Introduction
Glioblastoma is the most common primary brain tumor,
with an annual incidence of 3.9 cases per 100,000 individuals in
the Canton of Zurich (1) and the most
aggressive among all gliomas. The standard of care includes
radiotherapy plus concomitant and maintenance temozolomide
chemotherapy (TMZ/RT→TMZ) (2),
resulting in a median survival time of 16 months in clinical trial
populations (3). Several factors
account for the tumorigenicity of gliomas, including mutations in
selected genes, the amplification of signaling pathway genes, and
the exploitation of surrounding non-transformed brain cells induced
to provide molecules essential for glioma growth and invasiveness
(4,5).
Among these, cytokines and their receptors have received
significant attention (6,7). For instance, interleukin (IL)-1β, IL-6
and IL-8 are expressed by glioma cells and regulate glioma cell
survival, migration and invasion (8,9). However,
the expression and function of other IL-1 family members, such as
IL-33, in human glioma cells have not been addressed in detail.
IL-33 is recognized for its pro-inflammatory role in
inflammatory diseases of mucosal tissues, including allergic
asthma, inflammatory bowel diseases and eosinophilic esophagitis
(10–14), but also in rheumatoid arthritis and
sarcoidosis (15). IL-33 signals
through a heterodimeric receptor consisting of IL-1 receptor-like 1
protein (IL1RL1) and IL-1 receptor accessory protein (IL1RAcP).
Binding of IL-33 to the receptor activates nuclear factor-κB and
mitogen-activated protein kinases, in a myeloid differentiation
primary response gene 88-dependent manner (16–18).
IL-33 is predominantly and constitutively found in
the nucleus of endothelial or epithelial cells of barrier tissues
(17,19–21). It
mediates the recruitment and activation of immune cells such as
mast cells, monocytes, eosinophils, neutrophils, dendritic cells
and lymphocyte subsets (16,22). IL-33 can also exert multiple effects
on non-immune cells (19–21). Beyond the extracellular
receptor-mediated function of IL-33, these effects also appear to
be receptor-independent. In this case, IL-33 acts as an
intracellular repressor of transcription (12,20,21,23,24).
The role of IL-33 in the human brain is not well
known. IL-33 levels were found to be overexpressed in the brain of
patients with Alzheimer's disease (25) and multiple sclerosis (26). In the central nervous system (CNS) of
mice, IL-33 is constitutively expressed by astrocytes and
endothelial cells (17,27), and it can be induced in astrocytes in
response to inflammatory and necrotic stimuli (28). Since growing evidence indicates that
IL-33 plays a role in the maintenance and homeostasis of astrocytes
(27–30), it may also contribute to the
development and progression of tumors derived from astrocytes. The
function of IL-33 in human cancer has remained controversial, but
elevated serum levels of IL-33 have been reported in malignancies
such as gastric cancer, hepatocellular carcinoma and non-small cell
lung cancer (31–33). Recently, it was shown that growth rate
and colony formation of tumorigenic C6 rat glioma cells were
attenuated by the inhibition of IL-33 gene expression and that
IL-33 induced migration in these cells (34). In the present study, the expression
and prognostic significance of IL-33 in gliomas was
investigated.
Materials and methods
Patients
In accordance with the Institutional Review Board,
and following the retrieval of informed consent, the surgical
specimens and clinical records were retrieved from 95 patients who
underwent brain tumor resection between January 2000 and December
2009 at the Department of Neurosurgery, University Hospital Zurich
(Zurich, Switzerland). In total, 10 astrocytomas of World Health
Organization (WHO) grade I (AI), 14 astrocytomas of WHO grade II
(AII), 23 anaplastic astrocytomas (astrocytomas of WHO grade III;
AAIII) and 48 glioblastomas were analyzed. The group of
glioblastoma patients included 26 newly diagnosed patients and 22
patients with recurrent tumors. In 1 patient, primary and recurrent
tumor specimens were obtained. All tumors were classified and
graded according to the WHO classification of tumors of the central
nervous system (35). Tissues of 4
patients, not diagnosed with brain tumors, were retrieved during
autopsy and used as normal brain controls.
Immunohistochemistry
Immunohistochemistry was performed on a tissue
microarray (TMA) with archival formalin-fixed 4-µm thick sections
on SuperFrost slides (Menzel-Glaser, Braunschweig, Germany).
Deparaffinized, rehydrated sections were preincubated on the
BondMax system (Leica, Mannheim, Germany) in Bond Epitope Retrieval
Solution 2 (pH 9.0) for 30 min at 95°C and then stained for IL-33
using goat anti-human IL-33 immunoglobulin (Ig)G (catalog no.
AF3625; R&D Systems Inc., Minneapolis, MN, USA) at a dilution
of 1:400. The specificity of IL-33 staining was confirmed by
negative staining with isotype-matched IgG (catalog no. AB-108-C;
R&D Systems Inc.). The immunostaining results were assessed as
the percentage of IL-33-positive (IL-33+) cells (nuclear
staining) (21). Scoring was
performed by one scientist in a blinded manner.
