Introduction
Treatment of metastatic colorectal cancer (mCRC) has
progressed considerably over the past decade. In particular,
advances in the understanding of the molecular mechanisms involved
in carcinogenesis have led to the development of targeted therapy
(1). Clinical studies have shown the
validity of the epidermal growth factor receptor (EGFR) and
vascular endothelial growth factor (VEGF) as therapeutic targets
for mCRC patients. Several studies have shown the survival benefits
of anti-EGFR therapy for wild-type Kirsten rat sarcoma viral
oncogene homolog (KRAS) mCRC (2–4). Anti-VEGF
therapy has also shown survival benefits in first- and second-line
settings (5,6). Recently, several head to head
comparisons between anti-EGFR and anti-VEGF therapy were reported
for the first-line setting. Although one trial showed higher
overall survival (OS) times for anti-EGFR therapy (7), another phase III trial failed to show
any survival differences (8).
According to these controversial results, anti-EGFR and anti-VEGF
therapy are considered of equal importance in the first-line
setting. When considering the cosmetic aspects, treatments with
anti-EGFR therapy for long period tend to be avoided due to the
decrease in skin quality. Due to cumulative skin reactions,
anti-EGFR therapy is not preferred in the first-line setting in
Japan. However, in cases where a quick response or marked tumor
shrinkage is required, anti-EGFR therapy is chosen by oncologists
(9). A lack of evidence for the
successful use of anti-VEGF as salvage therapy and the impressive
results of using bevacizumab beyond progression (BBP) have led to
certain oncologists preferring first-line anti-VEGR therapy with
the BBP strategy (10,11). However, several detrimental results in
anti-EGFR trials have appeared to have resulted in an aversion to
using combined therapies as first-line treatment (12,13).
Based on the biological synergistic effect with
irinotecan, anti-EGFR tends to be combined even though it is a
salvage treatment (14). Two
monoclonal antibodies of anti-EGFR have been approved for use in
mCRC. The two agents have similar activity, but with certain
different results in clinical trials. There is little data on the
use of panitumumab with irinotecan in the salvage setting, although
cetuximab with irinotecan shows a survival benefit. Recent advances
have shown the appeal of a skin toxicity prevention program using
panitumumab (15). Panitumumab has
several advantageous aspects with regard to schedule and toxicity.
Therefore, as further clinical data on salvage panitumumab with
irinotecan is required, the present phase II trial for mCRC with a
skin toxicity prevention program was conducted to evaluate its
clinical efficacy.
Patients and methods
Trial details
The present study is a phase II, single-arm,
multicenter study of panitumumab and irinotecan combination
therapy. Approval for the study was obtained from the Ethics
Committee of Jichi Medical University (Shimotsuke, Japan) and each
facility involved, and written informed consent was obtained from
all enrolled patients (trial ID, UMIN000004500).
Patients
The inclusion criteria were as follows: Histological
diagnosis of unresectable advanced and/or recurrent colorectal
cancer; presence of a measurable target lesion; absence of KRAS
mutation in exon 2 (codons 12 and 13); disease progression or
intolerance to irinotecan- and oxaliplatin-based therapy, and had
previously received fluoropyrimidines; Eastern Cooperative Oncology
Group performance status of 0–2; age ≥20 years; neutrophil count
≥1,000/mm3; platelet count ≥100,000/mm3;
total bilirubin level ≤1.5 mg/dl; aspartate transaminase (10–35 U)
and alanine transaminase levels (5–40 U) ≤2.5 times the facility's
upper limit of normal (≤5 times the respective facility's upper
limit in the case of hepatic metastasis); serum creatinine level
≤2.0 mg/dl; at least 2 weeks having passed with no treatment since
completion of any of the following treatments: and radiotherapy,
surgical treatment with organ resection, chemotherapy, hormone and
immunotherapy. The presence or absence of a
UDP-glucuronosyltransferase 1–1 measurement was not taken into
consideration.
Treatment
Panitumumab (Takeda Pharmaceutical Company, Tokyo,
Japan) was administered at a dose of 6 mg/kg every 2 weeks by
intravenous infusion over 60 min, and irinotecan was administered
at a dose of 100–180 mg/m2 every 2 weeks by intravenous
infusion over 90 min, depending on the preceding treatment dose. As
prophylaxis for anti-emesis, topical steroids and 5-HT3 receptor
antagonists were administered prior to the chemotherapy regimen.
