Introduction
Thyroid cancer (TC) is one of the most common
endocrine malignancies (1), and its
incidence has significantly increased in recent years (2). Thyroid tumors are usually classified
into four subtypes [papillary TC, follicular TC, anaplastic TC and
medullary TC (MTC) (3)], according to
their histopathological characteristics, and treatments are
selected depending on the subtype and stage of the tumor (4). MTC is a malignancy of the parafollicular
cells (also called C cells), which accounts for ≤10% of all thyroid
tumors (5). The majority of MTCs are
sporadic (80%), while ~20% of cases are inherited as a germline
mutation in the rearranged during transfection proto-oncogene
(6–8).
Metastases occur in ~70% of patients with MTC who have a palpable
thyroid nodule (>1.0-cm diameter) (9). MTCs may present as an aggressive
malignancy with metastases to the liver, lungs, bones and
mediastinum (8,10,11). At
that stage of the disease, patients cannot undergo surgical
resection, and do not receive radioactive iodine. In consequence,
biochemical cure rates drop to ≤30% (12,13).
Surgical resection results in a recurrence rate of almost 50%
(6). Therefore, it is important to
develop novel therapies for the treatment of MTC.
Carcinogenesis is a progression of events resulting
from the accumulation of genetic alterations and the disruption of
epigenetic modifications, including epigenetic silencing of tumor
suppressor genes, which is a common event during carcinogenesis and
often involves aberrant DNA methylation and histone modifications
(14) M-phase phosphoprotein 8
(MPHOSPH8 or MPP8), which is also known as hybrid-associated
protein 3 with Ran-binding protein in the microtubule organizing
center (RanBPM) and human source MPP8, was originally identified in
the RanBPM complex (15). MPP8 was
identified as a novel M phase phosphoprotein using expression and
cloning by Matsumoto-Taniura et al (16) in 1996. MPP8 is capable of recognizing
the methylated lysine 9 of histone H3, and it couples histone H3 K9
methylation with the promotion of DNA methylation for the silencing
of tumor suppressor genes and induction of metastasis by recruiting
DNA (cytosine-5)-methyltransferase 3A to target CpG sites (17). MPP8 predominantly localizes at the
heterochromatin region during the interphase, and is important in
the organization of heterochromatin by regulating the interplay
between DNA methylation and histone H3 methylation (18,19). In
addition, MPP8 causes cells in the G2 phase of the cell cycle to
enter the M phase (20). Recently,
targeted therapies for TC have been developed (21,22), and
several potential drugs are currently in preclinical testing or in
clinical use (23). However, the lack
of systematic studies regarding the underlying molecular mechanisms
of TC may lead to a high risk for TC patients to suffer unexpected
side effects. A recent study has suggested that MPP8 may
participate in the progression of MTC, and may be considered a
potential biomarker (24). However,
the role of MPP8 in MTC remains unclear. The present study
conducted several experiments to investigate the role of MPP8 in
MTC cells using an RNAi-based knockdown method. The depletion of
MPP8 significantly inhibited the proliferation of TT cells and
arrested the cell cycle at the G0/G1 phase. These findings may
provide a novel insight into the treatment of MTC.
Materials and methods
Cell lines and cell culture
Human MTC TT cells and human embryonic kidney 293T
cells were obtained from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). TT cells were cultured in F-12K medium
(catalogue no. 21127022; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 20% fetal bovine serum (FBS; catalogue no.
10099-14; Gibco; Thermo Fisher Scientific, Inc.). 293T cells were
maintained in Dulbecco's modified Eagle's medium (catalogue no.
SH30243.01B+; HyClone; GE Healthcare Life Sciences, Chalfont, UK)
with 10% FBS (catalogue no. 04-001-1a-1351574; Biological
Industries, Cromwell, CT, USA). Both cell lines were maintained at
37°C in a humidified 5% CO2 atmosphere.
