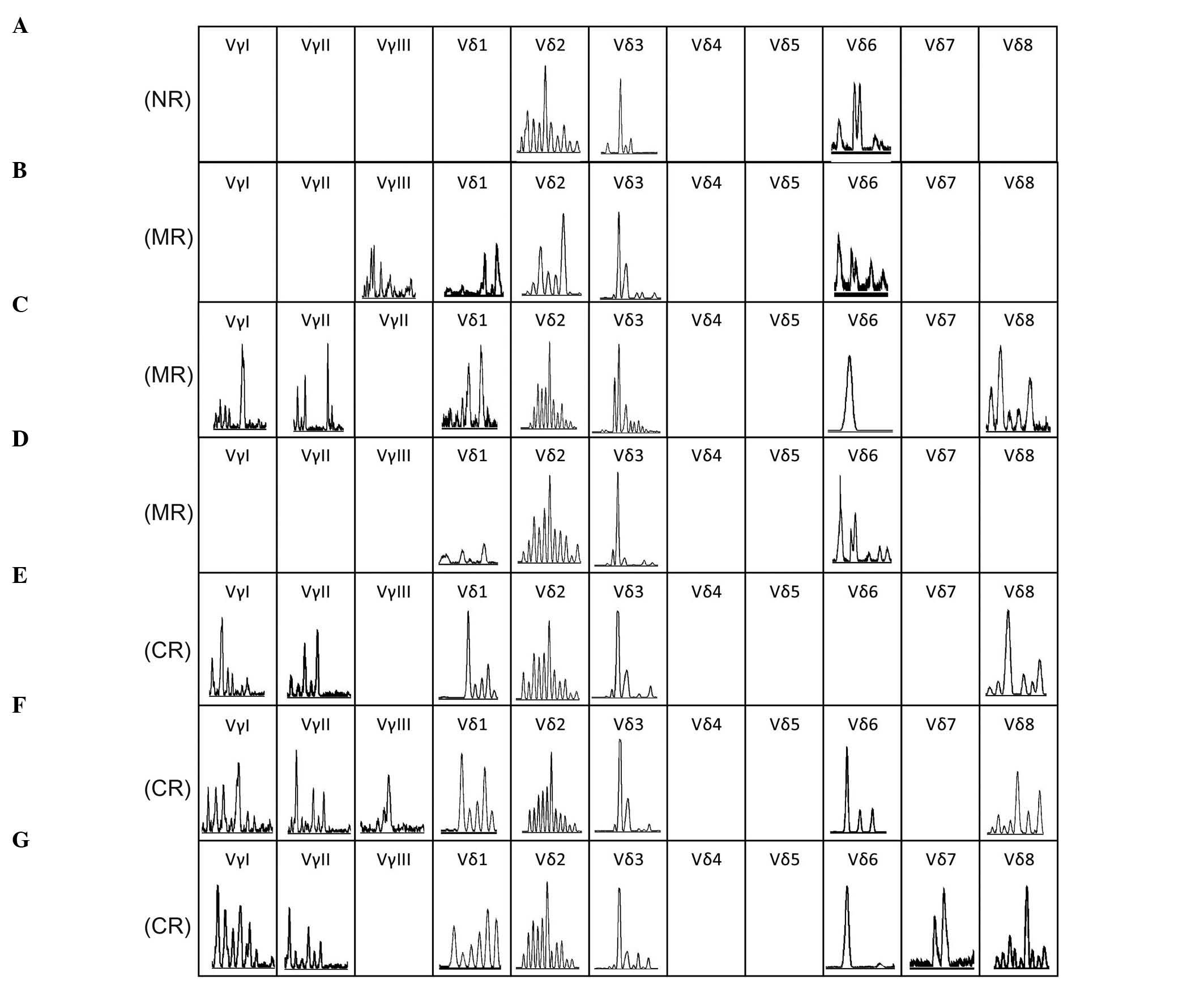 | Figure 3.Complementarity determining region 3
spectratyping of the T-cell receptor Vγ and Vδ subfamilies on
T-cells in the bone marrow at 7 time points. (A) Time of new
diagnosis with T-cell acute lymphoblastic leukemia. (B) Time of
achieving MR following treatment with vincristine, daunorubicin,
L-asparaginase, cyclophosphamide and prednisone. (C) Time of
achieving MR prior to treatment with the HyperCVAD-A regimen. (D)
Time of achieving MR upon treatment with the HyperCVAD-A regimen.