This treatment was continued until disease progression, intolerable
adverse events and/or upon patient refusal of treatment. If a
treatment course could not be initiated due to a neutrophil count
<1,000/mm3, a platelet count
<75,000/mm3 and/or other non-hematological toxicity,
the treatment was deferred or the dose decreased at the discretion
of the attending physician. In the event irinotecan could not be
continued due to adverse events, panitumumab monotherapy was
continued. To prevent skin toxicities, a moisturizer was applied to
the face, arms, legs, neck, back and chest every morning after
waking up; sunscreen was applied prior to stepping outside; a
topical steroid was applied to the face, arms, legs, neck, back,
and chest at bedtime; and oral antibiotics (100 mg minocycline
twice daily) were initiated 24 h prior to the first course of
treatment. All these treatments were continuously administered for
6 weeks. The decision to continue antibiotics after 6 weeks was
left to the discretion of the attending physician.
Evaluation
Patients underwent regular examinations and blood
tests as outpatients, and an examination by computed tomography or
magnetic resonance imaging was performed every 8 weeks from
chemotherapy initiation. The primary endpoint of the present study
was the response rate (RR) determined by two independent reviewers.
The antitumor effect was determined using the Response Evaluation
Criteria in Solid Tumors v. 1.0 (16)
by the attending physician, researchers and two independent
radiologists. Secondary endpoints included the disease control rate
(DCR), the progression-free survival (PFS) time, the OS time and
adverse events. PFS time was defined as the period from
chemotherapy initiation until progression, and OS time was defined
as the period from chemotherapy initiation until mortality. Adverse
events were evaluated using the National Cancer Institutes' Common
Toxicity Criteria v. 3.0 (Japanese edition) (17). To ascertain each patient's requirement
for prophylactic treatment of skin toxicities, patients were asked
