Introduction
It has previously been demonstrated that connexin 26
(Cx26), Cx32 and Cx43 are aberrantly distributed (in the cytoplasm
instead of membranous locations) and that their expression levels
are reduced in human endometrial hyperplasia and endometrial cancer
(1). In addition, suppression of gap
junctional intercellular signaling (GJIC) has been shown to be
mediated via 5′-CpG island methylation in the promoter region of
E-cadherin gene in endometrial cancer cell lines (2).
As well as a reduction in E-cadherin expression,
stimulation of Src also results in inhibition of GJIC (3). Signal transducer and activator of
transcription-3 (STAT3) is a downstream molecule of Src that if
activated via phosporyltion acts as an oncogene (3). By contrast, connexins (Cx) are
considered as suppressors of cancer metastasis (4). For example, Cx32 expression reduced
anchorage independency and the invasion capacity of a human
metastatic renal cell carcinoma cell line (Caki-1 cell) (4). A previous study used RNA interference to
demonstrate that Cx32 expression inhibited Src via downregulation
of vascular epithelial growth factor (VEGF) and a reduction in
STAT3 phosporylation (4). Cx proteins
build gap junctions channels for intercellular communication, and
Src-mediated phosphorylation of Cx43 hinders such a communication
(5). Unlike the Src effector Cas,
phosphorylation of another Src effector, STAT3, does not influence
Src-mediated GJIC suppression but is still required for
preservation of gap junctional communication in cells with high
levels of GJIC (5). The potential
relationship between STAT3 and GJIC was investigated by comparing
the quantity of tyr705-phosphorylation of STAT3 with the speed and
intensity of fluorescent dye Lucifer yellow migration in
electrophorated non small cell lung cancer (NSCLC) cell lines, with
additional detection of tyr418-phosphorylation of Src (6). The study showed that gap junctions
require the activation of STAT3 in order to maintain intracellular
communication in non neoplastic and neoplastic lung cell lines.
Cell proliferation is generally associated with a reduction in GJIC
(7). Coexistent activation of src and
its effector molecule STAT3 is also observed in NSCLC cell lines.
STAT3 deactivation results in a reduction in gap junctional
communication exclusively in normal cells or in lines with low Src
activity and high levels of gap junction communication (7). Similarly, STAT3 blockage abrogated
junctional permeability in normal liver cells with high GJIC
(3). Recruitment of Src and GJIC were
inversely associated with each other but deactivation of STAT3 did
not restore GJIC despite activation of Src (6). By contrast, reduction of STAT3 activity
abolished GJIC in immortalized lung epithelial cells and in the
NSCLC lines with high levels of GJIC. In the light of these
findings, STAT3 appears to be indispensible for gap junctional
communication in the mentioned cell lines (6). STAT3 function is multidirectional and
could affect a number of cellular signaling pathways. Cx proteins
are one of numerous families of proteins that may cooperate with
STAT3. In cardiac tissues taken from Cx43 knockout mice, STAT3
remained active in the cell (8).
Namely, normoxemia did not alter levels of phosphorylation of
STAT3, but repeated ischemia and reperfusion increased
phosphorylation of STAT3 in cells without functional Cx43 (8). Cx43 is switched on during embryonic stem
cell-mediated cardiomyogenesis in a leukemia inhibitory factor
(LIF) and bone-morphogenic protein-2 (BMP-2)-induced mouse ES cell
(mES-D3 line) with engagement of STAT3 and MAP kinase (ERK1/2)
(9). Apart from the association
between STAT3 and Cx, it should be emphasized that STAT3 is a
versatile driver of endometrial carcinogenesis, while signaling via
gap junction channel proteins with involvement of Cx26 and Cx43 is
considered to be weakened in progression of carcinogenesis
(1,10). The present study aimed to compare the
expression levels of STAT3, Cx43 and Cx26 in 78 endometrioid
adenocarcinoma samples using immunohistochemical analysis.