Construction of MPHOSPH8 small hairpin
(sh)RNA lentivirus vectors and virus packaging
The following stem-loop-stem oligos were designed
(Genechem, Shanghai, China) and cloned into the lentiviral
expression vector pGP (ShanghaiBio China, Shanghai, China), which
was digested with EcoRI and BamHI (Takara Biotechnology, Dalian,
China): MPHOSPH8 small hairpin (sh)RNA, S1
5′-GCTGTTTATCTTCCATGCAAACTCGAGTTTGCATGGAAGATAAACAGCTTTTT-3′ and S2
5′-CAGTGTCCAGACTGCGTATTTCTCGAGAAATACGCAGTCTGGACACTGTTTTT-3′; and
scramble shRNA
5′-GCGGAGGGTTTGAAAGAATATCTCGAGATATTCTTTCAAACCCTCCGCTTTTTT-3′, which
was used as control. The above plasmids Lv-sh MPHOSPH8 (S1 and S2)
and Lv-sh control were transformed into competent cells
(Escherichia coli strain DH5α; Solarbio, Beijing, China), and
extracted with a plasmid purification kit (Qiagen, Inc., Valencia,
CA, USA). Successful ligation was determined by polymerase chain
reaction (PCR) and sequencing analyses. The recombinant expression
shRNA vectors and packaging helper plasmids pVSVG-I and pCMVΔR8.92
(ShanghaiBio China) were next co-transfected into 293T cells.
Culture supernatants were harvested at 96 h post-transfection to
purify lentiviruses expressing MPHOSPH8 shRNA or control shRNA. The
lentiviruses were purified via ultracentrifugation at 400 × g for 10
min (MIKRO 200/200R; Andreas Hettich GmbH & Co. KG, Tuttlingen,
Germany), and their titer was measured by end point dilution
through counting the numbers of infected green fluorescent protein
(GFP)-positive cells at ×100 magnification under a fluorescence
microscope (Olympus, Tokyo, Japan). Titer (IU/ml) = (the numbers of
green fluorescent cells) × (dilution factor) / (volume of virus
solution). TT cells were infected with concentrated viruses at a
multiplicity of infection of 60, and mock-infected cells were used
as negative control. The infection efficiency was determined by
observing GFP-positive cells under fluorescence microscope 96 h
after infection. The efficiency of knocking down MPHOSPH8 was
subsequently evaluated by reverse transcription-quantitative PCR
(RT-qPCR) and western blot analyses.
RT-qPCR
TT cells were harvested following 7 days of
lentivirus infection, and total RNA extraction was performed using
TRIzol reagent (catalogue no. 15596-026; Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
purity and integrity of the RNA was assessed by spectrophotometry
using a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific,
Inc.) and 1% agarose gel electrophoresis, respectively. The agarose
gel electrophoresis was run in an electrophoresis tank with MOPS
buffer (Dojindo Laboratories, Shanghai, China) at 100 V for 30min,
and the results were observed under a ultraviolet lamp.
First-strand complementary DNA was synthesized from 2 µg total RNA
and the following PCR primers (Kangbeibio, Zhejiang, China):
MPHOSPH8, forward 5′-AGTTATTGCTCGGCTCTGTG-3′ and reverse
5′-CAGTCCCTTCTGTTTGGTCA-3′; and β-actin, forward
5′-GTGGACATCCGCAAAGAC-3′ and reverse 5′-AAAGGGTGTAACGCAACTA-3′.
RT-qPCR was performed in the linear range using SYBR®
Green PCR Core Reagents (Applied Biosystems; Thermo Fisher
Scientific, Inc.) on a CFX96 Touch™ Real-Time PCR Detection System
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The PCR cycling
conditions were as follows: Initial denaturation at 95°C for 60
sec, followed by 40 cycles of denaturation at 95°C for 5 sec, and
annealing and extension at 60°C for 20 sec. Data analysis was
performed using the 2−ΔΔCq method (25).
Western blotting
TT cells were harvested and washed twice with
ice-cold phosphate-buffered saline (PBS) following 7 days of
lentivirus infection. Then, cells were lysed in 2X sodium dodecyl
sulfate (SDS) sample buffer (100 mM Tris-HCl pH 6.8, 10 mM
ethylenediaminetetraacetic acid, 4% SDS and 10% glycine). The total
protein concentration in the cell lysate was quantified by BCA
Protein Assay Kit (catalogue no. 23235; Pierce; Thermo Fisher
Scientific, Inc.). A total of 30 µg cellular protein per lane was
resolved on 10% SDS-polyacrylamide gel electrophoresis and
transferred to a polyvinylidene fluoride membrane (catalogue no.
