Introduction
Human breast cancer is a malignant breast tumor that
primarily affects females. Effective control of breast cancer has
been hampered by a lack of specific tumor targets. One potential
way to improve the specificity of targeting is to develop novel
vectors that specifically bind to and are internalized by tumor
cells.
In recent years, receptor-mediated endocytosis
proteins, including vascular endothelial growth factor receptor
(VEGFR), epidermal growth factor receptor (EGFR), human epidermal
growth factor receptor 2 (HER2) and the Arg-Gly-Asp (RGD) motif
(1–4),
and protein transduction domains (including human immunodeficiency
virus trans-activator of transcription protein and VP22) (5–7), have
attracted considerable interest in the drug delivery field due to
their ability to translocate across biological membranes. Evidence
suggests that certain short peptides have promising intracellular
delivery activity, and a number of these proteins have been
modified and used as drug carriers in preclinical antitumor trials
(8,9).
However, the low cell specificity of this approach limits its
application in tumor-targeted therapy (10,11).
Dysregulation of oncogenes and tumor suppressor genes in tumor
cells results in abnormal transcription processes, which in turn
result in the expression of novel or uncovered ligands on the tumor
cell surface.
Recently, Ivanenkov et al (12) used phage display to identify a novel
peptide that showed a high affinity to HEp-2 human epithelial
cells, but no affinity to other types of cells. Subsequently,
peptide sequences with unique cell-type specificities have been
reported. However, little attention has been focused on the
potential uses of these peptides (13–15).
We hypothesized that tumor-targeting efficiency may
be greatly improved with the availability of a cell membrane
transduction peptides that are able to bind to tumor-specific
receptors and provide a higher tumor cell internalization rate.
This strategy may provide a novel addition to current antitumor
approaches. In the present study, using phage display technology
(16,17), we attempted to select a
tumor-targeting peptide with high cell specificity and delivery
capacity. In order to study the transmembrane transduction
mechanism of the peptide, the peptide was synthesized and labeled
with fluorescein isothiocyanate (FITC) green fluorescence at its
N-terminus. The association of the specific internalization of the
peptide into MDA-MB-231 cells with macropinocytosis and caveolin-
and clathrin-mediated endocytosis was investigated. Our previous
study found that a lung cancer cell line, Calu-1, also demonstrated
an affinity for the peptide, similarly to the human breast cancer
cell line MDA-MB-231 (18); thus, the
present study investigated the hypothesis that major
histocompatibility complex class I (MHC-I) antigen molecules and
the cytomembrane proteins of these two cell lines may also be
candidate proteins that are involved in the process of
transmembrane transduction of PI. MHC-I antigen-mediated
transmembrane transduction was investigated, and the membrane
proteins of MDA-MB-231 and Calu-1 were extracted and compared by
two-dimensional (2-D) electrophoresis (19) to identify those that were common to
both cell lines and may be involved in the process of transmembrane
transduction of PI. Further, to investigate the delivery efficiency
of PI to specific cancer cells, PI-glutathione-S-transferase (GST)
was constructed and the internalization of the fusion protein was
visualized by immunofluorescence microscopy.
Materials and methods
Chemicals and reagents
The pC89 phage display library of random peptides
was provided by Dr Alessandra Luzzago (Integrated Research Biotech
Model, Rome, Italy). The RGD-integrin was supplied by Dr Peter J.
Stambrook (Department of Cell Biology, Neurobiology, and Anatomy,
Vontz Center for Molecular Studies, College of Medicine, University
of Cincinnati, Cincinnati, OH, USA). Escherichia coli BL21
(DE3) were provided by Invitrogen (Thermo Fisher Scientific,
Waltham, MA, USA). The plasmid pGEX-2T was maintained in the Key
Laboratory of Translational Medicine of Cell Therapy Technology of
Yunnan Province (Department of Internal Medicine Oncology, First
Affiliated Hospital of Kunming Medical University, Kunming, Yunnan,
China). The GST agarose affinity chromatography column was
purchased from GE Healthcare Life Sciences (Tokyo, Japan). The
mouse anti-Schistosoma japonicum GST monoclonal antibody was
provided by Thermo Fisher Scientific (catalog no., MA4-004-1MG;
dilution, 1:500). Horseradish peroxidase (HRP)-labeled rabbit
anti-mouse IgG polyclonal antibody was also supplied by Thermo
Fisher Scientific (catalog no., 61-6520; dilution, 1:1,000).
ProteoPrep® Membrane Extraction Kit and sodium dodecyl
sulfate (SDS) were products of Sigma-Aldrich (St. Louis, MO, USA).
Polyclonal mouse anti-human MHC-I antibody (catalog no., ab76795;
dilution, 1:500) was purchased from Abcam (Cambridge, MA, USA).
RPMI-1640 medium, Dulbecco's modified Eagle's medium and fetal
bovine serum were purchased from Gibco (Thermo Fisher Scientific).
The human breast cancer cell lines MDA-MB-231, MDA-MB-435 and
MCF-7, and all other tumor cell lines (HeLa, A431, SCC-29, Calu-1,
Calu-3, GLC and U251) were purchased from the American Type Culture
Collection.
Peptide synthesis
Using the manual solid-phase Fmoc method, PI
(sequence, CASPSGALRSC) (18) was
synthesized to determine whether the phage-coating protein was
required for internalization into target cells. For cellular
localization, the synthesized peptide was labeled with FITC at the
N-terminus of PI (designated PI-FITC). Purification of the crude
product was applied by reverse phase high-performance liquid
chromatography (Agilent Technologies, Santa Clara, CA, USA), and
identification of chemical structures was conducted by
matrix-assisted laser desorption/ionization time-of-flight mass
spectrometry (JMS-S3000; Bruker Daltonic, Inc., Billerica, MA,
USA).