Materials and methods
Patient samples
Tumor specimens of human uterine endometrioid
adenocarcinomas were obtained from postoperative material of 78
total hysterectomies with bilateral adnexectomies. The study was in
accordance with the 2004 revision of the Declaration of Helsinki
(11), and was approved by the
Bioethical Committee for Studies on Humans of the Medical
University of Bialystok (Waszyngtona, Poland). Written informed
consent was obtained from all patients included in this study. The
tumor samples were categorized into 3 groups according to grade of
histopathological differentiation: 15 were well differentiated G1
neoplasms, 52 were moderately differentiated G2 neoplasms and 11
were poorly differentiated G3 neoplasms. Cancers were staged as
International Federation of Gynecology and Obstetrics (FIGO) IA
tumors (depth of tumor infiltration: tumor invades less than half
of myometrium). Tumors that invaded >50% of myometrium (FIGO IB)
or spread to the endocervix (FIGO II) comprised a common group for
statistical evaluation (2009 FIGO classification of endometrial
cancer) (9). Node-negative and
node-positive patients were not included in the statistical
evaluation, due to scarcity of node-positive endometrioid
adenocarcinomas in the present study. The tumors were also divided
into subgroups with lack or presence progesterone receptor (PR) and
estrogen receptor (ER) positivity. Postoperative material was fixed
in 10% buffered formalin for 48 h prior to sampling and embedding
in paraffin blocks at 56.8°C. Tissue sections (3–5 µm thick) were
sliced from paraffin blocks and then mounted on
3-aminopropyltriethoxysilane-coated slides, dewaxed and rehydrated.
Such processed slides were stained with hematoxylin and eosin
(Dako, Glostrup, Denmark) for standard diagnosis given by two
pathologists.
Immunohistochemistry
Standard immunohistochemical procedure for STAT3,
Cx43, Cx26 ER and PgR was performed in endometrioid adenocarcimonas
according to previously described protocols (10, 12–13).
Briefly, endogenous peroxidase activity was blocked with 60 sec
treatment of slides 3% hydrogen peroxide (Santa Cruz Biotechnology,
Inc. Santa Cruz, CA, USA, and then the slides were exposed to 1.5%
normal blocking serum (Dako) for 90 min to inhibit unspecific
binding reaction. Anti-STAT3 rabbit polyclonal antibodies (sc-7179)
(and goat polyclonal antibodies to Cx26 (sc-7261) and Cx43
(sc-6560) were diluted (1:500 for STAT3 and 1:100 for Cx26 and
Cx43) and incubated with specimens in PBS for 24 h. The secondary
antibodies used were goat anti-rabbit IgG-B (sc-2040) and rabbit
anti-goat IgG-B (sc-2774) (dilution, 1:100 for both).
Immunohistochemistry was performed as previously described
(14). All primary and secondary
antibodies were purchased from Santa Cruz Biotechnology, Inc..
EnVision method (Dako) was used for visual detection of complexes
of antibodies with STAT3 antigens with 10 min long incubation with
diaminobenzidine as a chromogen (DAB). Other proteins were
visualized with streptavidin-biotin-peroxidase complex method (LSAB
kit; Dako). Slides were counterstained with hematoxylin. Normal
endometrium and breast glandular tissue served as weak positive
control for STAT3 Cx43, Cx26, ER and PR. The normal non-neoplastic
endometrium was obtained from uterine corpses surgically removed
from premenopausal women with uterine leiomyomas, whereas the
normal, non-neoplastic breast glandular tissue samples were
obtained from the tissue margins adjacent to benign fibroadenomas
that were surgically removed with margin of uninvolved tissue. For
negative controls, the primary antibodies were omitted.