(E) Time of achieving CR, but with MRD subsequent to treatment with
cyclophosphamide, cytarabine and topotecan and ATO. (F) Time of
achieving CR without MRD detected following treatment with a second
course of ATO. (G) Time of CR that was maintained for 4 months in
which no significant MRD could be detected by flow cytometry. ND,
newly diagnosed; CR, complete remission; MR, minor remission; MRD,
minimal residual disease; HyperCVAD-A, cyclophosphamide,
vincristine, doxorubicin and dexamethasone; ATO, arsenic
trioxide. |
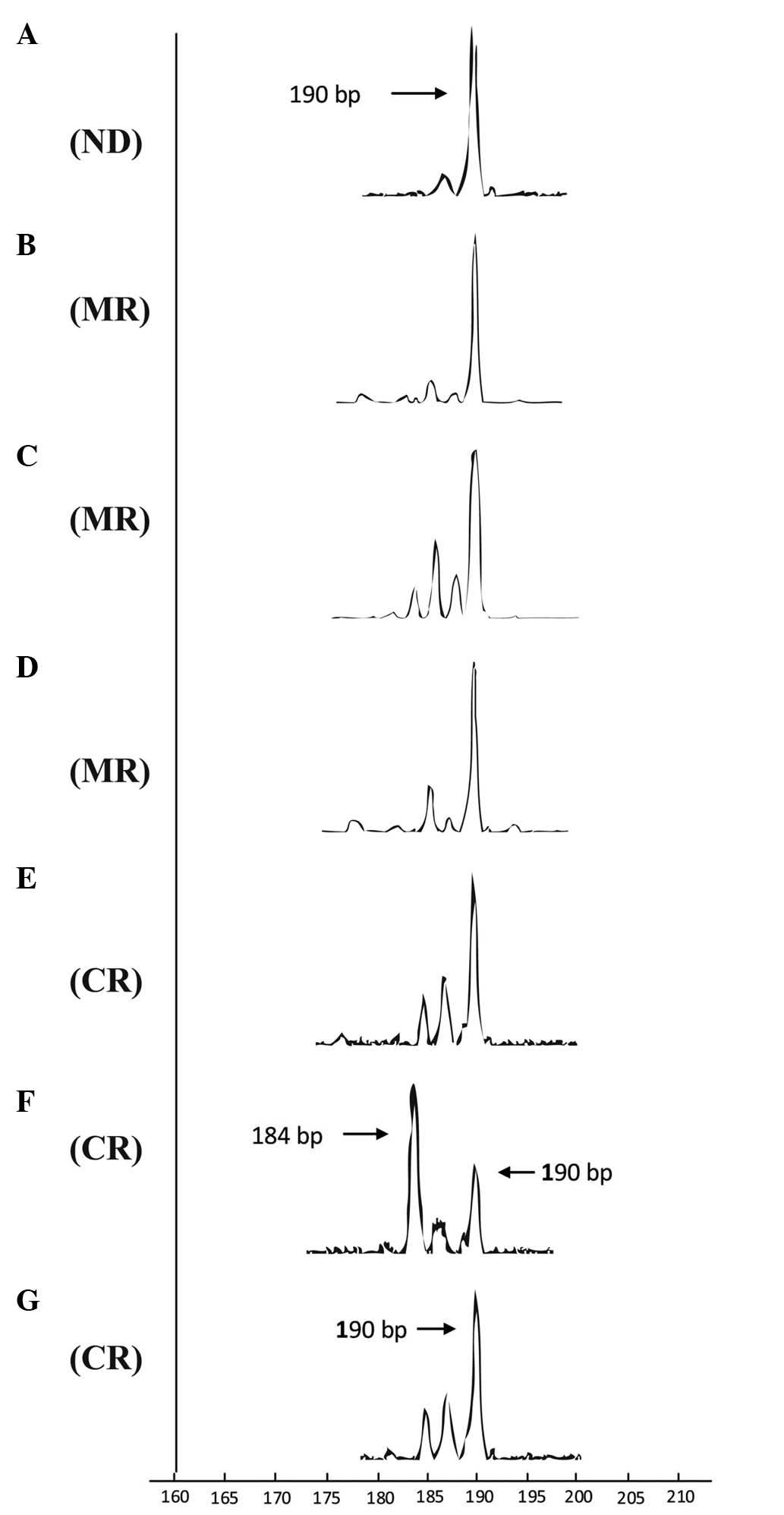 | Figure 4.Dynamic changes in complementarity
determining region 3 spectratyping of Vβ1 T-cells in samples at
various time points. The horizontal axis represent the product size
and the height of a single peak in each graph represents the
fluorescence intensity of product in corresponding size. (A) Time
of new diagnosis with T-cell acute lymphoblastic leukemia. (B) Time
of achieving MR following treatment with the vincristine,
daunorubicin, L-asparaginase, cyclophosphamide and prednisone
regimen. (C) Time of achieving MR prior to treatment with the
HyperCVAD-A regimen. (D) Time of achieving MR following treating
with the HyperCVAD-A regimen. (E) Time of achieving CR but with MRD
upon treatment with cyclophosphamide, cytarabine and topotecan and
ATO. (F) Time of achieving CR with no MRD detected following
treating with a second course of ATO. (G) Time of CR that was
maintained for 4 months in which no significant MRD could be
detected by flow cytometry. ND, newly diagnosed; MD, minor
remission; CR, complete remission; MRD, minimal residual disease;
HyperCVAD-A, cyclophosphamide, vincristine, doxorubicin and
dexamethasone; ATO, ATO, arsenic trioxide. |
Malignant T-ALL clones may express alternative TCRαβ
or γδ receptors (8); therefore, the
TCR Vγ and Vδ repertoires were also analyzed in all samples of the
present case. Since skewed Vγ and Vδ subfamily distribution is a
common characteristic of the leukemia patients (19), similar results were expected and
detected in the present study. In the present case, clonally
expanded Vδ3 T-cell clones of the same size as CDR3 were identified
in all samples at various time points (Fig. 3); however, no Vγ subfamily members
were detected in 3 of the samples (Fig.
3). This inconsistency may be due to a relatively low frequency
of γδ+ T-cells in the samples, which were not detected
using the present techniques. Furthermore, it appears unlikely that
the Vδ3 T-cell clone is the malignant T-ALL clone in this case, as
Vγ subfamily members that should pair with Vδ to form
γδ+ T-cell clones could not be detected at the time of
diagnosis (Fig. 3A), which also
supports the theory that malignant T-ALL clones should be