162-0177; Bio-Rad Laboratories, Inc.). The blots were probed
overnight at 4°C with primary rabbit anti-MPHOSPH8 (1:500;
catalogue no. HPA039701; Sigma-Aldrich, St. Louis, MO, USA) and
rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
1:100,000; catalogue no. 10494-1-AP; ProteinTech Group, Inc.,
Chicago, IL, USA) antibodies, and successively incubated with
horseradish peroxidase-conjugated goat anti-rabbit (1:5,000;
catalogue no. SC-2054; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) secondary antibody for 2 h at room temperature. GAPDH served
as internal standard. Signals were detected using the ECL Plus™ kit
(catalogue no. RPN2132; GE Healthcare Life Sciences). Images were
captured of the results (37X–V; Shanghai 5th optical factory,
Shanghai, China).
Growth curve determination by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The effect of MPHOSPH8 on cell viability was
determined based on growth curves of TT cells obtained by MTT
assay. TT cells were seeded at a density of 10,000 cells/well in
96-well plates at 96 h post-lentivirus infection. Cell growth was
examined by MTT assay once a day for 5 days. For that purpose, 20
µl MTT solution (5 mg/ml in PBS; Sigma-Aldrich) was added to each
well, followed by incubation for 4 h at 37°C. Then, 100 µl stop
buffer (0.01 M HCl, 10% SDS and 5% isopropanol; Sigma-Aldrich) was
added to each well, which was then gently agitated for 10 min,
prior to be analyzed on an Epoch Microplate Spectrophotometer
(BioTek Instruments, Inc., Winooski, VT, USA) at a wavelength of
595 nm.
Cell cycle analysis
Cell cycle analysis was performed with propidium
iodide (PI) staining (Sigma-Aldrich), following the manufacturer's
protocol. TT cells were seeded in 6-well plates at a density of
3×105 cells/well subsequent to 4 days of lentivirus
infection. The cell density was 50% after 72 h of culture, cells
were washed and resuspended in PBS containing 50 µg/ml RNase A
(Sigma-Aldrich), and next in cell cycle dying solution (50 µg/ml PI
and 50 µg/ml RNase A) at room temperature in the dark for 1 h.
Analysis of the cell cycle phase distribution was conducted on a
FACScan™system (BD Biosciences, Franklin Lakes, NJ, USA) using
ModFit LT 3.2 software (Verity Software House, Topsham, ME,
USA).
Apoptosis analysis by Annexin V
staining
To identify apoptotic cells, the Annexin V-APC/7-AAD