Scoring system and statistical
evaluation
Immunoreactions were scored under light microscopy
(Nikon Eclipse Ni-U; Nikon Corporation, Tokyo, Japan) in 10
different fields under magnification of ×200 to record the mean
percentage of immunoreactive malignant cells. The cut-off was 10%
malignant positive cells for separation between positive and
negative results. A three-grade scale was applied: 0, (negative
cancers) <10% positive malignant cells; 1+, with
immunoreactivity between 10–50% positive malignant cells (moderate
positivity); 2+, with >50% positive tumor cells (strong
positivity). Spearman's correlation rank test served for the
comparison between pairs of proteins in different groups. The
statistical software Statistica 12 64-Bit (StatSoft Polska Sp. z
o.o., Cracow, Poland) was used for the statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
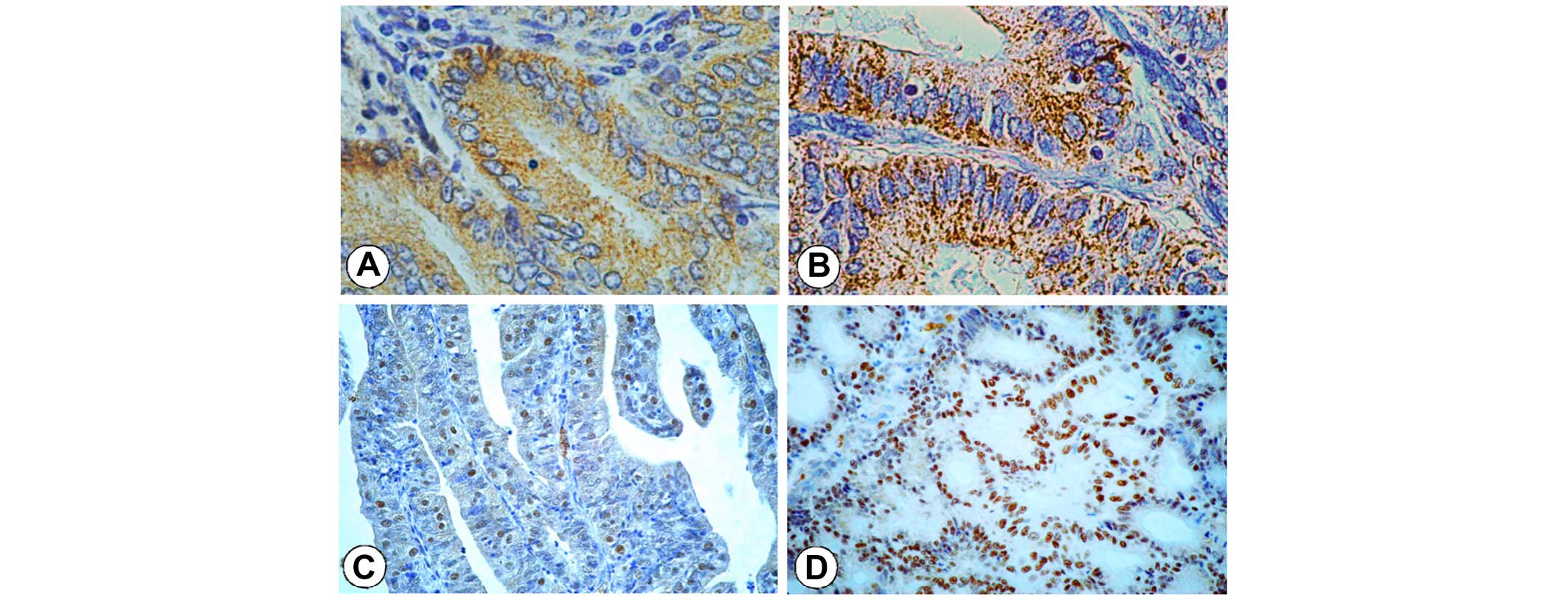
The cytoplasm was a site of cellular accumulation of
Cx26 and Cx43 in endometrioid adenocarcinomas, instead of its
functional membranous presence in normal endometrium (Fig. 1A and B). Nuclear expression of STAT3
positively correlated with Cx43 (P=0.004, r=0.318) and Cx26
(P=0.006, r=0.309) in the examined samples (Fig. 1C and D). STAT3 expression correlated
with Cx43 (P=0.022, r=0.411) and Cx26 (P=0.008 r=0.466) in
well-differentiated tumors. A statistically significant linkage
remained in moderately differentiated G2 cancers with STAT3
correlating with Cx43 (P=0.061, r=0.262) and with Cx26 (P=0.016,
r=0.331). In G3 tumors, however, no correlation was observed
between STAT3 and Cx43 (P=0.415, r=0.274) and Cx26 (P=0.200,
r=0.418). ER negativity was associated with sustained statistically
significant relationships between STAT3 and Cx43 (P=0.003, r=0.684)
and Cx26 (P=0.049, r=0.500). ER positivity coexisted with loss of
significance in STAT3 and Cx43 coexpression (P=0.074, r=0.229),
while STAT3 remained correlated with Cx26 (P=0.035, r=0.268). A
similar trend was observed in PR negative tumors where statistical
correlations remained for STAT3 and Cx43 (P=0.035, r=0.451) or
STAT3 and Cx26 (P<0.0001, r=0.707). In PR positive
adenocarcinomas statistical significance persisted in relation to
STAT3 and Cx43 (P=0.03, r=0.290) but was completely lost between
STAT3 and Cx26 (P=0.166, r=0.188) (Table
I).