Interrogations of The Cancer Genome
Atlas (TCGA) database
Microarray and outcome data were obtained from the
glioblastoma data set of the TGCA network available on December 11,
2014 (http://cancergenome.nih.gov/)
(36). The gene expression data in
this database were obtained using Affymetrix array. The query was
based on the reporter with the highest mean geometric intensity for
the target gene. The Affymetrix probesets used in the TCGA database
were 209821_at for IL-33, 205227_at for IL1RAcP and 234066_at for
IL1RL1. The TCGA database used for this study contains data of 276
gliomas of different histologies and 8 control samples (37). Survival analysis within the
glioblastoma data set of the TCGA database was performed using the
Kaplan-Meier analysis module of the R2 microarray analysis and
visualization platform (http://r2.amc.nl).
The cut-off to segregate glioblastoma patients into two groups with
high or low expression of the target gene was defined by the
average expression level of the target gene.
Statistics
Progression-free survival (PFS), overall survival
(OS) and post-recurrence survival (PRS) curves were estimated by
the Kaplan-Meier method and compared with the two-sided log-rank
test. PFS time was calculated from the date of surgery to the date
of recurrence. OS time was measured from the date of first surgery
to the date of mortality. PRS time was measured from the date of
the surgery in which the tissue was obtained to the date of
mortality. Patients without confirmed mortality were censored for
OS and PRS at the last follow-up visit. Patients without documented
progression were censored at the last follow-up visit for PFS, PRS
and OS. The Kruskal-Wallis test (one-way analysis of variance for
non-parametric values), in combination with Dunn's multiple
comparison test, was used to compare protein or mRNA levels within
groups. Survival-associated analyses were calculated with the
log-rank test. All statistical analyses were performed using Prism
5 (GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient and tumor characteristics
Tissue sections of 95 patients from a single center
with newly diagnosed or recurrent glioma were analyzed, and the
patient characteristics are presented in Table I. Glioblastoma patients were older
(median age, 56.5 years) than patients diagnosed with WHO grade I
(median age, 20 years), WHO grade II (median age, 37.5 years) or
WHO grade III (median age, 41 years) tumors. In the group of WHO
grade II–IV patients, males were more often affected than females.
The median OS time in the group of glioblastoma patients (14
months) was worse than that for patients diagnosed with WHO grade
III gliomas (41 months). The median OS time for patients with WHO
grade II was 14.3 years, whereas the median survival time for
patients diagnosed with WHO grade I was 22 years. The median OS
time in the group of newly diagnosed glioblastoma patients was 10
months, while in the group of recurrent glioblastoma patients, the
median OS time was 22 months. The PRS time for recurrent
glioblastoma patients was 5 months.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| WHO grade |
|---|
|
|
|
|---|
| Characteristic | I (n=10) | II (n=14) | III (n=23) | IV (n=48) | IV nd (n=26) | IV rec (n=22) |
|---|
| Age at diagnosis,
years |
|
|
|
|
|
|
|
Median | 20.0 | 37.5 | 41.0 | 56.5 | 58.5 | 53.0 |
|
Range | 1–29 | 23–69 | 24–75 | 18–80 | 21–80 | 18–79 |
| Gender, n |
|
|
|
|
|
|
|
Female | 5 | 4 | 10 | 15 | 10 | 5 |
|
Male | 5 | 10 | 13 | 33 | 16 | 17 |
| Survival, months
(events) |
|
|
|
|
|
|
| Median
OS | 264 (2) | 172 (3) | 41 (12) | 14 (39) | 10 (21) | 22 (18) |
|
(95% CI) | – | – | – | (2.9–7) | (2.9–6) | (3–14.2) |
| Median
PRS | n. a. | n. a. | n. a. | n. a. | n. a. | 5 (18) |
|
(95% CI) |
|
|
|
|
| (4.0–10.1) |
IL-33 is expressed in human gliomas
and is associated with inferior survival in patients with recurrent
glioblastoma
Protein levels of IL-33 were assessed by
immunohistochemistry using a TMA (Fig. 1A
and B). The TMA cores of normal brain tissue showed a number of
IL-33-negative (IL-33−) neuronal cells and only very
few, scattered, normal astrocytes with weak nuclear IL-33
expression. When captured on the TMA, infiltrating immune cells
were negative. The IL-33 mean labeling indexes in the tumor cells
were 13.3% [range, 0–65%; 95% confidence interval (CI), 2.2–28.7)
for AI, 15.4% (range 0–70%; 95% CI, 2.6–28.1) for AII, 19.7%
(range, 0–60%; 95% CI, 9.2–30.2) for AAIII and 21.7% (range,
0–92.5%; 95% CI, 13.1–30.3) for glioblastoma (Fig. 1B). Thus, the highest mean IL-33
labeling indexes were observed in glioblastoma, but these did not
differ significantly from gliomas of other grades. When newly
diagnosed and recurrent tumor samples for glioblastoma patients
were analyzed separately, the mean percentages were 19.4% (range,
0–92.5%; 95% CI, 8.9–29.9) for newly diagnosed tumors and 24.4%
(range, 0–90%; 95% CI, 9.4–39.5) for recurrent tumors (Fig. 1C). Based on the percentages of the
IL-33 labeling indexes, patients were divided into two groups:
Negative (0%) and positive (≥1%) for IL-33. The ratios of
IL-33− to IL-33+ patients per WHO grade were
as follows: AI, 50/50; AII, 57/43; AAIII, 57/43; and glioblastoma,
48/52. Nuclear IL-33 was also regularly found in endothelial cells
of vessels that were randomly captured on TMA cores of tumor and
normal brain tissues (data not shown).