Apoptosis Detection Kit (catalogue no. KGA1026; Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China) was used. TT cells were seeded
in 6-well plates at a density of 3×105 cells/well
following 4 days of lentivirus infection. Upon 48 h of culture,
cells were harvested and stained according to the manufacturer's
protocol. The cells were analyzed on a FACSCalibur™ (BD
Biosciences) using CellQuest Pro 5.1 software (BD Biosciences). The
percentage of cells in each quadrant was calculated.
Statistical analysis
The results were expressed as the mean ± standard
deviation. Differences between two groups were assessed using a
two-tailed t-test. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed with SPSS version 13.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
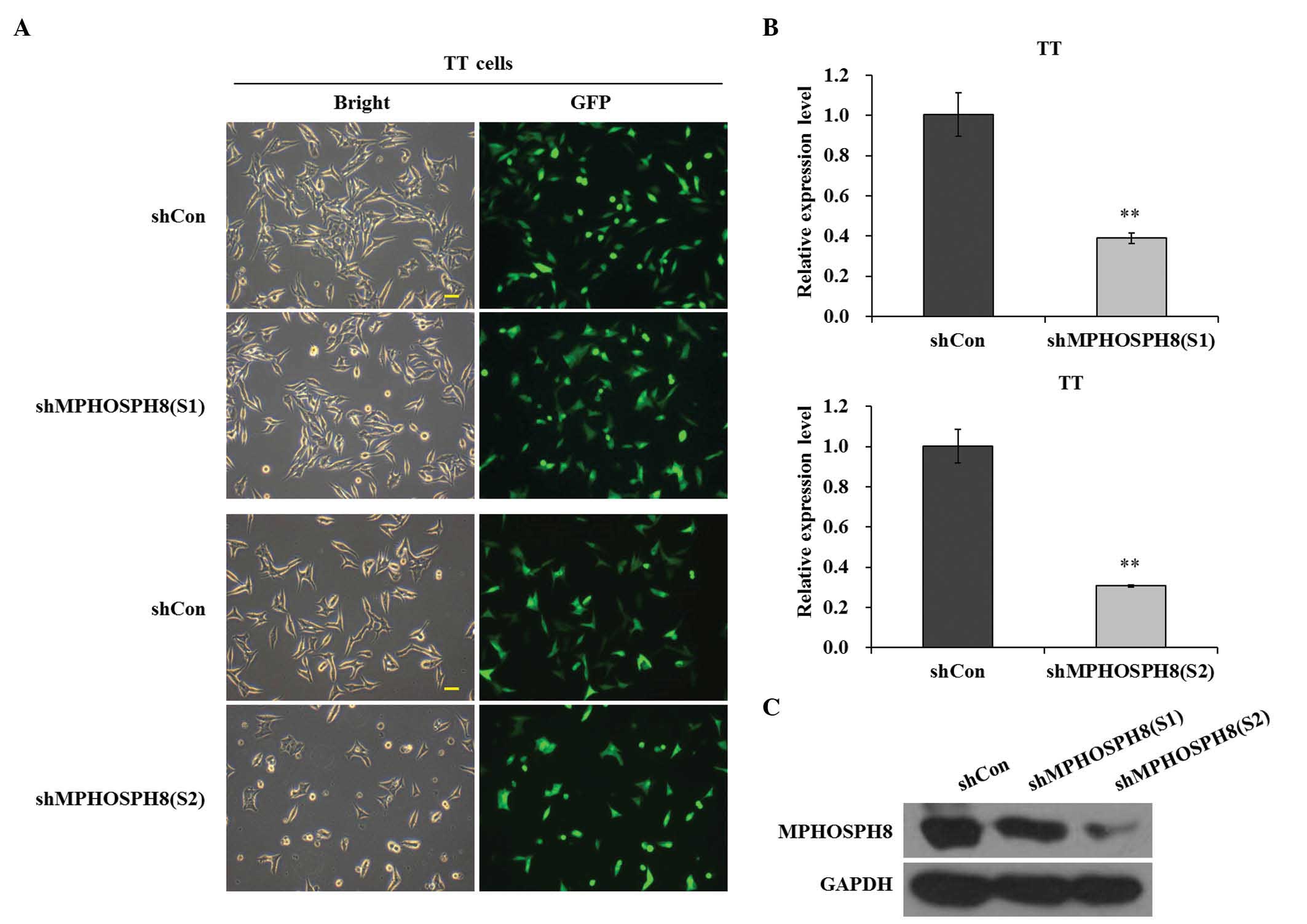
Lv-sh MPHOSPH8 strongly suppressed
MPHOSPH8 expression in TT cells
To explore the role of MPHOSPH8 in human MTC,
lentivirus-mediated shRNA was used to silence the expression of
MPHOSPH8 in TT cells. GFP was as used as a reporter gene. Lv-sh
MPHOSPH8 (S1 and S2) was successfully infected into TT cells, since
>61.2 and 69.4% cells, respectively, were GFP-positive under
fluorescence microscopy at 96 h post-infection (Fig. 1A). RT-qPCR revealed that the
lentiviruses containing S1 and S2 led to notable suppression of
MPHOSPH8 expression (P<0.01), compared with the Lv-sh control
group (Fig. 1B). In addition, Lv-sh
MPHOSPH8 (S1 and S2) was efficiently transduced into TT cells and
strongly reduced the expression of MPHOSPH8 protein, compared with
the Lv-sh control group (Fig. 1C).
Furthermore, the efficacy of S2 in knocking down MPHOSPH8 protein
expression was higher than that of S1. These results indicated that
Lv-sh MPHOSPH8 exerted successful knockdown effects on MPHOSPH8
expression in TT cells.
Lv-sh MPHOSPH8 suppressed the
viability and proliferation of TT cells
To assess the inhibitory effect of silencing
MPHOSPH8 on cell proliferation, a continuous 5-day MTT assay was
performed. Both S1 and S2 lentiviruses exhibited a remarkable
inhibition of proliferation in TT cells, compared with Lv-sh
control (Fig. 2). Compared with cells
infected with Lv-sh control, TT cell proliferation was markedly
reduced from day 2 to day 5 (P<0.001). These data indicated that
MPHOSPH8 depletion significantly decreased the proliferation of TT
cells.