Cell culture
MDA-MB-231 cells were cultured in Leibovitz's L-15
Medium (Gibco; Thermo Fisher Scientific) containing 100 ml/l fetal
bovine serum and 50 ml/l CO2 at 37°C. Calu-3 cells were
cultured in Eagle's Minimum Essential Medium (American Type Culture
Collection, Manassas, VA, USA) containing 150 ml/l fetal bovine
serum at 37°C in 50 ml/l CO2. MDA-MB-435, HeLa, U251,
MCF-7, SCC-29 and GLC cells were cultured and maintained in
RPMI-1640 (Gibco; Thermo Fisher Scientific) supplemented with 100
ml/l fetal bovine serum at 37°C in 50 ml/l CO2. A431
cells were cultured in DMEM-H (Gibco; Thermo Fisher Scientific)
containing 150 ml/l fetal bovine serum and 50 ml/l CO2
at 37°C. Calu-1 cells were cultured in McCoy's 5A (modified) medium
(Gibco; Thermo Fisher Scientific) containing 100 ml/l fetal bovine
serum and 50ml/l CO2 at 37°C.
Cell-type affinity assay of
PI-FITC
MDA-MB-231 cells in the logarithmic phase were grown
in a 96-well tissue culture plate for 12 h, and cells were treated
with PI-FITC at concentrations of 200, 500 and 1,000 ng/ml.
RGD-integrin labeled with FITC was used as a control. The ability
of PI-FITC to internalize into other breast cancer cells
(MDA-MB-435 and MCF-7) and other solid tumor cells (HeLa, A431,
SCC-29, Calu-3, GLC and U251) in the logarithmic phase was also
tested by laser scanning confocal microscopy. For microscopy, the
MDA-MB-231, MCF-7, HeLa, A431, SCC-29, Calu-3, GLC and U251 cells
were treated with PI-FITC and RGD-integrin separately at
concentrations of 200, 500 or 1,000 ng/ml. The duration of
incubation with PI-FITC and RGD-integrin was 12 h. In this
experiment, RGD-integrin was also labeled with FITC. Green
fluorescence signals of FITC were observed by scanning confocal
microscopy (Zeiss LSM 800; Carl Zeiss AG, Oberkochen, Germany).
Concentration-, temperature- and
time-dependence of PI internalization to MDA-MB-231 cells
As it is possible that the affinity of PI-FITC for
MDA-MB-231 may be influenced by varying PI-FITC concentrations,
incubation times and temperatures, experiments were conducted to
investigate the effects of these parameters. For the
concentration-dependence experiment, 5×104 MDA-MB-231
cells in the logarithmic phase were grown in 24-well tissue culture
plates and divided into four subgroups; 0, 2, 5 or 10 µmol/l of
PI-FITC was added to each subgroup, respectively. Following 1 h of
incubation, cells were harvested, washed in phosphate-buffered
saline (PBS; Beijing Jimei Biotechnology Co., Ltd., Beijing,
China), fixed in formaldehyde (Beijing Jimei Biotechnology Co.,
Ltd.) and permeabilized with Triton X-100 (Sigma-Aldrich). The
PI-FITC distribution of each subgroup was observed by flow
cytometry (FACSCanto II; catalog no., 338960; BD Biosciences,
Franklin Lakes, NJ, USA).
For the temperature-dependence experiment,
5×104 MDA-MB-231 cells in the logarithmic phase were
grown in three 24-well tissue culture plates, 2 µmol/l of PI-FITC
was added to each plate, and the plates were incubated for 1 h at
temperatures of 4, 25 or 37°C, respectively. Following incubation,
flow cytometry was used to observe the results. MDA-MB-231 cells
without PI-FITC were used as a control.
In the time-dependence experiment, 5×104
MDA-MB-231 cells in the logarithmic phase were grown in three
24-well tissue culture plates, 2 µmol/l of PI-FITC was added to
each plate, and the plates were incubated for 1, 6 or 12 h. A plate
containing MDA-MB-231 cells without PI-FITC was used as a control,
and flow cytometry was applied to analyze the results.
Transmembrane transduction inhibition
analysis
To investigate whether the transmembrane
transduction mechanism of PI was associated with macropinocytosis
or caveolin- or clathrin-mediated endocytosis, three inhibitors of
cytomembrane transport, consisting of amiloride (Sanofi China,
Hangzhou, China) (19),
methyl-β-cyclodextrin (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) (20) and chlorpromazine
(Amresco, Cleveland, OH, USA) (21)
were incubated with MDA-MB-231 cells. An MTT assay (Sigma-Aldrich)
was used to determine the toxicity of these membrane channel
inhibitors to MDA-MB-231 cells.
For each experiment, 5×104 MDA-MB-231
cells in the logarithmic growth phase were grown in 24-well tissue
culture plates and equally divided into three subgroups, designated
A, B and C; cells in each subgroup were treated with PI-FITC,
PI-FITC plus an inhibitor (amiloride, methyl-β-cyclodextrin or
chlorpromazine), or normal saline, respectively. The distribution
of PI-FITC in MDA-MB-231 cells in each experimental group was
subsequently investigated by flow cytometry.
MHC-I antigen analysis
There are 250,000 molecules of each type of human
leukocyte antigen (HLA) on the surface of each human cell (22). Our preliminary studies indicated that
the human lung cancer cell line Calu-1 had high affinity to PI,
similarly to MDA-MB-231 cells. Therefore, the present study
compared the MHC-I antigen molecules of these two cell lines and
further analyzed whether the MHC-I antigen molecules may be
involved in transmembrane transduction. PI-FITC was added to the
experimental cultural system of MDA-MB-231 cells and positive
cultural system of Calu-1 cells, and both were incubated with an
anti-MHC-I antibody. After the MHC-I molecules on the cell surfaces
were blocked by the anti-MHC-I antibody, the distributions of
PI-FITC in these two cell types were detected under fluorescence
microscopy (DM-IL; Leica, Wetzlar, Germany). DNA from MDA-MB-231
and Calu-1 cells were extracted using the QIAamp DNA Mini Kit
(Qiagen, Hilden, Germany), according to the manufacturer's
protocol. HLA-A and -B DNA fragments were amplified and
sequence-specific primer (SSP)-polymerase chain reaction (PCR) was
applied to analyze the MHC-I antigen molecules. The MHC-I antigen
molecules were analyzed using the AB/DR/DQ SSP UniTray-96 kit from
Texas BioGene, Inc. (Richardson, TX, USA). PCR was performed for 21
cycles as follows: Denaturation at 96°C for 25 sec; annealing at
65°C for 50 sec; and extension at 72°C for 45 sec. Extension was
then performed at 72°C for another 10 min. Separation and
determination of PCR products were conducted by electrophoresis.