to keep a diary beginning 24 h prior to the first treatment day
until week 6.
Statistical analysis
With the RR threshold set at 10% and the anticipated
RR at 25%, 41 patients with measurable responsiveness were required
for the α error to be <0.10 (one-sided) when the true RR was
less than the RR threshold and for the statistical power to be
>90% when the true RR was above the anticipated RR (18,19).
Considering that certain patients would be excluded from the
analyses, the aim was to enroll 45 patients. PFS and OS were
estimated on a survival curve using the Kaplan-Meier method, and
the two-sided 95% confidence intervals (CIs) of the standard
deviation and median value were calculated using JMP®
software (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
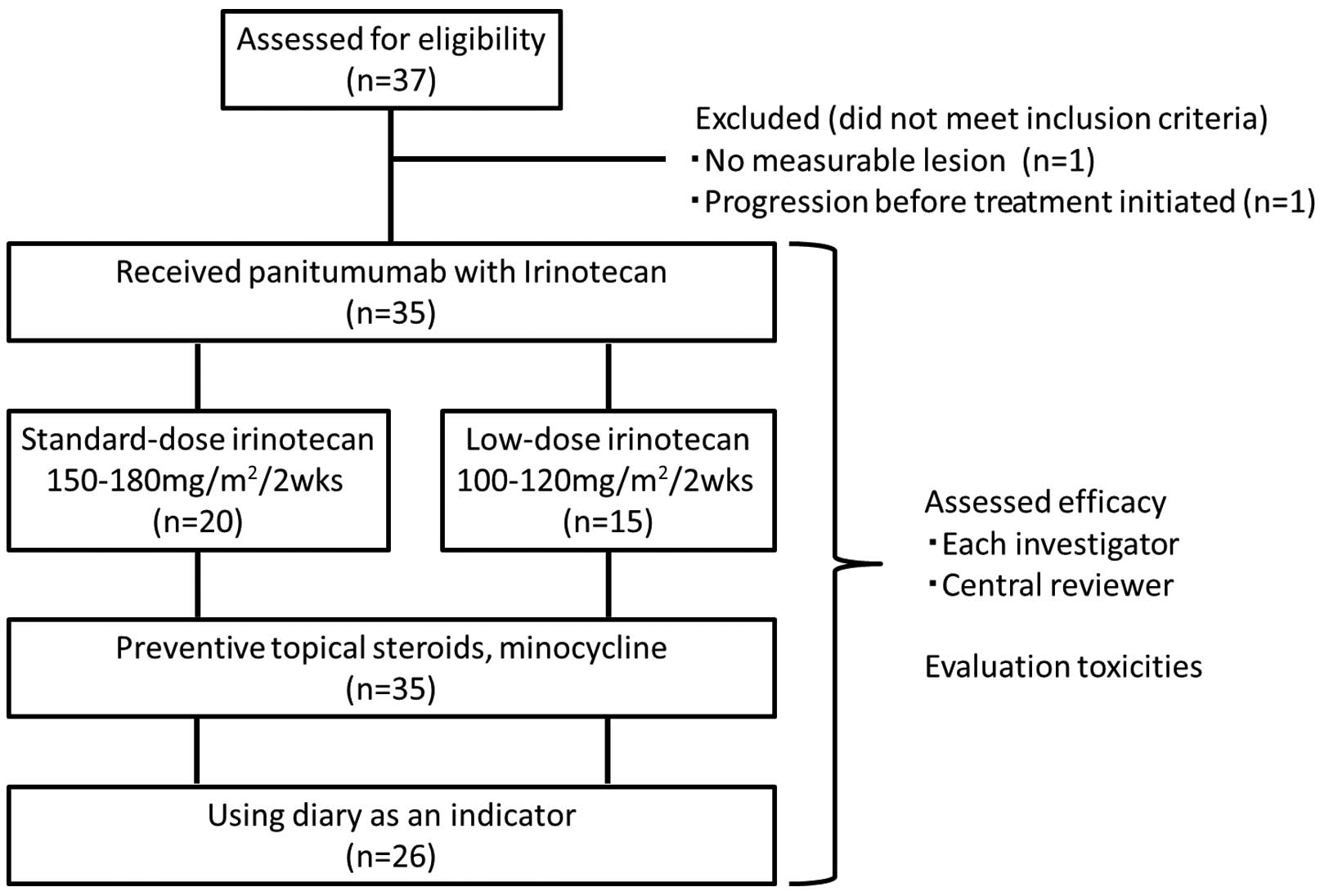
A total of 37 patients from 6 Japanese medical
institutions were enrolled between October 2010 and March 2012. The
institutions were as follows: Tochigi Cancer Center (Utsunomiya,
Japan), Jichi Medical University Hospital (Shimotsuke, Japan),
Chiba Cancer Center (Chiba, Japan), Saitama Cancer Center (Saitama,
Japan), Ibaraki Central Hospital (Mito, Japan) and Saku Central
Hospital (Saku, Japan). Delays in the enrollment process made it
difficult for data to be maintained by the study coordinator; as a
result, enrollment was terminated. Of the 37 patients enrolled, 1
patient was excluded due to the lack of a target lesion, and 1
patient was excluded due to the inability to receive treatment as a
result of disease progression. As a result, the chemotherapy
regimen was administered to 35 patients in total (Fig. 1). Patient characteristics are shown in
Table I; the median age was 61 years
(range, 41–76 years), with 25 male and 10 female patients. The
initial irinotecan dose was 150 mg/m2 in 19 patients
(54%) and 180 mg/m2 in 1 patient. The median number of
treatment courses administered was 6 (range, 3–41).
 | Table I.Patient characteristics (n=35). |
Table I.
Patient characteristics (n=35).