 | Table I.Association between STAT3 and connexin
expression levels in uterine endometrioid adenocarcinoma
samples. |
Table I.
Association between STAT3 and connexin
expression levels in uterine endometrioid adenocarcinoma
samples.
|
| STAT3-Cx43 | STAT3-Cx26 |
|---|
|
|
|
|
|---|
| Patients' group | P-value | r | P-value | r |
|---|
| All patients;
n=78 | 0.004 | 0.318 | 0.006 | 0.309 |
| Age <60 years;
n=33 | 0.167 | 0.246 | 0.439 | 0.139 |
| Age >60 years;
n=45 | 0.011 | 0.375 | 0.003 | 0.438 |
| IA; n=40 | 0.002 | 0.478 | 0.097 | 0.266 |
| IB+II; n=38 | 0.341 | 0.159 | 0.034 | 0.344 |
| G1; n=15 | 0.022 | 0.411 | 0.008 | 0.466 |
| G2; n=52 | 0.061 | 0.262 | 0.016 | 0.331 |
| G3; n=11 | 0.415 | 0.274 | 0.200 | 0.418 |
| ERα (−); n=16 | 0.003 | 0.684 | 0.049 | 0.500 |
| ERα (+); n=62 | 0.074 | 0.229 | 0.035 | 0.268 |
| PR (−); n=22 | 0.035 | 0.451 |
<0.0001 | 0.707 |
| PR (+); n=56 | 0.030 | 0.290 | 0.166 | 0.188 |
Discussion
It has previously been demonstrated in IK-ER1
overexpressing ER-alpha endometrial carcinoma cells that estradiol
suppressed formation of gap channels via Cx26 and Cx32 (15). In the present study using human
endometrial cancer tissues, sex hormone receptor positivity
contributes to loss of statistically significant linkage between
STAT3 and Cx43 in the case of ER positive cancers as well as loss
of association between STAT3 and Cx26 in PR-positive cancers. The
findings reflect impaired function of aberrantly expressed Cx
proteins and complete lack of association between STAT3 and Cx43
expression in PR-positive and ER-positive endometrial cancer. It
should be remembered that the compared proteins have opposite
functions. Namely, STAT3 as a driver of endometrial carcinogenesis
and connexins as suppressors of endometrial growth (2,3,10,13,16), but
such a role is mediated only by nuclear STAT3 and membranous Cx
proteins. It should not be remarkable that nuclear expression of
STAT3 positively correlated with expression of Cx proteins,
however, the expression of Cx43 and Cx26 was observed to be
cytoplasmic indicating loss of their growth suppressing properties
that require a membranous location. In spite of their opposite
functions, expression of these proteins could be switched on
together. For instance, heterodimer ciliary neurotrophic factor
expression increased Cx43 mRNA and protein expression and caused
nuclear localization of phosphorylated STAT3 in astrocytes
(17).