 | Figure 1.IL-33 protein labeling in human
gliomas. (A) Representative tissue sections demonstrating no
nuclear staining of IL-33 in normal brain tissues, but strong
nuclear staining in glioblastoma. (B) The percentage of IL-33
nuclear-positive cells was assessed by immunohistochemistry of
human normal brain tissues (NB), World Health Organization grade I
(AI), II (AII) and III astrocytomas (AAIII), and glioblastoma
(grade IV astrocytomas) samples. Each tissue sample is indicated by
a dot. The black bar marks the mean in each group. (C) The
percentage of IL-33 nuclear-positive cells was assessed in nd and
rec glioblastomas. (D) Progression-free survival and (E) overall
survival are shown in nd (left) and rec (right) patients. (F)
Post-recurrence survival data. IL-33+ patients were
compared with IL-33− patients (P<0.05 was considered
significant). IL-33, interleukin-33; n.s., not significant; nd,
newly diagnosed; rec, reccurrent; IL-33+,
IL-33-positive; IL-33−, IL-33-negative. |
To search for an association between IL-33 labeling
indexes and survival, patients with glioblastoma were divided into
two groups, defined as positive or negative for this cytokine.
Patient characteristics of IL-33− or IL-33+
patients separated for newly diagnosed or recurrent tumors are
summarized in Table II. In the group
of newly diagnosed glioblastomas (n=26), the median age was 63
years in the IL-33− group and 57 years in the
IL-33+ group. There was a male predominance in the group
of IL-33− patients. The pre-operative Karnofsky
performance score (KPS) was similar in the two groups. Treatment
regimes in the groups differed: While the majority of
IL-33− patients had received TMZ/RT→TMZ (66.7%), the
majority of IL-33+ patients had received RT only (42.9%)
or TMZ/RT (21.4%), due to early tumor progression (Table II). The median PFS time was 6 months
for the IL-33− patients and 3 months for the
IL-33+ patients (P=0.709) (Fig. 1D). The OS time was 13 months for the
IL-33− patients and 9 months for the IL-33+
patients (P=0.815) (Fig. 1E). In the
recurrent glioblastoma group (n=22), the median age was 50 years in
the IL-33− group and 59 years in the IL-33+
group. The pre-operative KPS was similar in the two groups. Prior
to second surgery, the majority of IL-33− patients had
received TMZ/RT→TMZ (27.3%) or RT→TMZ (27.3%), whereas the group of
IL-33+ patients received TMZ/RT→TMZ (54.5%) (Table II). In total, 45.5% of the recurrent
patients in each group, IL-33+ or IL-33−,
received TMZ alone as post-surgery treatment at the time of
recurrence (Table II). The median
PFS time was 13 months for the IL-33− patients and 6
months for the IL-33+ patients (P=0.308) (Fig. 1D). The median OS time was 34 months
for the IL-33− patients and 14 months for the
IL-33+ patients (P=0.045) (Fig. 1E). The median PRS time was 10 months
for the IL-33− patients and 4 months for the
IL-33+ patients (P=0.026) (Fig. 1F).
 | Table II.Clinical characteristics, treatment
and outcome in human glioblastoma by IL-33 protein labeling
indexes. |
Table II.
Clinical characteristics, treatment
and outcome in human glioblastoma by IL-33 protein labeling