| Characteristic | Value |
|---|
| Gender, n (%) |
|
| Male | 25 (71.4) |
|
Female | 10 (28.6) |
| Age, years |
|
|
Median | 61 |
|
Range | 41–76 |
| ECOG performance
status, n (%) |
|
| 0 | 30 (85.7) |
| 1 | 5
(14.3) |
| Primary tumor
resected, n (%) |
|
| Yes | 26 (74.3) |
| No | 9
(25.7) |
| Previous adjuvant
therapy, n (%) |
|
| Yes | 13 (37.1) |
| No | 22 (62.9) |
| First-line treatment,
n (%) |
|
|
Oxaliplatin-based therapy | 25 (71.4) |
|
Irinotecan-based therapy | 5
(14.3) |
|
Others | 6
(17.1) |
| Second-line
treatment, n (%) |
|
|
Irinotecan-based therapy | 28 (80.0) |
|
Oxaliplatin-based therapy | 4
(11.4) |
|
Others | 3 (8.6) |
| Prior treatment with
bevacizumab, n (%) |
|
| No | 4
(11.4) |
| Yes | 31 (88.6) |
| Last dose of
irinotecan of the previous treatment in mg/m2, n
(%) |
|
| 180 | 1 (2.9) |
| 150 | 19 (54.3) |
| 120 | 8
(22.9) |
| 100 | 7
(20.0) |
Efficacy
A central review indicated that, of the 35 patients
included, 8 patients (22.9%) presented with a partial response and
6 patients (17.1%) with stable disease, with an RR of 22.9% (95%
CI, 12.1–39.0) and a DCR of 40% (Table
II). An RR of 17.1% (95% CI, 8.1–32.7) and a DCR of 42.9% were
recorded. The objective RR of the patients with standard-dose
irinotecan (150 or 180 mg/m2) was 30.0% (6/20), although
that of low-dose irinotecan (100–120 mg/m2) was 13.3%
(2/15). In 2 patients, the chemotherapy was terminated due to
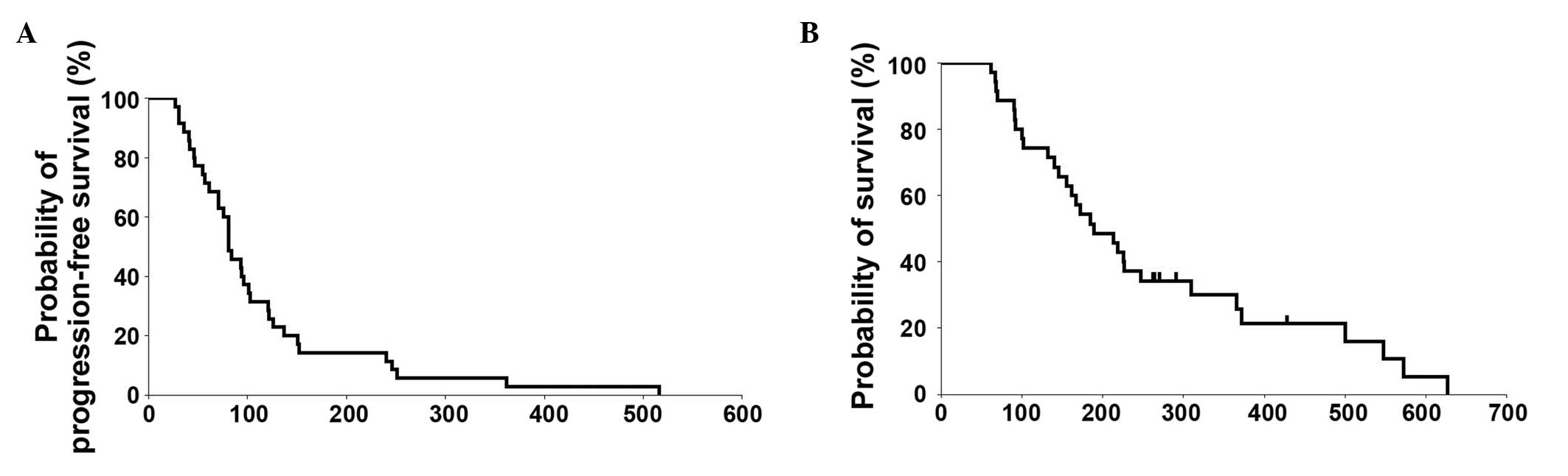
surgery being performed. The median PFS time was 81 days (2.7
months; 95% CI, 71–101) and the median OS time was 189 days (6.3
months; 95% CI, 146–247) (Table II).
The survival curve is shown in Fig.
2.
 | Table II.Efficacy (n=35). |
Table II.