αβ+ T-cell clones expressing Vβ1.
Written informed consent was obtained from the
patient for publication of the present study and any accompanying
images.
Discussion
The present study may be the first documented case
of a patient with T-ALL that achieved CR by ATO induction
treatment, although the achieved CR may not hold true on a
molecular level (indicated by the results of the T-cell repertoire
analysis). Medicinal uses of arsenic have been documented for
>2,000 years. ATO has been previously shown to be dramatically
effective for treating patients with APL (22,23), and
is also effective in ~20% of patients with myelodysplastic syndrome
(MDS) (24,25). The mechanisms of ATO-induced apoptosis
include the degradation of promyelocytic leukemia (PML)-retinoic
acid receptor α (RARα), which may occur with or without the
interference of B cell lymphoma-2 (Bcl-2) family genes (8,26,27). Several other mechanisms of ATO
anti-leukemic effects have been identified, including the induction
of PML-RARα-independent apoptosis, cell cycle arrest, growth
inhibition, induction of stress related processes, direct
mitochondrial damage and the inhibition of nuclear factor-κB
(NF-κB) (8,26–31). Based
on the dramatic effects of ATO on APL and the partial effects on
MDS, several studies have tested the efficacy of ATO on other
malignant T-cell lines. ATO has been demonstrated to selectively
inhibit growth and induce apoptosis in several cell lines,
including the megakaryocytic leukemia cell lines HEL, Meg-01, UT7,
and M07e (32), the myeloid leukemia
cell lines U937 and KG-1, plasma cells and cell lines from myeloma
patients (33) and B cell leukemia
cell lines (34). Notably, ATO has
been demonstrated to be capable of inducing apoptosis in certain
solid tumor cells, for example non-small cell lung cancer cells and
sarcoma cells (35,36), demonstrating the broad antitumor
activity of arsenic. Due to a lack of effective treatments against
adult T-cell leukemia/lymphoma (ATLL), ATO activity was also tested
in vitro in several HTLV-1-infected cell lines. In 1998, a
Japanese group conducted the first in vitro studies on the
comparative effects of ATO and retinoic acid (RA) on
HTLV-1-infected cell lines and fresh ATL cells (37). Subsequently, several in vitro
studies indicated that ATO combined with interferon-α (IFN-α) is a
promising therapy for ATLL (38–41). The
surprising specificity of the cellular effects of ATO, by
exclusively targeting the viral oncoprotein p40, human
T-lymphotropic virus (Tax) and the NF-κB pathway, provided a
biological basis for dual IFN-α/As2O3
treatment for ATLL patients (42). At
present, several studies have reported phase II trial results of
dual IFN-α/As2O3 treatment in
relapsed/refractory ATLL (23,43,44).