indexes.
|
| Newly
diagnosed | Recurrent |
|---|
|
|
|
|
|---|
| Characteristic | IL-33−
(n=12) | IL-33+
(n=14) | IL-33−
(n=11) | IL-33+
(n=11) |
|---|
| Age, years |
|
|
|
|
|
Median | 63 | 57 | 50 | 59 |
|
Range | 21–77 | 41–80 | 21–63 | 18–79 |
| Age groups, n
(%) |
|
|
|
|
| ≤40
years | 1 (8.3) | 0 (0.0) | 3
(27.3) | 3
(27.3) |
| 41–50
years | 2
(16.7) | 4
(28.6) | 3
(27.3) | 0 |
| 51–60
years | 3
(25.0) | 4
(28.6) | 3
(27.3) | 4
(36.4) |
| 61–70
years | 1 (8.3) | 3
(21.4) | 2
(18.2) | 3
(27.3) |
| >70
years | 5
(41.7) | 3
(21.4) | 0 (0.0) | 1 (9.1) |
| Gender, n (%) |
|
|
|
|
|
Female | 1 (8.3) | 9
(64.3) | 1 (9.1) | 4
(36.4) |
|
Male | 11 (91.7) | 5
(35.7) | 10 (90.9) | 7
(63.6) |
| KPS
(pre-operative), n (%) |
|
|
|
|
|
90–100 | 1 (8.3) | 1 (7.1) | 5
(45.5) | 3
(27.3) |
|
70–80 | 8
(66.7) | 11 (78.6) | 5
(45.5) | 7
(63.6) |
|
<70 | 3
(25.0) | 2
(14.3) | 1 (9.1) | 1 (9.1) |
| Tumor localization,
n (%) |
|
|
|
|
|
Frontal | 2
(16.7) | 3
(21.4) | 1 (9.1) | 4
(36.4) |
|
Parietal | 0 (0.0) | 2
(14.3) | 3
(27.3) | 0 (0.0) |
|
Temporal | 3
(25.0) | 7
(50.0) | 5
(45.5) | 5
(45.5) |
|
Occipital | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Brainstem | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Cerebellar | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Spinal | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Multifocal (>2
regions) | 5
(41.7) | 2
(14.3) | 2
(18.2) | 2
(18.2) |
| Surgery, n (%) |
|
|
|
|
|
Biopsy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Partial
resection | 2
(16.7) | 2
(14.3) | 1 (9.1) | 2
(18.2) |
|
Subtotal resection | 9
(75.0) | 2
(14.3) | 3
(27.3) | 6
(54.5) |
| Gross
total resection | 1 (8.3) | 10 (71.4) | 7
(63.6) | 2
(18.2) |
| No
data | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (9.1) |
| First-line therapy,
n (%) |
|
|
|
|
| No
therapy | 2
(16.7) | 2
(14.3) | 1 (9.1) | 2
(18.2) |
| RT
alone | 1 (8.3) | 6
(42.9) | 2
(18.2) | 1 (9.1) |
|
RT→TMZ | 3
(25.0) | 1 (7.1) | 3
(27.3) | 2
(18.2) |
|
TMZ/RT | 0 (0.0) | 3
(21.4) | 2
(18.2) | 0 (0.0) |
|
TMZ/RT→TMZ | 8
(66.7) | 1 (7.1) | 3
(27.3) | 6
(54.5) |
| TMZ
alone | 0 (0.0) | 1 (7.1) | 0 (0.0) | 0 (0.0) |
| No
data | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Post-second or
-third surgery treatmenta, n (%) |
|
|
|
|
| No
therapy |
|
| 2
(18.2) | 1 (9.1) |
|
TMZ/RT→TMZ |
|
| 1 (9.1) | 1 (9.1) |
| TMZ
alone |
|
| 5
(45.5) | 5
(45.5) |
|
Lomustine alone | n. a. | n. a. | 2
(18.2) | 0 (0.0) |
|
Gefitinib alone |
|
| 0 (0.0) | 1 (9.1) |
|
Bevacizumab plus
irinotecan |
|
| 0 (0.0) | 2
(18.2) |
| No
data |
|
| 1 (9.1) | 1 (9.1) |
| Survival |
|
|
|
|
| Median
follow-up, months | 12 | 7.5 | 27 | 14 |
| Median
PFS, months (events) | 6
(11) | 3 (13) | 13
(11) | 6
(11) |
|
(95% CI) | (1.9–8.9) | (0.9–5) | (3–27.9) | (3–12) |
| Median
PRS, months (events) | n.a. | n.a. | 10 (7) | 4
(11) |
|
(95% CI) |
|
| (3.9–54.9) | (−) |
| Median
OS, months (events) | 13 (11) | 9 (10) | 34 (7) | 14 (11) |
|
(95% CI) | (1.9–19.8) | (3.8–11.9) | (9.9–30.5) | (−) |
| Alive
at last follow up, n (%) | 1 (8.3) | 4 (28.6) | 4 (36.4) | 0 (0.0) |