Efficacy (n=35).
| Overall response
rate | Central review | Investigator
assessment |
|---|
| CR, n (%) | 0 (0.0) | 0 (0.0) |
| PR, n (%) | 8
(22.9) | 6
(17.1) |
| SD, n (%) | 6
(17.1) | 9
(25.7) |
| PD, n (%) | 21 (60.0) | 20 (57.1) |
| PFS time, days (95%
CI) | 81 (71–101) |
| OS time, days (95%
CI) | 189 (146–247) |
Safety
Diaries could be collected from 26 patients, among
whom prophylactic treatment for a rash was properly administered in
22 patients (84.6%). Other diaries could not be collected as they
had been lost, the patients had refused to complete them or as the
patients' symptoms had deteriorated prior to collection. Adverse
events are shown in Table III. A
grade 3 or above acne-like rash developed in 25.7% of patients,
paronychia in 11.4% and dryness in 8.6%. Recovery from the majority
of adverse events was possible with the appropriate cessation of
the chemotherapy and active interventional therapy. In total, 1
patient succumbed 15 days after the final course of treatment. In
this patient, a causal association between mortality and treatment
could not be ruled out. In addition, the chemotherapy regimen was
discontinued by the attending physician in 4 patients, as these
patients refused further treatment due to adverse events.
 | Table III.Toxicity (n=35). |
Table III.
Toxicity (n=35).
|
| Grade, n |
|
|---|
|
|
|
|
|---|
| Symptom | 1 | 2 | 3 | 4 | Grade 3/4, % |
|---|
| Leukopenia | 5 | 6 | 2 | 0 |
5.7 |
| Anemia | 23 | 3 | 3 | 0 |
8.6 |
|
Thrombocytopenia | 12 | 1 | 0 | 0 |
0.0 |
| Neutropenia | 4 | 6 | 1 | 1 |
5.7 |
| Febrile
neutropenia | 0 | 0 | 0 | 0 |
0.0 |
| Hypomagnesemia | 14 | 5 | 1 | 0 |
2.9 |
| Anorexia | 8 | 7 | 2 | 0 |
5.7 |
| Nausea | 5 | 5 | 2 | 0 |
5.7 |
| Vomiting | 4 | 3 | 0 | 0 |
0.0 |
| Diarrhea | 7 | 4 | 2 | 0 |
5.7 |
| Fatigue | 5 | 9 | 2 | 0 |
5.7 |
| Acneiform rash | 11 | 10 | 9 | 0 | 25.7 |
| Pruritis | 4 | 11 | 1 | 0 |
2.9 |
| Paronychia | 8 | 10 | 4 | 0 | 11.4 |
| Stomatitis | 6 | 9 | 1 | 0 |
2.9 |
| Dry skin | 1 | 4 | 3 | 0 |
8.6 |
| Infusion-related
reaction | 0 | 0 | 0 | 0 |
0.0 |
Discussion
The present study was designed to assess the
efficacy of anti-EGFR antibody administered in combination with
irinotecan in the salvage setting with skin protection. The
objective RR was 23%, the PFS time was 2.7 months and the grade 3
skin reaction rate was >20%. Relatively low-dose irinotecan
cases tended to exhibit a limited response. The study did not
reveal any additional efficacy for use of irinotecan and skin
toxicity prevention for KRAS wild-type patients. Several
explanations of these results should be considered with regard to
recent oncological topics.
First, the additional effect of salvage irinotecan
with panitumumab is controversial. Only small studies have
concluded the efficacy of this doublet. GERCOR conducted a
single-arm prospective trial showed that, based on biomarker
results, the combination of panitumumab and irinotecan was an
active third-line regimen in a well-defined population (20). Another Japanese study also suggested
promising results with acceptable toxicities (21). Notably, the two studies were
non-randomized single-arm study and the sample size was not high.
Therefore, the selection bias of these previous studies should be
considered.
The next concern is that anti-EGFR had a limited
additional efficacy when combined with irinotecan even in several
second-line trials. Although panitumumab showed a benefit in
overall response and PFS, the change in OS was not significant
(22). The PICOOLO trial failed to
show any additional effect of irinotecan plus panitumumab compared
with irinotecan alone (23).
Cetuximab also failed to confer any additional effect in the
second-line setting (24).
Third, the positive results of cetuximab with
irinotecan that have been noted in salvage therapy (14) require further investigation. The study
design of this previous study was of a randomized phase II trial
and the primary endpoint was RR (14). The sample size of the study was
relatively small in order to draw a definitive conclusion. No other
controlled data follows irinotecan beyond progression combined with
anti-EGFRs. It is therefore time to reconsider the additional
effect of irinotecan in the salvage setting.