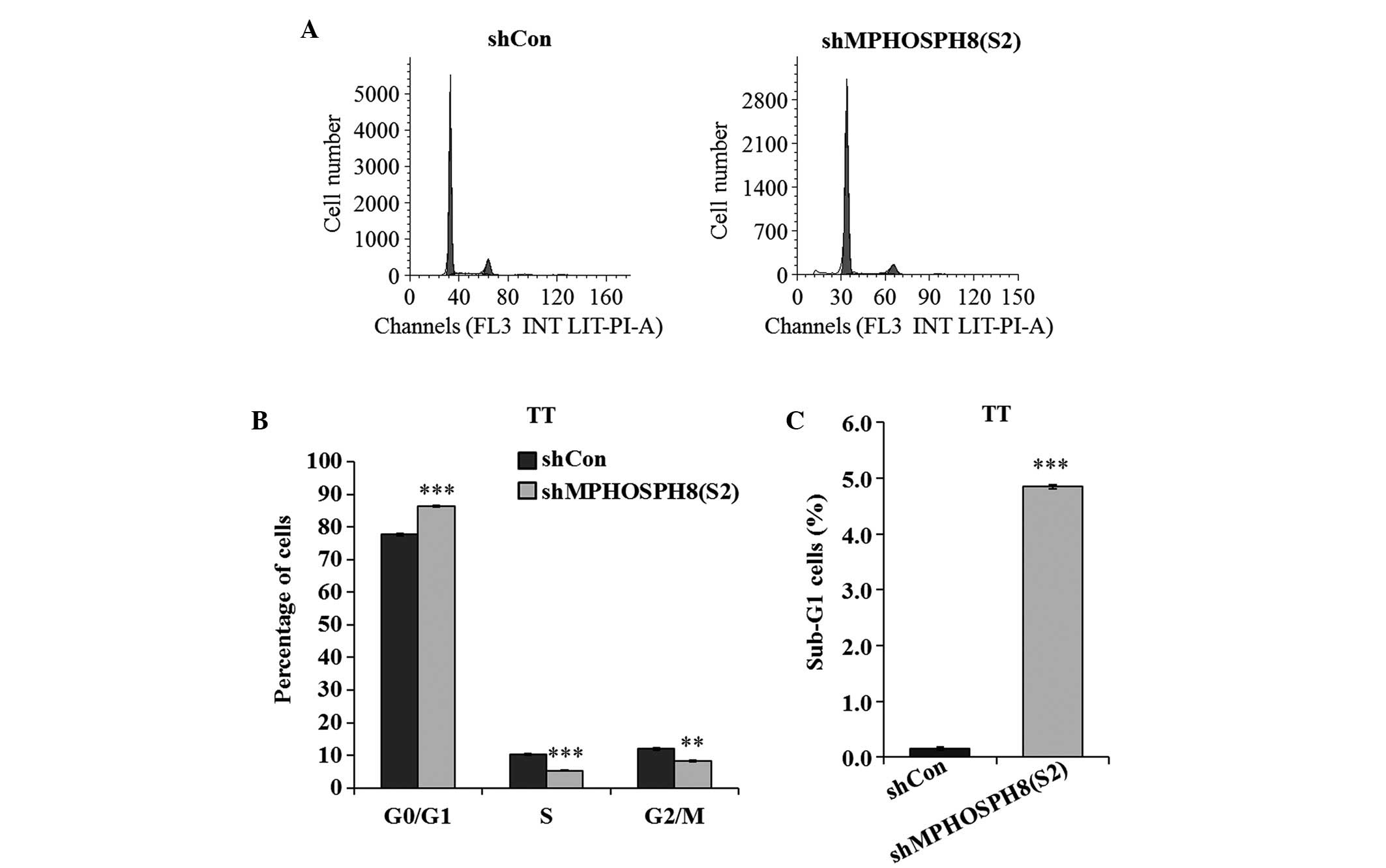
Lv-sh MPHOSPH8 affected the cell cycle
progression in TT cells
To explore the underlying mechanism of inhibition of
cell growth, cell cycle progression was evaluated by flow
cytometry. As indicated in Fig. 3,
the percentage of cells in different phases of the cell cycle
(G0/G1, S and G2/M phases) was significantly different in the three
groups (P<0.01 and P<0.001). Thus, Lv-sh MPHOSPH8
(S2)-infected TT cells exhibited a significant increase in the
fraction of cells in the G0/G1 phase, and a reduction in the
fraction of cells in the G2/M and S phases, compared with the Lv-sh
control group. In addition, TT cells infected with Lv-sh MPHOSPH8
(S2) displayed a remarkable increase in the number of cells in the
sub-G1 phase, suggesting that knockdown of MPHOSPH8 could induce
cell apoptosis.