IL-33 expression in human gliomas: An
analysis of the TCGA database
Microarray data were acquired from the TCGA database
(36) and a glioma population
(37), including AI (n=8), AII
(n=24), AAIII (n=85) and glioblastoma (n=159) samples selected for
validation of IL-33 expression and its prognostic significance.
Another 8 normal brain samples were included. The median mRNA
expression levels of IL-33 were 6.2 (95% CI, 5.3–6.9) for normal
brain tissues, 6.8 (95% CI, 5.31–6.85) for AI, 7.3 (95% CI,
6.5–7.5) for AII, 6.4 (95% CI, 5.8–6.6) for AAIII and 7.0 (95% CI,
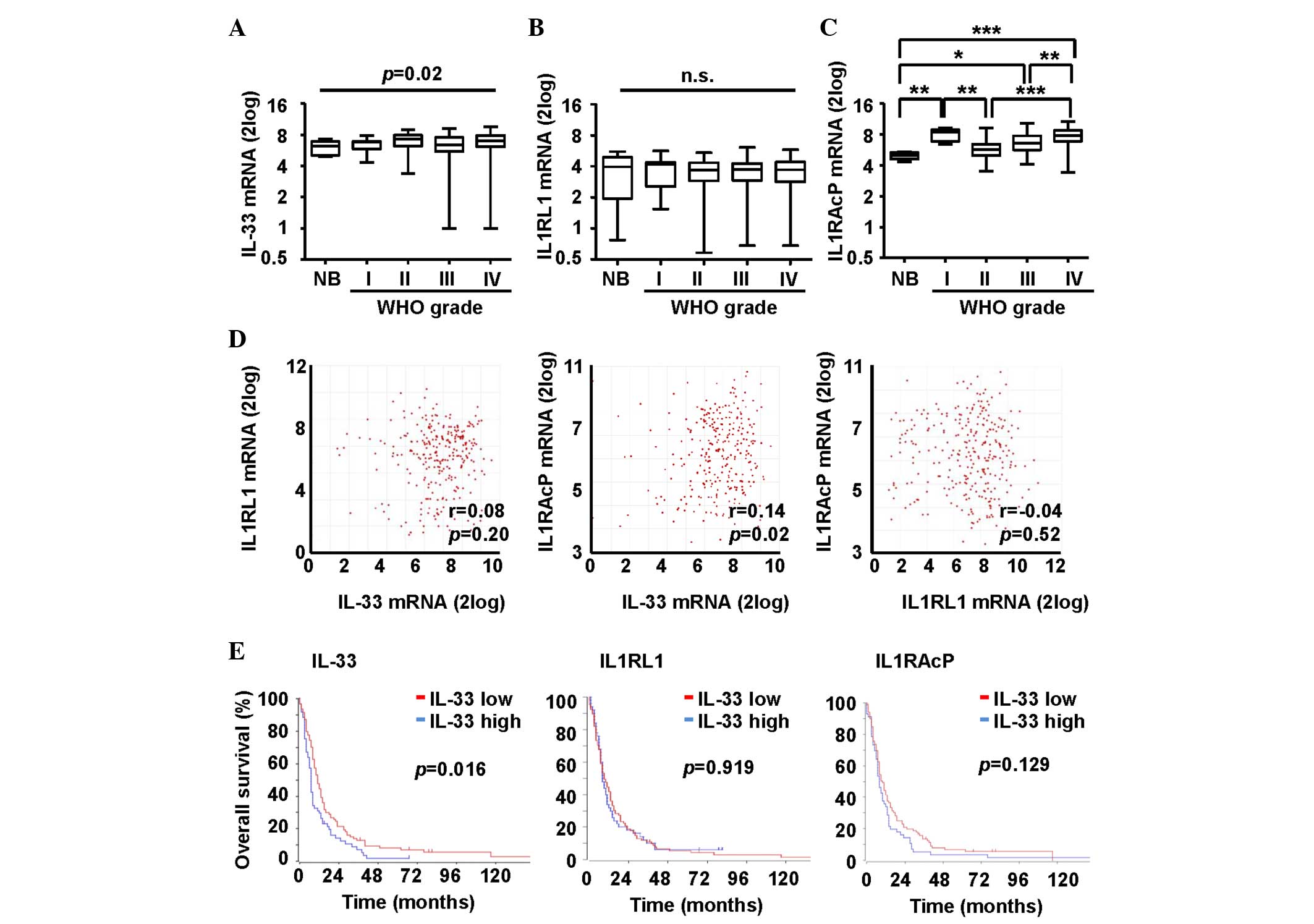
6.7–7.1) for glioblastoma (Fig. 2A).
The median IL-33 mRNA expression levels varied significantly
(P=0.02), but multiple comparisons between each tumor grade did not
demonstrate statistically significant results (Fig. 2A).
Next, the IL-33 receptors, IL1RL1 and IL1RAcP, were
analyzed. The median mRNA expression levels of IL1RL1 were 6.2 (95%
CI, 5.3–6.9) for normal brain tissues, 6.8 (95% CI, 5.31–6.85) for
AI, 7.3 (95% CI, 6.5–7.5) for AII, 6.4 (95% CI, 5.8–6.6) for AAIII
and 7.0 (95% CI, 6.7–7.1) for glioblastoma (Fig. 2B). mRNA levels were not increased or
decreased in any of the analyzed groups compared with each other.
By contrast, the median IL1RAcP mRNA levels were increased in the
glioblastoma patients compared with that of the patients diagnosed
with AII (P<0.01) or AAIII (P<0.01). The median mRNA
expression levels of IL1RAcP were 5 (95% CI, 4.6–5.3) for normal
brain tissues, 8.4 (95% CI, 7.1–8.9) for AI, 5.7 (95% CI, 5.3–6.4)
for AII, 6.6 (95% CI, 6.4–7.1) for AAIII and 7.9 (95% CI, 7.6–8)
for glioblastoma (Fig. 2C). A
correlation in mRNA expression levels was found between IL-33 and
IL1RAcP (r=0.14, P=0.02), but not between IL-33 and IL1RL1 (r=0.08,
P=0.20). There were no correlations between the IL-33 receptors
(r=−0.04, P=0.52) (Fig. 2D). Finally,
microarray and outcome data for glioblastoma were obtained from the
TCGA database. Patients were divided into two groups with high or
low IL-33 mRNA expression. The cut-off was defined by the average
mRNA expression level of the target gene. Patients with higher
IL-33 mRNA expression levels experienced inferior survival
(P=0.016). IL1RL1 and IL1RAcP mRNA expression levels were not
associated with survival in the group of glioblastoma patients
(Fig. 2E).
Discussion
Members of the IL-1 family (e.g., IL-1β, IL-6 and
IL-8) are expressed by glioma cells and modulate survival,
migration and invasion (8). IL-33,
another IL-1 family member, is a pleiotropic cytokine that may be
involved in the pathogenesis of brain diseases (38). IL-33 is a pro-inflammatory mediator
activating microglia, and inducing inflammatory cytokines and
chemokines, and thus may have neuroprotective or neurotoxic effects
depending on tissue conditions. In a previous study, the increased
expression of IL-33 contributed to enhanced cell growth of rat
glioma cells and the invasion of microglia (34). Moreover, exogenously administrated
IL-33 enhanced primary tumor growth and inhibited innate antitumor
immunity in a metastatic breast cancer model (39).