The ProFlex PCR machine was provided by Applied Biosystems (Thermo
Fisher Scientific).
Membrane protein analysis
To investigate whether the transmembrane
transduction mechanism of PI is associated with the same membrane
proteins in the MDA-MB-231 and Calu-1 cell lines, the membrane
proteins were extracted from the two types of cell using the
ProteoPrep Membrane Extraction Kit, according to the instructions,
and were compared by 2-D electrophoresis (23). The protein profiles of the two cell
lines were analyzed by PDQuest Software for 2-D Gel Analysis,
version 7.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA), with
the help of the Institute of Medical Biology, Chinese Academy of
Medical Sciences (Beijing, China).
Construction and expression of the
recombinant GST fusion protein PI-GST
The encoding region
(5′-TGCGCATCCCCATCTGGCGCCCTTCGTTGTTGC-3′) of PI was synthesized
with the addition of a BamHI site upstream and an
EcoRI site downstream by Takara Bio Inc. (Otsu, Japan). The
synthesized product was ligated into the pGEX-2T plasmid at the
BamHI and EcoRI sites. The insert, cut with the
restriction enzymes (Thermo Fisher Scientific), was subject to
SDS-polyacrylamide gel electrophoresis (PAGE) and was identified by
DNA sequence analysis. The Escherichia coli strain BL21
(DE3) was transformed with the recombinant plasmid pGEX-2T-PI or
vector alone using the CaCl2 transformation method
(24) and then grown in a lysogeny
broth solution (Sigma-Aldrich) containing 100 mg/ml of ampicillin
(Amresco) at 37°C. Isopropyl-β-D-thiogalactopyranoside (IPTG;
Amresco), an inducer of β-galactosidase activity in bacteria, was
added to a final concentration of 1 mM, and the solution was
incubated at 37°C for 1–6 h. Following IPTG induction, bacterial
pellets were obtained by centrifugation at 2,000 × g at 4°C.
Western blot analysis of PI-GST
Fusion protein products expressed in bacterial
pellets were released by ultrasonic membrane rupture and
centrifuged at 5,000 × g at 4°C. The supernatant was collected and
purified by GST affinity column. One-dimensional SDS-PAGE was
performed using 12.5% (wt/vol) polyacrylamide gels. For
immunoblotting, proteins were transferred from polyacrylamide gels
to Immobilon™ polyvinylidene difluoride membranes (Sigma-Aldrich)
using Tris-glycine electroblotting buffer (Sigma-Aldrich) at 15–20
mA overnight. The membranes were blocked with 5% evaporated skimmed
milk for 30 min at 37°C to prevent non-specific binding. The
membranes were then incubated with the primary mouse anti-GST
monoclonal antibody (dilution, 1:500) overnight at 4°C and with the
secondary HRP-conjugated goat anti-mouse IgG (dilution, 1:1,000)
for 30 min at 37°C. The expressed product was identified by
treating with dextran sulfate and 4-methylbenzamide for ~5 min.
Purification of PI-GST
The fused protein products in the supernatant
fraction were purified on GST-sepharose columns. Purification was
conducted as described by the manufacturer. Briefly, 100 ml of
sample was centrifuged to remove any undissolved membranes and
cellular debris before being added to the column. Triton X-100 was
then added to the collected supernatants. The column was washed
with 5–10 bed volumes of PBS to remove azide. The gel bed was
equilibrated with 3–5 bed volumes of PBS containing 1% Triton
X-100. Subsequently, the sample was added to the prepared column.
The flow-through was collected as a control. The column was washed
with 10 bed volumes of PBS until no protein could be detected in
the elution. The setup included sufficient Eppendorf tubes for a
standard curve with 0, 5, 10, 15, 20 and 25 g of bovine serum
albumin (BSA) and the samples to be tested. The harvest was subject
to western blotting for identification, as described.
Transduction activity of fusion
protein
MDA-MB-231 cells were grown in 24-well tissue
culture plates at 37°C for 12 h. Cells were incubated with PI-GST
at 20, 50, 100, 200 or 500 ng/ml for 8–12 h at 37°C. The cultured
cells were fixed in 10% Triton X-100 for 10 min and treated with
buffer (1% BSA, 0.025% NaN3, 0.1% saponin) for 15 min.
The GST monoclonal antibody (dilution, 1:800) was added to the
cells for 30 min at 4°C. The cells were then incubated with
HRP-labeled rabbit anti-mouse IgG antibody (dilution, 1:1,000) for
30 min at 37°C, subsequent to washing the cells 3 times.
Internalization was visualized by immunofluorescence microscopy
(DM-IL; Leica). MDA-MB-231 cells incubated with GST were used as a
blank control.