Characteristically, in our previous study, there was
no linkage between Cx26 expression and patients' age, histological
type of cancer and histological grade except for a positive
correlation between Cx26 expression and tumor size (18). GJA1/Cx43 and GJA6/Cx30 have emerged as
prognostic factors in breast cancer, which similarly to endometrial
cancer is regarded as an estrogen dependent malignancy (19). In addition diminished Cx26 expression
was associated with improved overall survival rates for breast
cancer patients following chemotherapy, while Cx46 expression was a
marker of significantly improved survival of selected groups of
breast cancer patients in pre-chemotherapy and post-chemotherapy
periods (20). In this perspective of
prognostic significance of Cx, the present findings connect Cx
expression with the carcinogenesis driver STAT3 in another estrogen
dependent neoplasm, endometrial cancer.
The association between STAT3 and Cx43 expression is
also gradually lost in the process of dedifferentiation from G1 to
G3 endometrioid adenocarcinomas in the current study. Generally
weakening of STAT3 expression was not observed within grading in
our previous studies (10,13) however expression of Cx43 was reduced
with tumor grade in endometrial cancer samples in a study by
Schlemmer et al (21).
In the context of associations between STAT3 and
Cx43 and Cx26, therapeutic perspectives have emerged that aim to
downregulate STAT3 expression (particularly reducing STAT3 nuclear
accumulation where it acts as an activator of transcription) and
upregulation of expression of Cx proteins (particularly encouraging
a membranous location of Cx where they act as cell growth
suppressors). For instance, kaempferol regulates STAT3 and Cx,
inducing differentiation in colon cancer cells expressing low
levels of Cx43 (keratin-negative cells) (22). Kaempferol resulted in differentiation
that was accompanied with increased quantity of Cx43 and its
phosporylation, an increase in GJIC and a decrease in activation of
STAT3 and ERK. In cancer cells that, even weakly, expressed Cx43,
kaempferol recruited STAT3 and triggered resultant overexpression
and phosporylation of this connexin, which led to the restoration
of GJIC (22). Kaempferol mediated
restoration of GJIC via STAT3-dependent overexpression and
phosporylation of Cx43, if only cancer cells expressed this
connexin at least at low level (22).
In addition, gap junctional communication was not restored if STAT3
was inhibited in lung cell lines with a high stimulated Src level
(7); similarly, gap junctional
communication was not restored in rat liver epithelial cells
habouring activated Src when trichloronitritodiammineplatinum(IV),
STAT3 inhibitor or a retroviral vector (expressing a STAT3-specific
shRNA) decreased expression of STAT3 (3). This evidence indicates a pleiotropic
significance of STAT3: That it enables permeability of gap
junctions and contributes to maintenance of gap junction
communication (3,7). It should be emphasized that, besides Cx
expression, STAT3 is an extraordinarily versatile mediator and it
interacts with a number of molecules, including leptin and its
receptor OB-R, IL-11 or oncostatin M that affect the invasive
properties of malignant cells via their impact on cellular
adhesion, motility and survival in gynecological cancers (10,23–27). Thus,
there may be multiple and complex associations between STAT3 and
other cellular mediators, and therefore any statistically
significant coexpression of engaged proteins should be carefully
evaluated. Nevertheless, further studies are required to elucidate
the role of STAT3 signaling in endometrial cancer.
In conclusion, ER and PR-positivity defines
endometrioid adenocarcinomas as sex steroid hormone dependent
neoplastic growth and is associated with deregulation of the link
between STAT3 and Cx expression. By contrast, lack of
immunoreactivity for the presence of mentioned receptors is
associated with the preservation of the statistically significant
relationship between STAT3 and the studied Cx. These findings
provide evidence that hormone dependent acceleration of cancer
growth breaks the association between STAT3 and Cx expression. Such
associations are gradually lost in the progression of
dedifferentiation from G1 to G3 endometrioid adenocarcinomas. In
conclusion, it appears that loss of correlation between STAT3 and
selected Cx proteins occurs in ER and PR-positive tumors and also
in tumors with more aggressive behavior.