The purpose of the present study was to assess IL-33
expression in human astroglial brain tumors and to evaluate its
prognostic significance. Normal brain sections were found to be
negative for IL-33 protein expression. The high level IL-33
detection in certain astrocytomas suggested a pathogenetic role for
this cytokine (Fig. 1B). On the other
hand, similar levels of IL-33 across all WHO grades showed IL-33
production to be independent of the grade of malignancy.
Furthermore, protein levels of IL-33 were markedly similar among
newly diagnosed and recurrent glioblastomas, indicating that
treatment in this patient population does not significantly affect
its expression (Fig. 1C). IL-33
protein labeling indexes were not associated with PFS in the
glioblastoma patients (Fig. 1D).
However, in the subgroup of recurrent glioblastoma patients,
IL-33+ tumors demonstrated inferior OS and PRS compared
with IL-33− tumors (Fig. 1E
and F). This could indicate that IL-33 expression may be an
important feature for advanced glioblastoma patients. In line with
these indications, IL-33 is known to enhance tumor surveillance and
antitumor immunity (40,41). Therefore, IL-33 may contribute to
tumor progression and antitumor activity, depending on the levels
of IL-33 and the microenvironment. The microenvironment of gliomas
in situ comprises not only stromal CNS-intrinsic cell types,
such as astrocytes and microglial cells, but also inflammatory
cells that have infiltrated into the tumor from the circulation,
including lymphocytes, macrophages, neutrophils and eosinophils
(42,43). Although these infiltrating immune
cells may seek to curb tumor growth initially, evidence points to
the tumor subsequently affecting the immune cells to allow its
growth. Necrosis or inflammation in the CNS causes the release of
IL-33, particularly from astrocytes and glioma cells. However,
since there is a population of resident mast cells in the CNS
(44,45), it would also lead to the secretion of
IL-33 by the mast cells. This could have profound effects in glioma
progression since IL-33 may then in turn act not only on
astrocytes, but also on tumor cells and possibly other CNS glial
cells to induce STAT6-responsive genes to complete an important,
neural-immune circuit (46).
TCGA interrogation in the present study indicated
that the mRNA levels of IL-33, as well as those of IL1RAcP, are
upregulated in human glioblastoma (Fig.
2A and C), and that IL-33 mRNA expression correlates with
IL1RAcP mRNA expression (Fig. 2D),
suggesting that there may be an IL-33 autocrine loop in CNS tumors
derived from glial cells, particularly astrocytes (28). TCGA also made it possible to delineate
an association between IL-33 mRNA expression and inferior survival
in glioblastoma patients (Fig. 2E),
suggesting that the IL-33-expressing phenotype defines a glioma
subset with a more aggressive course and a worse prognosis.
Angiogenesis is another hallmark of glioblastoma.
Notably, Choi et al demonstrated that IL-33 promotes
angiogenesis and vasopermeability in vivo and in
vitro (47). Endothelial cells
are a source of IL-33 and also express the heterodimeric IL1RL1
(27). In the present study, IL-33
was not expressed in all endothelial cells of normal brain and
intra-/peritumoral glioma vessels, but this could not be addressed
systematically since vessels were not captured on all TMA cores,
and the TMA was designed to study tumor tissues. The TMA-based
approach allowed for large-scale analysis of multiple human glioma
cases. However, the approach is by nature limited to the study of
IL-33 expression in the tumor area selected on the TMA. Therefore,
a systematic evaluation of different tumor subregions (e.g.,
necrotic or vascular areas) was not possible.
The present study data suggested that IL-33 may play
a role in human glioma development, growth and progression. This
could be due to IL-33-mediated communication between glioma cells
and components of the tumor microenvironment, such as resident
astrocytes, microglia and inflammatory or endothelial cells.
Alternatively, IL-33 overexpression in glioma could be a bystander
effect reflecting a more general disease-associated phenomenon, as
IL-33 is upregulated in other neurological conditions as well
(48). The complex interactive
network of IL-33 and the extent to which the IL-33/IL1RL1/IL1RAcP
axis can be affected by therapeutic intervention will require
elucidation in human glioma.
Acknowledgements
The authors thank Melanie Sachs (Institute of
Pathology Liestal, Cantonal Hospital Baselland, Liestal,
Switzerland) and Martina Storz (Department of Pathology, Institute
of Surgical Pathology, University Hospital Zurich, Zürich,
Switzerland) for their expert technical assistance. This study was
supported by a grant from Oncosuisse (no. KLS 3110-02-2013) and by
a Filling-the-Gap personal grant from the University of Zurich.
References
|
1
|
Gramatzki D, Dehler S, Rushing EJ, Zaugg
K, Hofer S, Yonekawa Y, Bertalanffy H, Valavanis A, Korol D,
Rohrmann S, et al: Glioblastoma in the Canton of Zurich,
Switzerland revisited: 2005 to 2009. Cancer. Apr 18–2016.(Epub
ahead of print). View Article : Google Scholar
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weller M, van den Bent M, Hopkins K, Tonn
JC, Stupp R, Falini A, Cohen-Jonathan-Moyal E, Frappaz D,
Henriksson R, Balana C, et al: EANO guideline for the diagnosis and
treatment of anaplastic gliomas and glioblastoma. Lancet Oncol.