The majority of these studies exhibited feasible anti-leukemia
effects in ATL patients with a poor prognosis, even though the
treatment context should be optimized (23,43,44). In
addition, arsenic, IFN-γ and zidovudine were shown to be important
for restoring immunocompetent microenvironments, thus enhancing the
eradication of ATL cells (45). This
mechanism may be secondary to arsenic or IFN-γ-induced Tax
degradation and the reversal of NF-кB activation (45).
With the promising role of arsenic and arsenic
combined with dual or triple treatment in ATLL, and the specific
effects of arsenic compounds in the treatment of APL, one study
investigated the potential of using arsenic to treat relapsed or
refractory ALL (5). The disappointing
result showed that ATO was not active in the treatment of ALL in a
cohort of 11 patients [5 early pre-acute B-cell lymphoblastic
leukemia (B-ALL), 2 pre-B-ALL, 1 mature B-ALL and 3 T-ALL] in 2006
(5). However, this result should not
preclude additional evaluation of arsenic in combination therapies
for ALL. For example, a previous study reported that subtoxic doses
of ATO and glucocorticoids could be advantageous for the treatment
of glucocorticoid-resistant ALL cells from T- and precursor B-ALL
patients (6). The underlying
pro-apoptosis mechanism partially depended on the inhibition of the
protein kinase B/X-linked inhibitor of apoptosis protein pathway
and the activation of the pro-apoptosis Bcl-2 family member, Bcl-2
associated agonist of cell death (6).
In addition, the cytotoxicity effect with an 80% inhibition rate of
ATO in the Molt-4 cell line was reported to involve apoptosis and
autophagy via the upregulation of Beclin-1 at the
post-transcriptional level (8,9). The
pre-clinical evidence indicated that ATO alone or combined with
other chemotherapy regimens have a potential capability in treating
T-ALL patients via complicated mechanisms (6,8,9). In the present study, the T-ALL patient
had a poor response to induction remission regimens, so the patient
was treated with a protocol involving arsenic, which is a salvaging
treatment for refractory leukemia patients in the Department of
Hematology, Guangdong General Hospital. Notably, the patient
responded well to arsenic treatment regimens without evident
toxicity. At present, it is unknown whether the successful
treatment of the present case was due to the therapeutic strategy
(drug-dose, route of administration or drug-combination) or to
factors that rendered the patient sensitive to arsenic treatment.
However, the present rare case at least provides support for the
use of arsenic agents combined with other drugs, such as
glucocorticoids, to treat T-ALL, as alternative salvage
chemotherapy in the future.
In the present case, the restricted expression of
the TCR Vβ, Vγ and Vδ repertoire subsets displayed a common
characteristic of samples collected at various time points and
various disease states. For example, restricted use of the TCR Vβ
subfamilies lacking Vβ2, 5, 7, 10, 17 and 22 was shown (Fig. 4B). Previous studies have shown that
TCR repertoire deficiency is a common characteristic of patients
with leukemia, including those with T-ALL (12,46).
Potential reasons for deficiencies in the TCR repertoire include:
i) The prior proliferation of a malignant T-cell clones suppressed
the proliferation of normal T-cell clones, and ii) the tumor
microenvironment or other unknown factors affect the competency of
the immune system (47,48). However, the T-cell repertoire
deficiency was not significantly reconstituted even when the
patient achieved CR, as the reconstitution of the TCR repertoire is
slow due to the cytotoxicity of chemotherapy (49). In addition, little is known of the
mechanisms by which arsenic therapy inhibits T-cell proliferation
(6). However, the continued presence
of certain TCR subfamilies, including Vβ12 and Vβ19, at various
time points of CR may indicate the reconstitution of the TCR
repertoire to a certain extent.