Lv-sh MPHOSPH8 promoted cell apoptosis
in TT cells
Whether knockdown of MPHOSPH8 could enhance
apoptosis in TT cells was next examined. As represented in Fig. 4, the apoptosis rate was significantly
higher in Lv-sh MPHOSPH8 (S2)-transfected TT cells than in Lv-sh
control-transfected TT cells (P<0.001). Following transfection,
the apoptosis rate (early and late apoptotic cells) of TT cells was
20.45% for the Lv-sh MPHOSPH8 group, which was significantly higher
than that of the Lv-sh control group (8.58%) (P<0.001).
Therefore, the ratio of apoptotic TT cells was markedly increased
following knockdown of MPHOSPH8, compared with that in the Lv-sh
control group.
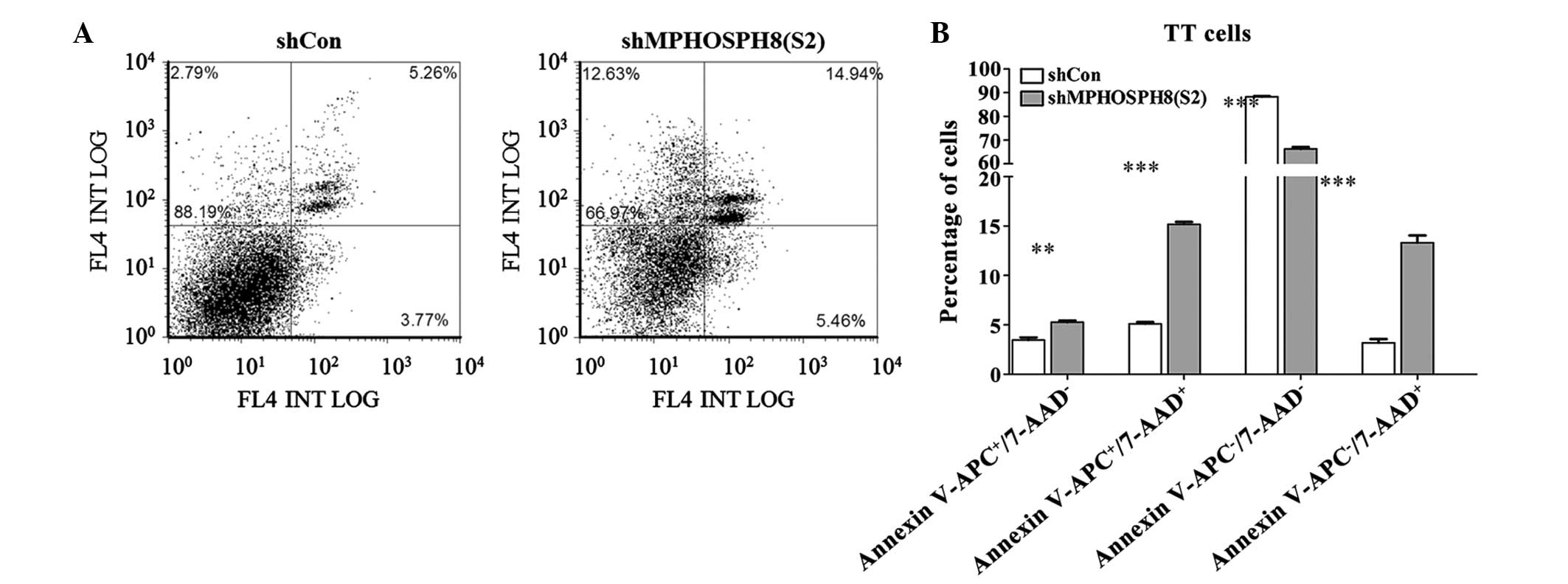 | Figure 4.Detection of apoptosis using FACS. TT
cells were assayed for apoptosis using Annexin V-APC/7-AAD staining
in combination with FACS. (A) Scatter plots representing the FACS
determination of the number of viable
(APC−/7-AAD−), necrotic
(APC−/7-AAD+), early apoptotic
(APC+/7-AAD−) and late apoptotic
(APC+/7-AAD+) TT cells following transfection
with Lv-sh MPHOSPH8 (S2) for 4 days. (B) Proportion of viable,
necrotic, early apoptotic and late apoptotic cells. Values are
presented as the mean ± standard deviation. Representative results
of three independent experiments are shown. **P<0.01;
***P<0.001 vs. control. FL4 INT LOG indicates the fluorescence
signal intensity from different channels. Con, control; sh, small
hairpin; MPHOSPH8, M-phase phosphoprotein 8; Lv, lentivirus; APC,
allophycocyanin; 7-AAD, 7-amino actinomycin D; FACS,
fluorescence-activated cell sorting. |
Discussion
TC is one of the most common malignancies in the
world, and the mortality of MTC is the second highest of all
thyroid tumors. Surgical resection results in a recurrence rate of
almost 50% (6). Therefore, the
identification of novel therapeutic targets and the development of
novel therapeutic regimens able to more effectively regulate the
cellular function of the target genes compared with traditional
treatments is important.