15:e395–e403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huse JT and Holland EC: Targeting brain
cancer: Advances in the molecular pathology of malignant glioma and
medulloblastoma. Nat Rev Cancer. 10:319–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Charles NA, Holland EC, Gilbertson R,
Glass R and Kettenmann H: The brain tumor microenvironment. Glia.
59:1169–1180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin Y, Zhang G, Zhang J, Gao G, Li M, Chen
Y, Wang J, Li G, Song SW, Qiu X, et al: A panel of four cytokines
predicts the prognosis of patients with malignant gliomas. J
Neurooncol. 114:199–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prosniak M, Harshyne LA, Andrews DW,
Kenyon LC, Bedelbaeva K, Apanasovich TV, Heber-Katz E, Curtis MT,
Cotzia P and Hooper DC: Glioma grade is associated with the
accumulation and activity of cells bearing M2 monocyte markers.
Clin Cancer Res. 19:3776–3786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yeung YT, McDonald KL, Grewal T and Munoz
L: Interleukins in glioblastoma pathophysiology: Implications for
therapy. Br J Pharmacol. 168:591–606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu VF, Yang J, Lebrun DG and Li M:
Understanding the role of cytokines in glioblastoma multiforme
pathogenesis. Cancer Lett. 316:139–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schiering C, Krausgruber T, Chomka A,
Fröhlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J,
Hegazy AN, et al: The alarmin IL-33 promotes regulatory T-cell
function in the intestine. Nature. 513:564–568. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beltrán CJ, Núñez LE, Díaz-Jiménez D,
Farfan N, Candia E, Heine C, López F, González MJ, Quera R and
Hermoso MA: Characterization of the novel ST2/IL-33 system in
patients with inflammatory bowel disease. Inflamm Bowel Dis.
16:1097–1107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Palmer G and Gabay C: Interleukin-33
biology with potential insights into human diseases. Nat Rev
Rheumatol. 7:321–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Préfontaine D, Nadigel J, Chouiali F,
Audusseau S, Semlali A, Chakir J, Martin JG and Hamid Q: Increased
IL-33 expression by epithelial cells in bronchial asthma. J Allergy
Clin Immunol. 125:752–754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Byers DE, Alexander-Brett J, Patel AC,
Agapov E, Dang-Vu G, Jin X, Wu K, You Y, Alevy Y, Girard JP, et al:
Long-term IL-33-producing epithelial progenitor cells in chronic
obstructive lung disease. J Clin Invest. 123:3967–3982. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kempf W, Zollinger T, Sachs M, Ullmer E,
Cathomas G, Dirnhofer S and Mertz KD: Granulomas are a source of
interleukin-33 expression in pulmonary and extrapulmonary
sarcoidosis. Hum Pathol. 45:2202–2210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moulin D, Donzé O, Talabot-Ayer D, Mézin
F, Palmer G and Gabay C: Interleukin (IL)-33 induces the release of
pro-inflammatory mediators by mast cells. Cytokine. 40:216–225.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmitz J, Owyang A, Oldham E, Song Y,
Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et
al: IL-33, an interleukin-1-like cytokine that signals via the IL-1
receptor-related protein ST2 and induces T helper type 2-associated
cytokines. Immunity. 23:479–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chackerian AA, Oldham ER, Murphy EE,
Schmitz J, Pflanz S and Kastelein RA: IL-1 receptor accessory
protein and ST2 comprise the IL-33 receptor complex. J Immunol.
179:2551–2555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Küchler AM, Pollheimer J, Balogh J,
Sponheim J, Manley L, Sorensen DR, De Angelis PM, Scott H and
Haraldsen G: Nuclear interleukin-33 is generally expressed in
resting endothelium but rapidly lost upon angiogenic or
proinflammatory activation. Am J Pathol. 173:1229–1242. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moussion C, Ortega N and Girard JP: The
IL-1-like cytokine IL-33 is constitutively expressed in the nucleus
of endothelial cells and epithelial cells in vivo: A novel
‘alarmin’? PLoS One. 3:e33312008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carriere V, Roussel L, Ortega N, Lacorre
DA, Americh L, Aguilar L, Bouche G and Girard JP: IL-33, the
IL-1-like cytokine ligand for ST2 receptor, is a
chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci
USA. 104:282–287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alves-Filho JC, Sônego F, Souto FO,
Freitas A, Verri WA Jr, Auxiliadora-Martins M, Basile-Filho A,
McKenzie AN, Xu D, Cunha FQ and Liew FY: Interleukin-33 attenuates
sepsis by enhancing neutrophil influx to the site of infection. Nat
Med. 16:708–712. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haraldsen G, Balogh J, Pollheimer J,
Sponheim J and Küchler AM: Interleukin-33-cytokine of dual function
or novel alarmin? Trends Immunol. 30:227–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roussel L, Erard M, Cayrol C and Girard
JP: Molecular mimicry between IL-33 and KSHV for attachment to
chromatin through the H2A-H2B acidic pocket. EMBO Rep. 9:1006–1012.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiong Z, Thangavel R, Kempuraj D, Yang E,
Zaheer S and Zaheer A: Alzheimer's disease: Evidence for the
expression of interleukin-33 and its receptor ST2 in the brain. J
Alzheimers Dis. 40:297–308. 2014.PubMed/NCBI
|
|
26
|
Christophi GP, Gruber RC, Panos M,
Christophi RL, Jubelt B and Massa PT: Interleukin-33 upregulation
in peripheral leukocytes and CNS of multiple sclerosis patients.