Unlike T-ALL patients following HSCT, in which T-ALL
clones are eradicated and not detected at the molecular level by
GeneScan analysis, Vβ1 T-cell clones of the same size could be
detected at all time points in the present case, even when the
patient achieved CR lasts for >4 months. This result suggests
that molecular CR cannot be achieved in cases treated with arsenic
and chemotherapy. The result also has significant implications for
MRD monitoring using specific clonal T-cell detection by GeneScan
and RT-PCR, which may provide dynamic information for disease
states and direct further therapy (12).
In the majority of cases with cancer or leukemia, an
antitumor T-cell clone could be identified in patient samples
(blood, BM or tumor tissue) even if the patient had
immunodeficiency (46,50), In the present study, clonally expanded
Vβ21, Vβ24, Vδ3 and Vδ6 were identified in certain samples
collected from various time points, and whether such clonally
expanded T-cells are special responders to T-ALL therapy requires
further investigation.
In conclusion, the present reported that arsenic
induced CR in a case with refractory T-ALL, which supports an
alternative salvage therapeutic method for clinically treating
refractory and relapsed T-ALL patients. However, the prolonged
response to arsenic associated with treatment and the associated
toxicity requires additional studies. The distribution and
clonality of the TCR β, γ and δ repertoires were characterized in
samples from various time points from the patient. The results
suggested that the evolution of a malignant T-ALL clone occurred,
indicating that arsenic therapy may be unable to induce molecular
CR in T-ALL patients. Therefore, dynamically monitoring the TCR
repertoire distribution and clonal evolution combined with clinical
course analysis would aid in predicting the prognosis of the
patient and in designing specific therapeutic strategies.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 91129720 and
81100384), the Collaborated Grant for HK-Macao-TW of the Ministry
of Science and Technology (grant no. 2012DFH30060) and the
Guangdong Science & Technology Project (Guangdong, China; grant
no. 2012B050600023).
Glossary
Abbreviations
Abbreviations:
|
T-ALL
|
acute T-cell lymphoblastic
leukemia
|
|
ATLL
|
adult T-cell leukemia/lymphoma
|
|
TCR
|
T-cell receptor
|
|
ND
|
newly diagnosed
|
|
MR
|
minor remission
|
|
CR
|
complete remission
|
|
MRD
|
minimal residual disease
|
|
ATO
|
arsenic trioxide
|
|
VDLCP
|
vincristine, daunorubicin,
L-asparaginase, cyclophosphamide and prednisone
|
|
HyperCVAD-A
|
cyclophosphamide, vincristine,
doxorubicin and dexamethasone
|
|
HyperCVAD-B
|
mitoxantrone and cytarabine
|
|
CAT
|
cyclophosphamide, cytarabine and
topotecan
|
|
CT
|
cyclophosphamide and topotecan
|
|
MP
|
methylprednisolone.
|
References
|
1
|
Van Vlierberghe P and Ferrando A: The
molecular basis of T cell acute lymphoblastic leukemia. J Clin
Invest. 122:3398–3406. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhojwani D and Pui CH: Relapsed childhood
acute lymphoblastic leukaemia. Lancet Oncol. 14:e205–e217. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van der Meulen J, Van Roy N, Van
Vlierberghe P and Speleman F: The epigenetic landscape of T-cell
acute lymphoblastic leukemia. Int J Biochem Cell Biol. 53:547–557.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koyama D, Kikuchi J, Hiraoka N, Wada T,
Kurosawa H, Chiba S and Furukawa Y: Proteasome inhibitors exert
cytotoxicity and increase chemosensitivity via transcriptional
repression of Notch1 in T-cell acute lymphoblastic leukemia.
Leukemia. 28:1216–1226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Litzow MR, Lee S, Bennett JM, Dewald GW,
Gallagher RE, Jain V, Paietta EM, Racevskis J, Rousey SR, Mazza JJ
and Tallman MS: A phase II trial of arsenic trioxide for relapsed
and refractory acute lymphoblastic leukemia. Haematologica.
91:1105–1108. 2006.PubMed/NCBI
|
|
6
|
Bornhauser BC, Bonapace L, Lindholm D,
Martinez R, Cario G, Schrappe M, Niggli FK, Schäfer BW and Bourquin
JP: Low-dose arsenic trioxide sensitizes glucocorticoid-resistant
acute lymphoblastic leukemia cells to dexamethasone via an
Akt-dependent pathway. Blood. 110:2084–2091. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qian W, Liu J, Jin J, Ni W and Xu W:
Arsenic trioxide induces not only apoptosis but also autophagic
cell death in leukemia cell lines via up-regulation of Beclin-1.