Results
Internalization assay of synthesized
peptides in MDA-MB-231 and other cell lines
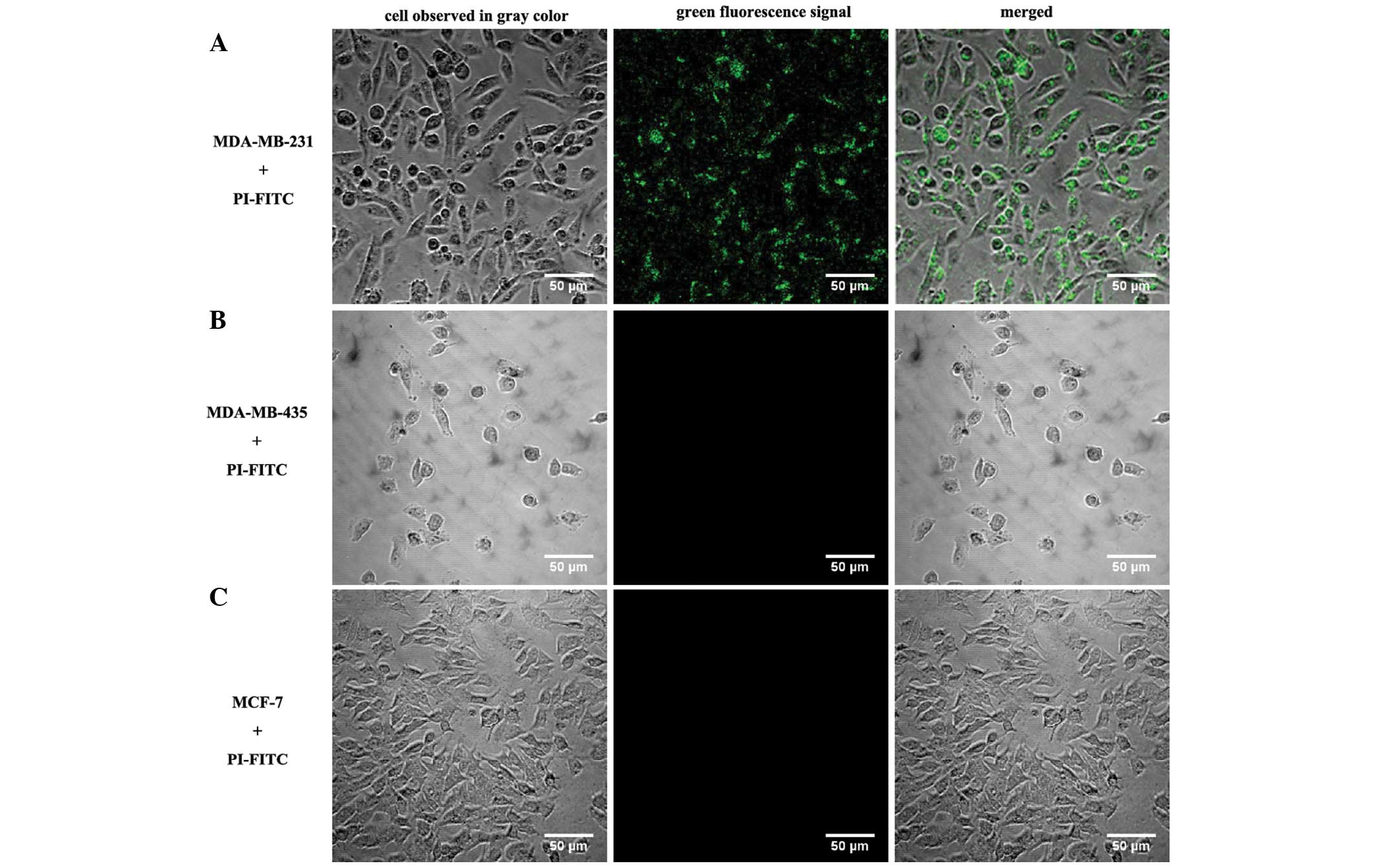
The green fluorescence signal of PI-FITC was easily
detectable in each MDA-MB-231 cell after 12–48 h of incubation,
indicating that PI-FITC was efficiently taken up by MDA-MB-231
cells; the internalized PI was predominantly located in the
cytoplasm or around the nuclear membrane. No green fluorescence
signal could be observed in the MDA-MB-231 cells without PI-FITC
(Fig. 1). Compared with RGD-integrin
labeled with FITC, the internalization activity of PI in MDA-MB-231
cells was similar (Fig. 2). In
contrast to MDA-MB-231 cells incubated with PI-FITC, no
fluorescence signal was observed in the other breast cancer cell
lines, MDA-MB-435 and MCF-7 (Fig. 3),
and the synthesized peptides exhibited no affinity to the other
types of tumor cell, HeLa, A431, SCC-29, Calu-3, GLC and U251
(Table I).
 | Table I.Results of an internalization assay
of the synthesized peptide, PI, into various cell lines. |
Table I.
Results of an internalization assay
of the synthesized peptide, PI, into various cell lines.
| Cell line | Fluorescence signal
of PI-FITC |
|---|
| MDA-MB-231 | + |
| MDA-MB-435 | − |
| MCF-7 | − |
| Hela | − |
| A431 | − |
| SCC-29 | − |
| Calu3 | − |
| GLC | − |
| U251 | − |
Concentration-, temperature- and
time-dependence of PI internalization to MDA-MB-231
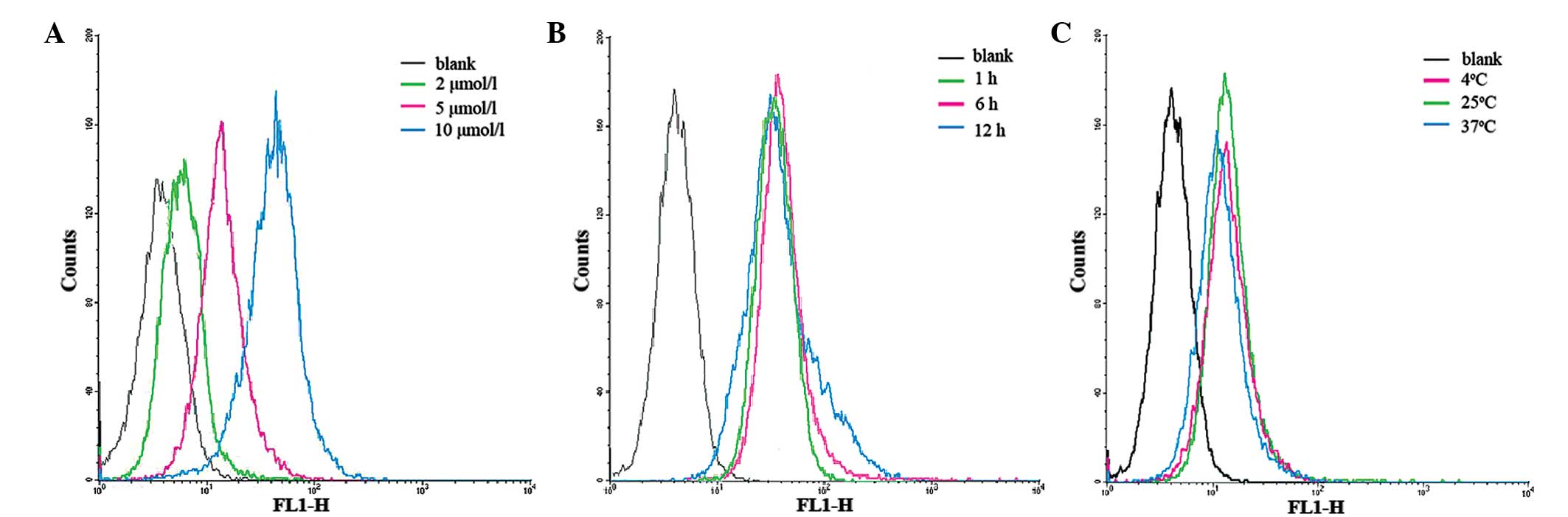
Flow cytometry results revealed an increase in
cell-associated fluorescence with increasing concentration of PI,
indicating that the concentration of PI is associated with its
internalization (Fig. 4A); both the
number of MDA-MB-231 cells and the concentration of PI-FITC were
influencing factors on the internalization of PI-FITC. However, the
incubation temperature and time had little influence on the
internalization of PI (Fig. 4B and
C)
Membrane channel inhibition
experiments
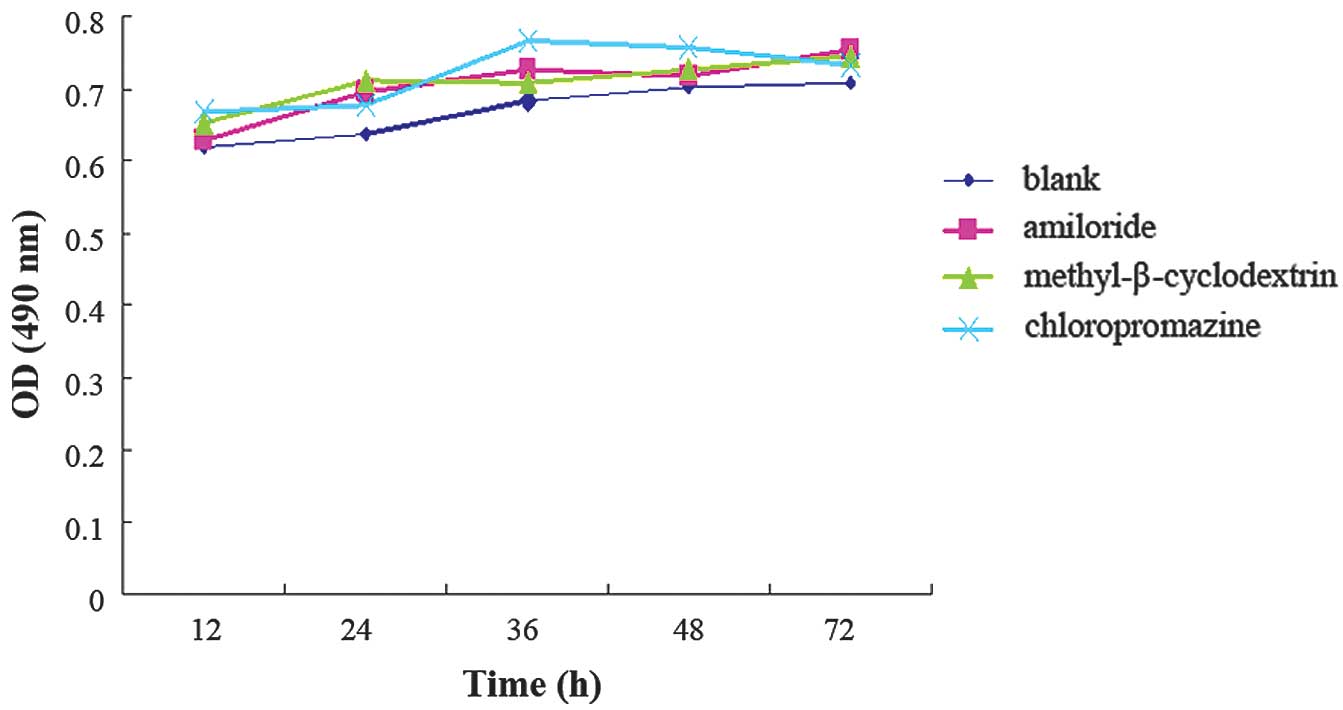
Following the addition of amiloride,