Clin Immunol. 142:308–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yasuoka S, Kawanokuchi J, Parajuli B, Jin
S, Doi Y, Noda M, Sonobe Y, Takeuchi H, Mizuno T and Suzumura A:
Production and functions of IL-33 in the central nervous system.
Brain Res. 1385:8–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hudson CA, Christophi GP, Gruber RC,
Wilmore JR, Lawrence DA and Massa PT: Induction of IL-33 expression
and activity in central nervous system glia. J Leukoc Biol.
84:631–643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han P, Mi WL and Wang YQ: Research
progress on interleukin-33 and its roles in the central nervous
system. Neurosci Bull. 27:351–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kempuraj D, Khan MM, Thangavel R, Xiong Z,
Yang E and Zaheer A: Glia maturation factor induces interleukin-33
release from astrocytes: Implications for neurodegenerative
diseases. J Neuroimmune Pharmacol. 8:643–650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun P, Ben Q, Tu S, Dong W, Qi X and Wu Y:
Serum interleukin-33 levels in patients with gastric cancer. Dig
Dis Sci. 56:3596–3601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bergis D, Kassis V, Ranglack A, Koeberle
V, Piiper A, Kronenberger B, Zeuzem S, Waidmann O and Radeke HH:
High serum levels of the interleukin-33 receptor soluble ST2 as a
negative prognostic factor in hepatocellular carcinoma. Transl
Oncol. 6:311–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu LA, Fu Y, Zhang DN and Zhang J: Serum
IL-33 as a diagnostic and prognostic marker in non-small cell lung
cancer. Asian Pac J Cancer Prev. 14:2563–2566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fang KM, Yang CS, Lin TC, Chan TC and
Tzeng SF: Induced interleukin-33 expression enhances the
tumorigenic activity of rat glioma cells. Neuro Oncol. 16:552–566.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Louis DN, Ohgaki H, Wiestler B and Cavenee
WK: WHO Classification of Tumours of the Central Nervous System.
IARC Press. Lyon: 2007.
|
|
36
|
Cancer Genome Atlas Research Network:
Comprehensive genomic characterization defines human glioblastoma
genes and core pathways. Nature. 455:1061–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gravendeel LA, Kouwenhoven MC, Gevaert O,
de Rooi JJ, Stubbs AP, Duijm JE, Daemen A, Bleeker FE, Bralten LB,
Kloosterhof NK, et al: Intrinsic gene expression profiles of
gliomas are a better predictor of survival than histology. Cancer
Res. 69:9065–9072. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singhal G, Jaehne EJ, Corrigan F, Toben C
and Baune BT: Inflammasomes in neuroinflammation and changes in
brain function: A focused review. Front Neurosci. 8:3152014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jovanovic IP, Pejnovic NN, Radosavljevic
GD, Arsenijevic NN and Lukic ML: IL-33/ST2 axis in innate and
acquired immunity to tumors. Oncoimmunology. 1:229–231. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Villarreal DO, Wise MC, Walters JN,
Reuschel EL, Choi MJ, Obeng-Adjei N, Yan J, Morrow MP and Weiner
DB: Alarmin IL-33 acts as an immunoadjuvant to enhance
antigen-specific tumor immunity. Cancer Res. 74:1789–1800. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao K, Li X and Zhang L, Bai L, Dong W,
Gao K, Shi G, Xia X, Wu L and Zhang L: Transgenic expression of
IL-33 activates CD8(+) T cells and NK cells and inhibits tumor
growth and metastasis in mice. Cancer Lett. 335:463–471. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Curran CS and Bertics PJ: Eosinophils in
glioblastoma biology. J Neuroinflammation. 9:112012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hussain SF, Yang D, Suki D, Aldape K,
Grimm E and Heimberger AB: The role of human glioma-infiltrating
microglia/macrophages in mediating antitumor immune responses.
Neuro Oncol. 8:261–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dong H, Zhang X and Qian Y: Mast cells and
neuroinflammation. Med Sci Monit Basic Res. 20:200–206. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Skaper SD, Facci L and Giusti P: Mast
cells, glia and neuroinflammation: Partners in crime? Immunology.
141:314–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Merk BC, Owens JL, Lopes MB, Silva CM and
Hussaini IM: STAT6 expression in glioblastoma promotes invasive
growth. BMC Cancer. 11:1842011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Choi YS, Choi HJ, Min JK, Pyun BJ, Maeng
YS, Park H, Kim J, Kim YM and Kwon YG: Interleukin-33 induces
angiogenesis and vascular permeability through ST2/TRAF6-mediated
endothelial nitric oxide production. Blood. 114:3117–3126. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gadani SP, Walsh JT, Smirnov I, Zheng J
and Kipnis J: The glia-derived alarmin IL-33 orchestrates the
immune response and promotes recovery following CNS injury. Neuron.
85:703–709. 2015. View Article : Google Scholar : PubMed/NCBI
|