Leuk Res. 31:329–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu XM, Hirano T and Oka K: Arsenic
trioxide induces apoptosis in cells of MOLT-4 and its
daunorubicin-resistant cell line via depletion of intracellular
glutathione, disruption of mitochondrial membrane potential and
activation of caspase-3. Cancer Chemother Pharmacol. 52:47–58.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiao Y, Zhang W, Liu J, Ni W, Xu W, Jin J
and Qian W: Telomere attrition and chromosome instability via
downregulation of TRF2 contributes to arsenic trioxide-induced
apoptosis of human T-Cell leukemia cell line molt-4 cells. Cancer
Biol Ther. 6:1186–1192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taghon T, Waegemans E and Van de Walle I:
Notch signaling during human T cell development. Curr Top Microbiol
Immunol. 360:75–97. 2012.PubMed/NCBI
|
|
11
|
Zheng H, Wang X, Ma Y, Xu B, Chen S, Yang
L, Wu X, Przybylski GK, Huang S, Ye T and Li Y: The TCR γδ
repertoire and relative gene expression characteristics of T-ALL
cases with biclonal malignant Vδ1 and Vδ2 T cells. DNA Cell Biol.
33:49–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen S, Huang X, Zheng H, Geng S, Wu X,
Yang L, Weng J, Du X and Li Y: The evolution of malignant and
reactive γδ+T cell clones in a relapse T-ALL case after allogeneic
stem cell transplantation. Mol Cancer. 12:732013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Langerak AW, van Den Beemd R,
Wolvers-Tettero IL, Boor PP, van Lochem EG, Hooijkaas H and van
Dongen JJ: Molecular and flow cytometric analysis of the Vbeta
repertoire for clonality assessment in mature TCRalphabeta T-cell
proliferations. Blood. 98:165–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prinz I, Thamm K, Port M, Weissinger EM,
Stadler M, Gabaev I, Jacobs R, Ganser A and Koenecke C: Donor Vδ1+
γδ T cells expand after allogeneic hematopoietic stem cell
transplantation and show reactivity against CMV-infected cells but
not against progressing B-CLL. Exp Hematol Oncol. 2:142013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Langerak AW, Szczepański T, van der Burg
M, Wolvers-Tettero IL and van Dongen JJ: Heteroduplex PCR analysis
of rearranged T cell receptor genes for clonality assessment in
suspect T cell proliferations. Leukemia. 11:2192–2199. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takeuchi J, Kyo T, Naito K, Sao H,
Takahashi M, Miyawaki S, Kuriyama K, Ohtake S, Yagasaki F, Murakami
H, et al: Induction therapy by frequent administration of
doxorubicin with four other drugs, followed by intensive
consolidation and maintenance therapy for adult acute lymphoblastic
leukemia: The JALSG-ALL93 study. Leukemia. 16:1259–1266. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kantarjian H, Thomas D, O'Brien S, Cortes
J, Giles F, Jeha S, Bueso-Ramos CE, Pierce S, Shan J, Koller C, et
al: Long-term follow-up results of hyperfractionated
cyclophosphamide, vincristine, doxorubicin and dexamethasone
(Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic
leukemia. Cancer. 101:2788–2801. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Chen S, Yang L, Yin Q, Geng S, Wu X,
Schmidt CA and Przybylski GK: TRAV and TRBV repertoire, clonality
and the proliferative history of umbilical cord blood T-cells.
Transpl Immunol. 18:151–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Chen S, Yang L, Li B, Chan JY and
Cai D: TRGV and TRDV repertoire distribution and clonality of T
cells from umbilical cord blood. Transpl Immunol. 20:155–162. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Assaf C, Hummel M, Dippel E, Goerdt S,
Müller HH, Anagnostopoulos I, Orfanos CE and Stein H: High
detection rate of T-cell receptor beta chain rearrangements in
T-cell lymphoproliferations by family specific polymerase chain
reaction in combination with the GeneScan technique and DNA
sequencing. Blood. 96:640–646. 2000.PubMed/NCBI
|
|
21
|
Gorski J, Yassai M, Zhu X, Kissela B,
Kissella B [corrected to Kissela B], Keever C and Flomenberg N:
Circulating T cell repertoire complexity in normal individuals and
bone marrow recipients analyzed by CDR3 size spectratyping.
Correlation with immune status. J Immunol. 152:5109–5119.