methyl-β-cyclodextrin and chlorpromazine to the MDA-MB-231 cells,
MTT results indicated that these three channel inhibitors had no
significant toxicity to the growth of MDA-MB-231 cells (Fig. 5). In the amiloride and
methyl-β-cyclodextrin experimental groups, flow cytometry results
suggested that the distribution of PI-FITC in MDA-MB-231 cells of
each group had decreased (Fig. 6A and
B). However, in the chlorpromazine group, no significant change
in intracellular distribution was observed (Fig. 6C).
MHC-I antigen analysis
After the MHC-I antigen had been blocked in the
MDA-MB-231+PI-FITC and Calu-1+PI-FITC culture systems, the
distributions of PI-FITC in the cells of the two culture systems
were consistent with the control groups without MHC-I antibody. DNA
samples of MDA-MB-231 cells and Calu-1 cells were detected by
PCR-SSP. The results revealed that none of the same MHC-I antigen
molecules could be detected. This indicated that the MHC-I antigen
molecules of the two cell lines may not be involved in the process
of transmembrane transduction of PI (Table II).
 | Table II.Analysis of the alleles of HLA-I in
the MDA-MB-231 and Calu-1 cell lines. |
Table II.
Analysis of the alleles of HLA-I in
the MDA-MB-231 and Calu-1 cell lines.
| MDA-MB-231 | Calu-1 |
|---|
|
|
|---|
| HLA-A | HLA-B | HLA-A | HLA-B |
|---|
| A*02:01 | B*41:01 | A*26:01 | B*44:03 |
| A*02:02 | B*41:03 | A*26:02 | B*44:04 |
| A*02:03 | B*41:05 | A*26:08 | B*44:05 |
| A*24:02 | B*40:02 | A*29:01 | B*15:01 |
| A*24:03 | B*40:03 | A*29:02 | B*15:04 |
| A*24:04 | B*40:04 | A*29:03 | B*15:27 |
Membrane protein comparison by 2-D
electrophoresis map
Following the extraction of membrane proteins from
the two cell lines, 2-D electrophoresis of each sample was repeated
four times, and the protein profiles of the two cell types were
analyzed by PDQuest Software for 2-D Gel Analysis. Around 260
protein spots of each sample were detected, and a total of 11
common protein spots were identified (Fig. 7).