Recently, MPHOSPH8 was identified in various human
carcinoma cells, whereby it displayed an elevated expression
(18). However, MPHOSPH8 as a
potential target in human MTC has not been reported to date. RNA
interference-mediated gene silencing is currently being tested in
clinical trials as a potential therapy for a number of diseases
(26). Thus, in order to investigate
the role of MPHOSPH8 in MTC, TT cells were employed and infected
with MPHOSPH8 lentivirus and control lentivirus to knockdown
MPHOSPH8 expression in the present study. The selected
shRNA-containing vector efficiently suppressed MPHOSPH8 expression
at both messenger RNA and protein levels. Next, the effect of
MPHOSPH8 knockdown on the cellular functions of TT cells was
explored. The results of MTT assay revealed that TT cells exhibited
a reduced proliferation ability following infection with
MPHOSPH8-targeted shRNA. In addition to cell growth and
differentiation, the effect of MPHOSPH8 knockdown on the cell cycle
was also studied. The results indicated that suppressed MPHOSPH8
expression in TT cells led to G1 phase cell cycle arrest and
decreased percentage of cells in S and G2/M phases, while flow
cytometry analysis revealed an increase in apoptosis in Lv-sh
MPHOSPH8 (S2)-treated cells. These results strongly suggest that
MPHOSPH8 may play a central role in MTC. Further understanding of
the molecular roles of MPHOSPH8 in human MTC may aid to clarify its
pathophysiology and to develop novel therapeutic strategies.
In conclusion, the present study demonstrated that
Lv-sh MPHOSPH8 successfully knocked down MPHOSPH8 expression in TT
cells, which exerted an anti-proliferative effect, caused cell
cycle arrest in the G0/G1 phase and induced cell apoptosis.
Although further studies are required, the present results suggest
that MPHOSPH8 knockdown may constitute a potential therapeutic
approach for the treatment of MTC, and may aid to improve the
understanding of MTC progression.
References
|
1
|
Nix P, Nicolaides A and Coatesworth AP:
Thyroid cancer review 1: Presentation and investigation of thyroid
cancer. Int J Clin Pract. 59:1340–1344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ito Y, Nikiforov YE, Schlumberger M and
Vigneri R: Increasing incidence of thyroid cancer: Controversies
explored. Nat Rev Endocrinol. 9:178–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lalami Y and Awada A: Recurrent thyroid
cancer: A molecular-based therapeutic breakthrough. Curr Opin
Oncol. 23:235–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Albero A, Lopéz JE, Torres A, de la Cruz L
and Martín T: Effectiveness of chemotherapy in advanced
differentiated thyroid cancer: A systematic review. Endocr Relat
cancer. 23:R71–R84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giuffrida D and Gharib H: Current
diagnosis and management of medullary thyroid carcinoma. Ann Oncol.
9:695–701. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pelizzo MR, Boschin IM, Bernante P,
Toniato A, Piotto A, Pagetta C, Nibale O, Rampin L, Muzzio PC and
Rubello D: Natural history, diagnosis, treatment and outcome of
medullary thyroid cancer: 37 years experience on 157 patients. Eur
J Surg Oncol. 33:493–497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA
and Chen H: Overexpression of the NOTCH1 intracellular domain
inhibits cell proliferation and alters the neuroendocrine phenotype
of medullary thyroid cancer cells. J Biol Chem. 281:39819–39830.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sippel RS, Kunnimalaiyaan M and Chen H:
Current management of medullary thyroid cancer. Oncologist.