References
|
1
|
Saito T, Nishimura M, Kudo R and Yamasaki
H: Suppressed gap junctional intercellular communication in
carcinogenesis of endometrium. Int J Cancer. 93:317–323. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nishimura M, Saito T, Yamasaki H and Kudo
R: Suppression of gap junctional intercellular communication via 5′
CpG island methylation in promoter region of E-cadherin gene in
endometrial cancer cells. Carcinogenesis. 24:1615–1623. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geletu M, Chaize C, Arulanandam R, Vultur
A, Kowolik C, Anagnostopoulou A, Jove R and Raptis L: Stat3
activity is required for gap junctional permeability in normal rat
liver epithelial cells. DNA Cell Biol. 28:319–327. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujimoto E, Sato H, Shirai S, Nagashima Y,
Fukumoto K, Hagiwara H, Negishi E, Ueno K, Omori Y, Yamasaki H, et
al: Connexin32 as a tumor suppressor gene in a metastatic renal
cell carcinoma cell line. Oncogene. 24:3684–3690. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Geletu M, Trotman-Grant A and Raptis L:
Mind the gap; regulation of gap junctional, intercellular
communication by the SRC oncogene product and its effectors.
Anticancer Res. 32:4245–4250. 2012.PubMed/NCBI
|
|
6
|
Geletu M, Arulanandam R, Greer S,
Trotman-Grant A, Tomai E and Raptis L: Stat3 is a positive
regulator of gap junctional intercellular communication in
cultured, human lung carcinoma cells. BMC Cancer. 12:6052012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guy S, Geletu M, Arulanandam R and Raptis
L: Stat3 and gap junctions in normal and lung cancer cells. Cancers
(Basel). 6:646–662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sánchez JA, Rodríguez-Sinovas A, Barba I,
Miró-Casas E, Fernández-Sanz C, Ruiz-Meana M, Alburquerque-Béjar JJ
and García-Dorado D: Activation of RISK and SAFE pathways is not
involved in the effects of Cx43 deficiency on tolerance to
ischemia-reperfusion injury and preconditioning protection. Basic
Res Cardiol. 108:3512013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rajasingh J, Bord E, Hamada H, Lambers E,
Qin G, Losordo DW and Kishore R: STAT3-dependent mouse embryonic
stem cell differentiation into cardiomyocytes: Analysis of
molecular signaling and therapeutic efficacy of cardiomyocyte
precommitted mES transplantation in a mouse model of myocardial
infarction. Circ Res. 101:910–918. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wincewicz A, Koda M, Sulkowska M,
Kanczuga-Koda L and Sulkowski S: Comparison of STAT3 with
HIF-1alpha, Ob and ObR expressions in human endometrioid
adenocarcinomas. Tissue Cell. 40:405–410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
World Medical Association. 2004.WMA
declaration of Helsinki: Ethical principles for medical research
involving human subjects. http://www.wma.net/en/30publications/10policies/b3/Accessed.
January. 2016
|
|
12
|
Lesniewicz T, Kanczuga-Koda L, Baltaziak
M, Jarzabek K, Rutkowski R, Koda M, Wincewicz A, Sulkowska M and
Sulkowski S: Comparative evaluation of estrogen and progesterone
receptor expression with connexins 26 and 43 in endometrial cancer.
Int J Gynecol Cancer. 19:1253–1257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wincewicz A, Baltaziak M, Kanczuga-Koda L,
Koda M, Sulkowska U, Famulski W and Sulkowski S: STAT3 and
apoptosis regulators: Bak and Bcl-xL in endometrioid
adenocarcinomas of different estrogen receptor-α immunoprofile.
Gynecol Endocrinol. 27:536–540. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kanczuga-Koda L, Wincewicz A, Fudala A,
Abrycki T, Famulski W, Baltaziak M, Sulkowski S and Koda M:
E-cadherin and β-catenin adhesion proteins correlate positively
with connexins in colorectal cancer. Oncol Lett. 7:1863–1870.