Expression of PI-GST
Expression of PI-GST and GST was induced by IPTG,
and crude bacterial extracts were analyzed by SDS-PAGE. Two
constructs produced protein of the expected molecular mass, 26–27
kDa (Fig. 8). Western blot analysis
of the crude extract indicated that the fusion protein reacted with
an antibody against GST. All data demonstrated that the GST-fused
protein PI-GST was present.
Delivery efficiency of fusion protein
into target cells
In the presence of increasing concentrations (20–500
ng/ml) of the PI-GST fusion protein for 72 h at 37°C, cellular
viability was 95%. Cellular toxicity was not observed in the
experimental cells. At 200 ng/ml, the optimal concentration of the
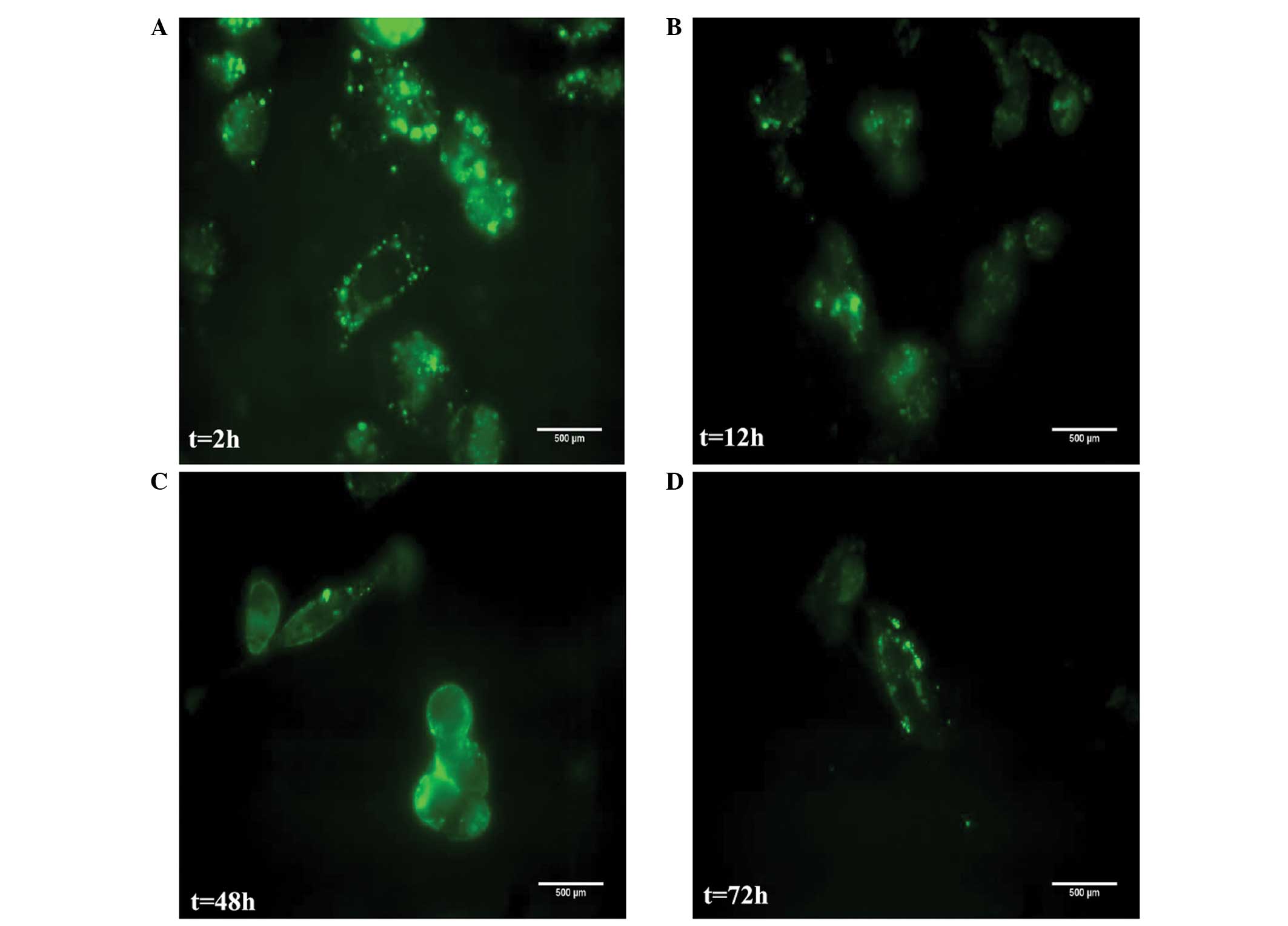
peptide PI-GST was taken up by MDA-MB-231 cells efficiently. In the
initial 12–18 h culturing period, the fluorescence intensity of
PI-GST in MDA-MB-231 cells was as strong as that observed for PI
itself (Fig. 9A); a strong
intracellular PI-GST signal was observed in the cytoplasm after 12
h (Fig. 9B). After 48–72 h, the
signal of the fusion protein in the majority of the cells became
weak and disappeared (Fig. 9C and
D).
Discussion
Breast cancer is the most commonly diagnosed cancer
in women and is the second leading cause of cancer-associated
mortality (25). The risks of local
recurrence, metastasis and drug resistance of tumors remain
difficult to overcome and control (26).
Recent targeted and biological therapies reflect
novel and promising improvements in the treatment of breast cancer;
for example, drugs including trastuzumab, bevacizumab, gefitinib
and celecoxib have been designed to focus on identified
tumor-associated targets and have demonstrated promising results in
clinical practice (27–32). However, these therapeutic targets are
expressed in numerous tissues, suggesting that the cellular
specificity is extremely low and may cause potential side effects
(33,34). Therefore, the development of agents
with cancer cell selectivity is urgently required to improve
targeted therapy.
It has been reported that use of the phage display
system allows selection of peptide sequences with unique cell-type
specificity (35,36). We hypothesized that a breast
cancer-specific peptide may also be screened through the phage
display system. The strategy adopted for the current study was to
screen a breast cancer-specific peptide, evaluate whether it has
potential as a specific delivery system for tumor-targeting
therapy, and investigate the delivery mechanism.