Finally, several imbalances in anti-EGFR trials
should be considered. Previous classical trials have not included
the status of the KRAS exon 2 mutation. Recent molecular
analysis has shown that other minor KRAS and NRAS
mutations, and BRAF status are also of importance in the
efficacy of anti-EGFR. In the present prospective trial, only
KRAS exon 2 mutations were tested, and other mutations was
not considered.
With regard to skin toxicity prevention, the present
study did not reveal a preventive effect with pre-emptive skin
treatment. However, in a previous randomized Japanese study, a
preventative effect was successfully reported (25). In this randomized study, the
importance of skin toxicity prevention was emphasized, although
several fundamental problems were evident. The study was a
randomized comparison of the conventional and pre-emptive methods
in an open-label, non-blinded manner. After previous reports, it
would be expected by the investigators and patients that the
preventive group would exhibit a higher potential. The lack of a
reactive skin program also could be a bias point of this trial.
Certain investigators have reported that intensive reactive skin
therapy is enough for skin toxicities (26).
Several limitations were noted in the present study.
Firstly, the study failed to collect the planned sample. The study
was originally designed with >90% statistical power. Only 35
patients were included in the study, the statistical power was
decreased to 87%. Furthermore, it was also limited by the fact that
the education in skin protection was dependent on the institution.
A lack of a full set of biomarker analyses and the low-dose
irinotecan administration are reasons for the poor outcome. The
present study is therefore not sufficient enough to draw definitive
conclusions.
Overall, the present trial was the first negative
report of panitumumab and irinotecan in salvage therapy with skin
toxicity prevention. The routine use of skin toxicity prevention is
not recommended and additional irinotecan also remains under
evaluation. In particular, the effect of low-dose irinotecan with
panitumumab appears to be clinically insignificant. Further
full-set biological analysis is warranted to consider the
additional effect of salvage irinotecan combined with anti-EGFR
treatment.
References
|
1
|
Winder T and Lenz HJ: Vascular endothelial
growth factor and epidermal growth factor signaling pathways as
therapeutic targets for colorectal cancer. Gastroenterology.
138:2163–2176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jonker DJ, O'Callaghan CJ, Karapetis CS,
Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, et
al: Cetuximab for the treatment of colorectal cancer. N Engl J Med.
357:2040–2048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giantonio BJ, Catalano PJ, Meropol NJ,
O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA and Benson AB III:
Eastern Cooperative Oncology Group Study E3200: Bevacizumab in
combination with oxaliplatin, fluorouracil and leucovorin (FOLFOX4)
for previously treated metastatic colorectal cancer: Results from
the eastern cooperative oncology group study E3200. J Clin Oncol.
25:1539–1544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Venook AP, Niedzwiecki D, Lenz HJ,
Innocenti F, Mahoney MR, O'Neil BH, Shaw JE, Polite BN, Hochster
HS, Atkins JN, et al: CALGB/SWOG 80405: Phase III trial of
irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin
(mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients
(pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma
of the colon or rectum (MCRC). J Clin Oncol. 32:abstract LBA3.
2014.
|
|
9
|
Van Cutsem E, Cervantes A, Nordlinger B
and Arnold D: ESMO, Guidelines Working Group: Metastatic colorectal
cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. (25 Suppl 3): iii1–iii9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bennouna J, Sastre J, Arnold D, Österlund
P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C,
et al: Continuation of bevacizumab after first progression in
metastatic colorectal cancer (ML18147): A randomised phase 3 trial.
Lancet Oncol. 14:29–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Cutsem E, Tabernero J, Lakomy R,
Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko
V, Ferry D, et al: Addition of aflibercept to fluorouracil,
leucovorin, and irinotecan improves survival in a phase III
randomized trial in patients with metastatic colorectal cancer
previously treated with an oxaliplatin-based regimen. J Clin Oncol.
30:3499–3506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Primrose J, Falk S, Finch-Jones M, Valle
J, O'Reilly D, Siriwardena A, Hornbuckle J, Peterson M, Rees M,
Iveson T, et al: Systemic chemotherapy with or without cetuximab in
patients with resectable colorectal liver metastasis: The New EPOC
randomised controlled trial. Lancet Oncol. 15:601–611. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Madi A, Fisher D, Wilson RH, Adams RA,
Meade AM, Kenny SL, Nichols LL, Seymour MT, Wasan H, Kaplan R, et
al: Oxaliplatin/capecitabine vs oxaliplatin/infusional 5-FU in
advanced colorectal cancer: The MRC COIN trial. Br J Cancer.