1994.PubMed/NCBI
|
|
22
|
Soignet SL, Frankel SR, Douer D, Tallman
MS, Kantarjian H, Calleja E, Stone RM, Kalaycio M, Scheinberg DA,
Steinherz P, et al: United States multicenter study of arsenic
trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol.
19:3852–3860. 2001.PubMed/NCBI
|
|
23
|
Mahieux R and Hermine O: In vivo and in
vitro treatment of HTLV-1 and HTLV-2 infected cells with arsenic
trioxide and interferon-alpha. Leuk Lymphoma. 46:347–355. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schiller GJ, Slack J, Hainsworth JD, Mason
J, Saleh M, Rizzieri D, Douer D and List AF: Phase II multicenter
study of arsenic trioxide in patients with myelodysplastic
syndromes. J Clin Oncol. 24:2456–2464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vey N, Bosly A, Guerci A, Feremans W,
Dombret H, Dreyfus F, Bowen D, Burnett A, Dennis M, Ribrag V, et
al: Arsenic trioxide in patients with myelodysplastic syndromes: A
phase II multicenter study. J Clin Oncol. 24:2465–2471. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ,
Si GY, Jin XL, Tang W, Li XS, Xong SM, et al: In vitro studies on
cellular and molecular mechanisms of arsenic trioxide (As2O3) in
the treatment of acute promyelocytic leukemia: As2O3 induces NB4
cell apoptosis with downregulation of Bcl-2 expression and
modulation of PML-RAR alpha/PML proteins. Blood. 88:1052–1061.
1996.PubMed/NCBI
|
|
27
|
Galimberti S, Guerrini F, Salvi F, Petrini
I, Gioia D, Messa E, Palumbo GA, Cilloni D, Petrini M and Levis A:
Arsenic trioxide and ascorbic acid interfere with the BCL2 family
genes in patients with myelodysplastic syndromes: An ex-vivo study.
J Hematol Oncol. 5:532012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yedjou C, Tchounwou P, Jenkins J and
McMurray R: Basic mechanisms of arsenic trioxide (ATO)-induced
apoptosis in human leukemia (HL-60) cells. J Hematol Oncol.
3:282010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rojewski MT, Körper S and Schrezenmeier H:
Arsenic trioxide therapy in acute promyelocytic leukemia and
beyond: From bench to bedside. Leuk Lymphoma. 45:2387–2401. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mathas S, Lietz A, Janz M, Hinz M, Jundt
F, Scheidereit C, Bommert K and Dorken B: Inhibition of NF-kappaB
essentially contributes to arsenic-induced apoptosis. Blood.
102:1028–1034. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumar S, Yedjou CG and Tchounwou PB:
Arsenic trioxide induces oxidative stress, DNA damage and
mitochondrial pathway of apoptosis in human leukemia (HL-60) cells.
J Exp Clin Cancer Res. 33:422014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu M, Levin J, Sulpice E, Sequeira-Le
Grand A, Alemany M, Caen JP and Han ZC: Effect of arsenic trioxide
on viability, proliferation, and apoptosis in human megakaryocytic
leukemia cell lines. Exp Hematol. 27:845–852. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rousselot P, Labaume S, Marolleau JP,
Larghero J, Noguera MH, Brouet JC and Fermand JP: Arsenic trioxide
and melarsoprol induce apoptosis in plasma cell lines and in plasma
cells from myeloma patients. Cancer Res. 59:1041–1048.
1999.PubMed/NCBI
|
|
34
|
Akao Y, Mizoguchi H, Kojima S, Naoe T,
Ohishi N and Yagi K: Arsenic induces apoptosis in B-cell leukaemic
cell lines in vitro: Activation of caspases and down-regulation of
Bcl-2 protein. Br J Haematol. 102:1055–1060. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li H, Zhu X, Zhang Y, Xiang J and Chen H:
Arsenic trioxide exerts synergistic effects with cisplatin on
non-small cell lung cancer cells via apoptosis induction. J Exp
Clin Cancer Res. 28:1102009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chiu HW, Tseng YC, Hsu YH, Lin YF, Foo NP,
Guo HR and Wang YJ: Arsenic trioxide induces programmed cell death
through stimulation of ER stress and inhibition of the
ubiquitin-proteasome system in human sarcoma cells. Cancer Lett.