The synthesized PI peptide (CASPSGALRSC) was
incubated with MDA-MB-231 cells in order to investigate it as a
peptide with tumor cell specificity. To improve comparability,
RGD-integrin, which is known to be recognized and internalized by
various types of human cells (37,38), was
set as a control. The synthesized PI exhibited no affinity to other
cancer cell lines MDA-MB-435, MCF-7, HeLa, A431, SCC-29, Calu-3,
GLC and U251. These affinity experiments suggested that PI was a
novel type of transmembrane peptide with MDA-MB-231 cell
specificity. Moreover, PI has no sequence similarity to
RGD-integrin or to any other protein sequence available in various
protein databases.
To further analyze the transduction mechanism of PI,
three inhibitors of cytomembrane transport (amiloride,
methyl-β-cyclodextrin and chlorpromazine) were used to investigate
whether the specific internalization of the peptide into MDA-MB-231
cells is associated with macropinocytosis, caveolin-mediated
endocytosis or clathrin-mediated endocytosis, using flow cytometry.
The results revealed that the transduction of the peptide into the
cell is partially mediated by macropinocytosis and
caveolin-mediated endocytosis. Our initial study established that
the lung cancer cell line Calu-1 also demonstrated high affinity to
the peptide, similarly to MDA-MB-231 (39). Thus, we hypothesized that MHC-I
antigen molecules may mediate transmembrane transduction, and the
cytomembrane proteins of the two cell lines may be involved in the
process of transmembrane transduction of PI. In the present study,
MHC-I antigen molecules and the cytomembrane proteins of the two
cell lines were investigated. The results revealed that MHC-I
antigen molecules of MDA-MB-231 and Calu-1 share no common alleles,
which indicated that these MHC-I antigen molecules have no
association with the transmembrane transduction of PI. The results
of 2-D electrophoresis identified 11 proteins spots that were
common to MDA-MB-231 and Calu-1 cells, which may also be candidates
for the transmembrane transduction of PI.
Given the notable cellular specificity of PI, its
use as a vector for a therapeutic protein delivery may be a
practical way to improve targeting efficiency in tumor therapy. To
investigate the ability of PI to deliver a potential therapeutic
protein, GST was introduced to represent an exogenous protein. GST
was fused with PI and was used as a marker to assess the protein
transduction ability of PI in vitro. PI was observed to be
capable of successfully delivering GST into the cytoplasm of
MDA-MB-231 cells, and the exogenous protein did not degrade for ≥72
h. Significant immunofluorescence signals for PI-GST were observed
in the MDA-MB-231 cell cytoplasm, while no GST signal was observed
in MDA-MB-231 cells. Thus, it may be concluded that PI is able to
deliver an exogenous protein of at least 26 kDa into MDA-MB-231
cells. This transduction procedure is also cell-specific, which was
confirmed by treating different human breast cancer cell lines with
PI-GST. Additionally, PI and PI-GST did not lead to any cytotoxic
effects when the cells were maintained in culture medium for 72 h.
Thus, the current research shows that PI has the necessary features
to be an efficient drug delivery vector for targeted cancer
therapy.
Taken together, our studies may lead to the
discovery of a novel tumor cell-specific receptor and provide a new
therapeutic target for cancer treatment and diagnosis (40). Such a peptide may serve as a specific
vehicle for tumor-targeting therapy and may complement other
molecular therapeutic approaches for localized and systemic
application. Further research is required in this area.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (no. 30860330), Science and Technology
Platform Construction Project of Yunnan Province (no. 2007DA006)
and Applied Basic Research Project of Yunnan Province (no.
2009CC023).
References
|
1
|
Cooke SP, Boxer GM, Lawrence L, Pedley RB,
Spencer DI, Begent RH and Chester KA: A strategy for antitumor
vascular therapy by targeting the vascular endothelial growth
factor: Receptor complex. Cancer Res. 61:3653–3659. 2001.PubMed/NCBI
|
|
2
|
Miller CR, Buchsbaum DJ, Reynolds PN,
Douglas JT, Gillespie GY, Mayo MS, Raben D and Curiel DT:
Differential susceptibility of primary and established human glioma
cells to adenovirus infection: Targeting via the epidermal growth
factor receptor achieves fiber receptor-independent gene transfer.
Cancer Res. 58:5738–5748. 1998.PubMed/NCBI
|
|
3
|
Baselga J, Norton L, Albanell J, Kim YM
and Mendelsohn J: Recombinant humanized anti-HER2 antibody
(Herceptin) enhances the antitumor activity of paclitaxel and
doxorubicin against HER2 overexpressing human breast cancer
xenografts. Cancer Res. 58:2825–2831. 1998.PubMed/NCBI
|
|
4
|
Ruoslahti E and Pierschbacher MD: New
perspectives in cell adhesion: RGD and integrins. Science.
238:491–497. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frankel AD and Pabo CO: Cellular uptake of
the tat protein from human immunodeficiency virus. Cell.
55:1189–1193. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Green M and Loewenstein PM: Autonomous
functional domains of chemically synthesized human immunodeficiency
virus tat trans activator protein. Cell. 55:1179–1188. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elliott G and O'Hare P: Intercellular
trafficking and protein delivery by a herpesvirus structural
protein. Cell. 88:223–233. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muthumani K, Lambert VM, Shanmugam M,
Thieu KP, Choo AY, Chung JC, Satishchandran A, Kim JJ, Weiner DB
and Ugen KE: Anti-tumor activity mediated by protein and peptide
transduction of HIV viral protein R (Vpr). Cancer Biol Ther.
8:180–187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu CS, Kong B, Ma DX, Wang WX, Mq W and
Kao E: VP22 enhanced intercellular trafficking of HSV thymidine
kinase promoted an effective cell killing effect at lower
concentration of ganciclovir. Chin J Cancer Biother. 8:93–97.
2001.
|
|
10
|
Albarran B, To R and Stayton PS: A
TAT-streptavidin fusion protein directs uptake of biotinylated
cargo into mammalian cells. Protein Eng Des Sel. 18:147–152. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiong F, Xiao S, Peng F, Zheng H, Yu M,
Ruan Y, Li W, Shang Y, Zhao C, Zhou W, et al: Herpes simplex virus
VP22 enhances adenovirus-mediated microdystrophin gene transfer to
skeletal muscles in dystrophin-deficient (mdx) mice. Hum Gene Ther.