13:539–547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tavares MR, Toledo SP, Montenegro FL,
Moyses RA, Toledo RA, Sekyia T, Cernea CR and Brandão LG: Surgical
approach to medullary thyroid carcinoma associated with multiple
endocrine neoplasia type 2. Clinics (Sao Paulo). 67(Suppl 1):
S149–S154. 2012. View Article : Google Scholar
|
|
10
|
Ball DW: Medullary thyroid cancer:
Monitoring and therapy. Endocrinol Metab Clin North Am. 36:823–837.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brassard M and Rondeau G: Role of
vandetanib in the management of medullary thyroid cancer.
Biologics. 6:59–66. 2012.PubMed/NCBI
|
|
12
|
Machens A, Lorenz K and Dralle H:
Individualization of lymph node dissection in RET (rearranged
during transfection) carriers at risk for medullary thyroid cancer:
Value of pretherapeutic calcitonin levels. Ann Surg. 250:305–310.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tavares MR, Michaluart P Jr, Montenegro F,
Arap S, Sodre M, Takeda F, Brandao L, Toledo S and Ferraz A: Skip
metastases in medullary thyroid carcinoma: A single-center
experience. Surg Today. 38:499–504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoo CB and Jones PA: Epigenetic therapy of
cancer: Past, present and future. Nat Rev Drug Discov. 5:37–50.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun L, Kokura K, Izumi V, Koomen JM, Seto
E, Chen J and Fang J: MPP8 and SIRT1 crosstalk in E-cadherin gene
silencing and epithelial-mesenchymal transition. EMBO Rep.
16:689–699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsumoto-Taniura N, Pirollet F, Monroe R,
Gerace L and Westendorf JM: Identification of novel M phase
phosphoproteins by expression cloning. Mol Biol Cell. 7:1455–1469.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Murata K, Sato S, Haruta M, Goshima T,
Chiba Y, Takahashi S, Sharif J, Koseki H, Nakanishi M and Shimada
M: Physical interaction between MPP8 and PRC1 complex and its
implication for regulation of spermatogenesis. Biochem Biophys Res
Commun. 458:470–475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kokura K, Sun L, Bedford MT and Fang J:
Methyl-H3K9-binding protein MPP8 mediates E-cadherin gene silencing
and promotes tumour cell motility and invasion. EMBO J.
29:3673–3687. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang Y, Sun L, Kokura K, Horton JR,
Fukuda M, Espejo A, Izumi V, Koomen JM, Bedford MT, Zhang X, et al:
MPP8 mediates the interactions between DNA methyltransferase Dnmt3a
and H3K9 methyltransferase GLP/G9a. Nat Commun. 2:5332011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishigaki M, Kawada Y, Misaki T, Murata K,
Goshima T, Hirokawa T, Yamada C, Shimada M and Nakanishi M: Mitotic
phosphorylation of MPP8 by cyclin-dependent kinases regulates
chromatin dissociation. Biochem Biophys Res Commun. 432:654–659.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Antonelli A, Fallahi P, Ferrari SM,
Ruffilli I, Santini F, Minuto M, Galleri D and Miccoli P: New
targeted therapies for thyroid cancer. Curr Genomics. 12:626–631.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sipos JA and Shah MH: Thyroid cancer:
Emerging role for targeted therapies. Ther Adv Med Oncol. 2:3–16.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Russo D, Damante G, Puxeddu E, Durante C
and Filetti S: Epigenetics of thyroid cancer and novel therapeutic
targets. J Mol Endocrinol. 46:R73–R81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kokura K, Sun L, Bedford MT and Fang J:
Methyl-H3K9-binding protein MPP8 mediates E-cadherin gene silencing
and promotes tumour cell motility and invasion. EMBO J.
29:3673–3687. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–8. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Manjunath N, Wu H, Subramanya S and
Shankar P: Lentiviral delivery of short hairpin RNAs. Adv Drug
Deliv Rev. 61:732–745. 2009. View Article : Google Scholar : PubMed/NCBI
|