107:1037–1043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lacouture ME, Mitchell EP, Piperdi B,
Pillai MV, Shearer H, Iannotti N, Xu F and Yassine M: Skin toxicity
evaluation protocol with panitumumab (STEPP), a phase II,
open-label, randomized trial evaluating the impact of a pre-Emptive
Skin treatment regimen on skin toxicities and quality of life in
patients with metastatic colorectal cancer. J Clin Oncol.
28:1351–1357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
James K, Eisenhauer E, Christian M,
Terenziani M, Vena D, Mudal A and Therasse P: Measuring response in
solid tumors: Unidimensional versus bidimensional measurement. J
Natl Cancer Inst. 91:523–528. 1991. View Article : Google Scholar
|
|
17
|
Common Terminology Criteria for Adverse
Events v3.0 (CTCAE). 2006.http://www.jcog.jp/doctor/tool/CTCAEv3J_070308.pdfAccessed.
March 08–2007
|
|
18
|
Van Cutsem E, Peeters M, Siena S, Humblet
Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J,
Richardson G, et al: Open-label phase III trial of panitumumab plus
best supportive care compared with best supportive care alone in
patients with chemotherapy-refractory metastatic colorectal cancer.
J Clin Oncol. 25:1658–1664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doi T, Ohtsu A, Tahara M, Tamura T, Shirao
K, Yamada Y, Otani S, Yang BB, Ohkura M and Ohtsu T: Safety and
pharmacokinetics of panitumumab in Japanese patients with advanced
solid tumors. Int J Clin Oncol. 14:307–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
André T, Blons H, Mabro M, Chibaudel B,
Bachet JB, Tournigand C, Bennamoun M, Artru P, Nguyen S, Ebenezer
C, et al: Panitumumab combined with irinotecan for patients with
KRAS wild-type metastatic colorectal cancer refractory to standard
chemotherapy: A GERCOR efficacy, tolerance and translational
molecular study. Ann Oncol. 24:412–419. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hasegawa H, Yoshida M, Taira K, Sugimoto
N, Kii T, Takeda K, Yoshinami T, Sakai D, Satou T, et al: A Phase
II study of pmab + IRI FOR mCRC PTS with WildKRAS, resistant to
fluoropyrimidine, oxaliplatin and IRI (OGSG1001). Ann Oncol.
25(Suppl 5): v44–v74. 2014.
|
|
22
|
Peeters M, Price TJ, Cervantes A, Sobrero
AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJ, et al:
Randomized phase III study of panitumumab with fluorouracil,
leucovorin and irinotecan (FOLFIRI) compared with FOLFIRI alone as
second-line treatment in patients with metastatic colorectal
cancer. J Clin Oncol. 28:4706–4713. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seymour MT, Brown SR, Middleton G, Maughan
T, Richman S, Gwyther S, Lowe C, Seligmann JF, Wadsley J, Maisey N,
et al: Panitumumab and irinotecan versus irinotecan alone for
patients with KRAS wild-type, fluorouracil-resistant advanced
colorectal cancer (PICCOLO): A prospectively stratified randomised
trial. Lancet Oncol. 14:749–759. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sobrero AF, Maurel J, Fehrenbacher L,
Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C,
Steinhauer EU, Prausova J, et al: EPIC: Phase III trial of
cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin
failure in patients with metastatic colorectal cancer. J Clin
Oncol. 26:2311–2319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kobayashi Y, Komatsu Y, Yuki S, Fukushima
H, Sasaki T, Iwanaga I, Uebayashi M, Okuda H, Kusumi T, Miyagishima
T, et al: Randomized controlled trial on the skin toxicity of
panitumumab in Japanese patients with metastatic colorectal cancer:
HGCSG1001 study; J-STEPP. Future Oncol. 11:617–627. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schimanski CC, Moehler M, Zimmermann T,
Wörns MA, Steinbach A, Baum M and Galle PR: Cetuximab-induced skin
exanthema: Improvement by a reactive skin therapy. Mol Med Rep.
3:789–793. 2010.PubMed/NCBI
|