2014.PubMed/NCBI
|
|
15
|
Saito T, Tanaka R, Wataba K, Kudo R and
Yamasaki H: Overexpression of estrogen receptor-alpha gene
suppresses gap junctional intercellular communication in
endometrial carcinoma cells. Oncogene. 23:1109–1116. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang JZ, Kong XJ, Banerjee A, Muniraj N,
Pandey V, Steiner M, Perry JK, Zhu T, Liu DX and Lobie PE:
STAT3alpha is oncogenic for endometrial carcinoma cells and
mediates the oncogenic effects of autocrine human growth hormone.
Endocrinology. 151:4133–4145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ozog MA, Bernier SM, Bates DC, Chatterjee
B, Lo CW and Naus CC: The complex of ciliary neurotrophic
factor-ciliary neurotrophic factor receptor alpha up-regulates
connexin43 and intercellular coupling in astrocytes via the Janus
tyrosine kinase/signal transducer and activator of transcription
pathway. Mol Biol Cell. 15:4761–4764. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lesniewicz T, Kanczuga-Koda L, Baltaziak
M, Sulkowska M, Rutkowski R, Koda M and Sulkowski S: Expression of
connexin 26 in endometrial adenocarcinoma-analysis of correlations
with some anatomoclinical features. Folia Histochem Cytobiol.
46:171–176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Teleki I, Szasz AM, Maros ME, Gyorffy B,
Kulka J, Meggyeshazi N, Kiszner G, Balla P, Samu A and Krenacs T:
Correlations of differentially expressed gap junction connexins
Cx26, Cx30, Cx32, Cx43 and Cx46 with breast cancer progression and
prognosis. PLoS One. 9:e1125412014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Teleki I, Krenacs T, Szasz MA, Kulka J,
Wichmann B, Leo C, Papassotiropoulos B, Riemenschnitter C, Moch H
and Varga Z: The potential prognostic value of connexin 26 and 46
expression in neoadjuvant-treated breast cancer. BMC Cancer.
13:502013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schlemmer SR, Novotny DB and Kaufman DG:
Changes in connexin 43 protein expression in human endometrial
carcinoma. Exp Mol Pathol. 67:150–163. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakamura Y, Chang CC, Mori T, Sato K,
Ohtsuki K, Upham BL and Trosko JE: Augmentation of differentiation
and gap junction function by kaempferol in partially differentiated
colon cancer cells. Carcinogenesis. 26:665–671. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Méndez-López LF, Dávila-Rodríguez MI,
Zavala-Pompa A, Torres-López E, González-Martínez BE and
López-Cabanillas-Lomelí M: Expression of leptin receptor in
endometrial biopsies of endometrial and ovarian cancer patients.
Biomed Rep. 1:659–663. 2013.PubMed/NCBI
|
|
24
|
Lay V, Yap J, Sonderegger S and
Dimitriadis E: Interleukin 11 regulates endometrial cancer cell
adhesion and migration via STAT3. Int J Oncol. 41:759–764.
2012.PubMed/NCBI
|
|
25
|
Yang J, Liu B, Wang Q, Yuan D, Hong X,
Yang Y and Tao L: Connexin 32 and its derived homotypic gap
junctional intercellular communication inhibit the migration and
invasion of transfected HeLa cells via enhancement of intercellular
adhesion. Mol Med Rep. 4:971–979. 2011.PubMed/NCBI
|
|
26
|
Zhu M, Che Q, Liao Y, Wang H, Wang J, Chen
Z, Wang F, Dai C and Wan X: Oncostatin M activates STAT3 to promote
endometrial cancer invasion and angiogenesis. Oncol Rep.
34:129–138. 2015.PubMed/NCBI
|
|
27
|
Sulkowska U, Wincewicz A, Kanczuga-Koda L,
Koda M and Sulkowski S: Eventual proapoptotic or anti-apoptotic
impact of aberrantly expressed Cx43 and Cx26 can depend on ER-alpha
overexpression in human endometrioid adenocarcinoma. Gynecol
Endocrinol. 31:604–608. 2015. View Article : Google Scholar : PubMed/NCBI
|