18:490–501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ivanenkov VV, Felici F and Menon AG:
Targeted delivery of multivalent phage display vectors into
mammalian cells. Biochim Biophys Acta. 1448:463–472. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwarze SR, Hruska KA and Dowdy SF:
Protein transduction: Unrestricted delivery into all cells? Trends
Cell Biol. 10:290–295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ho A, Schwarze SR, Mermelstein SJ, Waksman
G and Dowdy SF: Synthetic protein transduction domains: Enhanced
transduction potential in vitro and in vivo. Cancer Res.
61:474–477. 2001.PubMed/NCBI
|
|
15
|
Rajotte D and Ruoslahti E: Membrane
dipetidase is the receptor for a lung-targeting peptide dentified
by in vivo phage display. J Biol Chem. 274:11593–11598. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dente L, Vetriani C, Zucconi A, Pelicci G,
Lanfrancone L, Pelicci PG and Cesareni G: Modified phage peptide
libraries as a tool to study specificity of phosphorylation and
recognition of tyrosine containing peptide. J Mol Biol.
269:694–703. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu F, Guo XR, Gong HX, Ni YH, Fei L, Pan
XQ, Guo M and Chen RH: A resistin binding peptide selected by phage
display inhibits 3T3-L1 preadipocyte differentiation. Chin Med J
(Engl). 119:496–503. 2006.PubMed/NCBI
|
|
18
|
Pires D, Bemquerer M and Nascimento C:
Some mechanistic aspects on Fmoc solid phase peptide synthesis. Int
J Pept Res Ther. 20:53–69. 2014. View Article : Google Scholar
|
|
19
|
West MA, Bretscher MS and Watts C:
Distinct endocytotic pathways in epidermal growth factor-stimulated
human carcinoma A431 cells. J Cell Biol. 109:2731–2739. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Furuchi T and Anderson RG: Cholesterol
depletion of caveolae causes hyperactivation of extracellular
signal-related kinase (ERK). J Biol Chem. 273:21099–21104. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Subtil A, Hémar A and Dautry-Varsat A:
Rapid endocytosis of interleukin 2 receptors when clathrin-coated
pit endocytosis is inhibited. J Cell Sci. 107:3461–3468.
1994.PubMed/NCBI
|
|
22
|
Garcia-Lora A, Algarra I and Garrido F:
MHC class I antigens, immune surveillance and tumor immune escape.
J Cell Physiol. 195:346–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adessi C, Miege C, Albrieux C and
Rabilloud T: Two-dimensional electrophoresis of membrane proteins:
A current challenge for immobilized pH gradients. Electrophoresis.
18:127–135. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huebener N, Lange B, Lemmel C, Rammensee
HG, Strandsby A, Wenkel J, Jikai J, Zeng Y, Gaedicke G and Lode HN:
Vaccination with minigenes encoding for novel ‘self’ antigens are
effective in DNA-vaccination against neuroblastoma. Cancer Lett.
197:211–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dean-Colomb W and Esteva FJ: Her2-positive
breast cancer: Herceptin and beyond. Eur J Cancer. 44:2806–2812.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davoli A, Hocevar BA and Brown TL:
Progression and treatment of HER2-positive breast cancer. Cancer
Chemother Pharmacol. 65:611–623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fogelman DR, Kopetz S and Eng C: Emerging
drugs for colorectal cancer. Expert Opin Emerg Drugs. 13:629–642.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim R and Toge T: Changes in therapy for
solid tumors: Potential for overcoming drug resistance in vivo with
molecular targeting agents. Surg Today. 34:293–303. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ran JT, Zhou YN, Tang CW, Lu JR, Wu J, Lu
H and Yang GD: Celecoxib induces apoptosis and inhibites
angiogenesis in gastric cancer. Zhonghua Zhong Liu Za Zhi.
30:448–451. 2008.(In Chinese). PubMed/NCBI
|
|
32
|
Ortega J, Vigil CE and Chodkiewicz C:
Current progress in targeted therapy for colorectal cancer. Cancer
Control. 17:7–15. 2010.PubMed/NCBI
|
|
33
|
Mailliez A, Baldini C, Van JT, Servent V,
Mallet Y and Bonneterre J: Nasal septum perforation: A side effect
of bevacizumab chemotherapy in breast cancer patients. Br J Cancer.
103:772–775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takeda A, Loveman E, Harris P, Hartwell D
and Welch K: Time to full publication of studies of anti-cancer
medicines for breast cancer and the potential for publication bias:
A short systematic review. Health Technol Assess. 12:1–46. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brown KC: Peptidic tumor targeting agents:
The road from phage display peptide selections to clinical
applications. Curr Pharm Des. 16:1040–1054. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Laakkonen P and Vuorinen K: Homing
peptides as targeted delivery vehicles. Integr Biol (Camb).
2:326–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miller WH, Alberts DP, Bhatnagar PK,
Bondinell WE, Callahan JF, Calvo RR, Cousins RD, Erhard KF,
Heerding DA, Keenan RM, et al: Discovery of orally active
nonpeptide vitronectin receptor antagonists based on a
2-benzazepine gly-Asp mimetic. J Med Chem. 43:22–26. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao H, Wang JC, Sun QS, Luo CL and Zhang
Q: RGD-based strategies for improving antitumor activity of
paclitaxel-loaded liposomes in nude mice xenografted with human
ovarian cancer. J Drug Target. 17:10–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dong J, Liu WQ, Jiang AM, Zhang KJ and
Chen MQ: A novel peptide, selected from phage display library of
random peptides, can efficiently target into human breast cancer
cell. Chinese Science Bulletin. 53:860–867. 2008.
|
|
40
|
Zitzmann S, Krämer S, Mier W, Hebling U,
Altmann A, Rother A, Berndorff D, Eisenhut M and Haberkorn U:
Identification and evaluation of a new tumor cell-binding peptide,
FROP-1. J Nucl Med. 48:965–972. 2007. View Article : Google Scholar : PubMed/NCBI
|