356:762–772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishitsuka K, Hanada S, Suzuki S,
Utsunomiya A, Chyuman Y, Takeuchi S, Takeshita T, Shimotakahara S,
Uozumi K, Makino T and Arima T: Arsenic trioxide inhibits growth of
human T-cell leukaemia virus type I infected T-cell lines more
effectively than retinoic acids. Br J Haematol. 103:721–728. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bazarbachi A, El-Sabban ME, Nasr R,
Quignon F, Awaraji C, Kersual J, Dianoux L, Zermati Y, Haidar JH,
Hermine O and de Thé H: Arsenic trioxide and interferon-alpha
synergize to induce cell cycle arrest and apoptosis in human T-cell
lymphotropic virus type I-transformed cells. Blood. 93:278–283.
1999.PubMed/NCBI
|
|
39
|
El-Sabban ME, Nasr R, Dbaibo G, Hermine O,
Abboushi N, Quignon F, Ameisen JC, Bex F, de Thé H and Bazarbachi
A: Arsenic-interferon-alpha-triggered apoptosis in HTLV–I
transformed cells is associated with tax down-regulation and
reversal of NF-kappa B activation. Blood. 96:2849–2855.
2000.PubMed/NCBI
|
|
40
|
Ishitsuka K, Hanada S, Uozumi K,
Utsunomiya A and Arima T: Arsenic trioxide and the growth of human
T-cell leukemia virus type I infected T-cell lines. Leuk Lymphoma.
37:649–655. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mahieux R, Pise-Masison C, Gessain A,
Brady JN, Olivier R, Perret E, Misteli T and Nicot C: Arsenic
trioxide induces apoptosis in human T-cell leukemia virus type 1-
and type 2-infected cells by a caspase-3-dependent mechanism
involving Bcl-2 cleavage. Blood. 98:3762–3769. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nasr R, Rosenwald A, El-Sabban ME, Arnulf
B, Zalloua P, Lepelletier Y, Bex F, Hermine O, Staudt L, de Thé H
and Bazarbachi A: Arsenic/interferon specifically reverses 2
distinct gene networks critical for the survival of HTLV-1-infected
leukemic cells. Blood. 101:4576–4582. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ishitsuka K, Suzumiya J, Aoki M, Ogata K,
Hara S and Tamura K: Therapeutic potential of arsenic trioxide with
or without interferon-alpha for relapsed/refractory adult T-cell
leukemia/lymphoma. Haematologica. 92:719–720. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hermine O, Dombret H, Poupon J, Arnulf B,
Lefrère F, Rousselot P, Damaj G, Delarue R, Fermand JP, Brouet JC,
et al: Phase II trial of arsenic trioxide and alpha interferon in
patients with relapsed/refractory adult T-cell leukemia/lymphoma.
Hematol J. 5:130–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kchour G, Rezaee R, Farid R, Ghantous A,
Rafatpanah H, Tarhini M, Kooshyar MM, El Hajj H, Berry F, Mortada
M, et al: The combination of arsenic, interferon-alpha and
zidovudine restores an ‘immunocompetent-like’ cytokine expression
profile in patients with adult T-cell leukemia lymphoma.
Retrovirology. 10:912013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li Y, Geng S, Du X, Chen S, Yang L, Wu X,
Li B, Schmidt CA and Przybylski GK: Restricted TRBV repertoire in
CD4+ and CD8+ T-cell subsets from CML patients. Hematology.
16:43–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shi L, Chen S, Yang L and Li Y: The role
of PD-1 and PD-L1 in T-cell immune suppression in patients with
hematological malignancies. J Hematol Oncol. 6:742013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yawalkar N, Ferenczi K, Jones DA, Yamanaka
K, Suh KY, Sadat S and Kupper TS: Prof-ound loss of T-cell receptor
repertoire complexity in cutaneous T-cell lymphoma. Blood.
102:4059–4066. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eyrich M, Wiegering V, Lim A, Schrauder A,
Winkler B and Schlegel PG: Immune function in children under
chemotherapy for standard risk acute lymphoblastic leukaemia-a
prospective study of 20 paediatric patients. Br J Haematol.
147:360–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zha X, Chen S, Yang L, Li B, Chen Y, Yan X
and Li Y: Characterization of the CDR3 structure of the Vβ21 T cell
clone in patients with P210 (BCR-ABL)-positive chronic myeloid
leukemia and B-cell acute lymphoblastic leukemia. Hum Immunol.
72:798–804. 2011. View Article : Google Scholar : PubMed/NCBI
|


















