Introduction
Oral leukoplakia (OLK) is the most common oral
precancerous lesion, with a global prevelance of 1% (1) and a malignant transformation rate of
0.13–17% (2). Following OLK
transformation to oral cancer, the 5- and 10-year survival rates
are 59 and 48%, respectively (3).
Currently, the pathogenesis of OLK is unclear. Numerous studies
have demonstrated that OLK is closely associated with smoking,
drinking and betel chewing (4–8). Tobacco,
betel nut and alcohol all increase the expression of the oxidant
H2O2 in saliva and oral mucosal cells
(9,10), and H2O2
expression at a high level may result in oxidative damage of DNA
and activation of apoptotic genes, thus inducing apoptosis of cells
(11–13). Reactive oxygen species (ROS) is a
collective term that describes O2-derived non-radical
species, including H2O2, and
O2-derived free radicals, such as superoxide anion,
hydroxyl and peroxyl free radicals. At physiological low levels,
ROS functions as redox messengers in intracellular signaling and
regulation. However, excessive ROS induce oxidative modification of
macromolecules, inhibit protein functions and promote apoptosis of
cells (14).
Peroxiredoxins (Prxs) are thio-specific antioxidant
enzymes, and may be induced by several types of oxidative stress
conditions. They are associated with neutralizing cellular
hydroperoxides, which protect cells from oxidative damage. Prxs are
often identified in mammals, yeast and bacteria, which are
classified as 1-cys Prx and 2-cys Prx on the basis of one or two
conserved cysteine residues. Peroxiredoxin 1 (Prx1), as an
important member of Prxs, has two conserved cysteine residues
(15). Current evidence suggests that
Prx1, as a simple peroxidase, initiates the mechanistic switch from
peroxidase to chaperone function, meaning that it is closely
associated with a variety of biological processes including cell
proliferation, differentiation and apoptosis (16).
Yanagawa et al (17,18) have
identified that an overexpression of Prx1 is significantly
associated with the recurrence of oral squamous cell carcinoma
(OSCC). Previous studies by the present authors have confirmed that
Prx1 expression and 8-hydroxy-2′-deoxyguanosine (8-OHdG) expression
levels are elevated in human OLK tissues, and an increase in 8-OHdG
is consistent with the expression of Prx1 (19). This result indicates that there is a
significant association between Prx1 and oxidative damage in the
progression of OLK. Whether Prx1 is important in OLK remains
unknown, and the mechanism associated with Prx1 and apoptosis or
oxidative stress remains unclear.
Apoptosis signal-regulating kinase 1 (ASK1) is a
serine-threonine protein kinase that functions as a
mitogen-activated protein kinase (MAPK), which activates c-Jun
N-terminal kinase (JNK) and p38 MAPK signaling cascades. ASK1 may
be activated by various stresses and is critical in the regulation
of signaling in response to oxidative stress, which is a major
contributor to cell death (20–22). Kim
et al (23) have demonstrated
that Prx1 plays a negative role in regulating ASK1-induced
apoptosis. However, to the best of our knowledge, there is no
evidence that reveals similar results in vivo.
In the present study, 4-nitroquinoline-1-oxide
(4NQO) was used to establish a precancerous lesion model in
wild-type and Prx1 knockout mice, to investigate the apoptotic role
of Prx1 in oral precancerous lesions based on the hypothesis that
Prx1 may mediate the ASK1/p38 signalling pathway. In addition, the
effect of oxidative stress on Prx1 and apoptosis in oral
precancerous lesions was also determined. Understanding the
molecular mechanisms of Prx1 involved in the initiation and
progression to malignancy may benefit methods for the prognosis and
treatment of oral precancerous lesions.
Materials and methods
Experimental animals
A total of 50 wild-type C57BL/6 mice (Vital River
Laboratory Animal Technology Co., Ltd., Shenzhen, China) and 50
Prx1 knockout mice, which had been previously established (24), aged 6–8 weeks old, were used in the
present study. All the animals were kept in accordance with
institutional guidelines in specific pathogen free units at 24±2°C
room temperature with 40–60% humidity, in a 14 day light/10 day
dark cycle with freely accessible water food. The experimental
protocol for the present study was approved by the local Ethical
Committee for Animal Use. The experimental mice were randomly
divided into six groups that underwent various treatments as
follows: Wild-type control (n=10), treatment with vehicle
(distilled water); wild-type 4NQO group (n=20), treatment with 50
µg/ml 4NQO (Sigma-Aldrich, St. Louis, MO, USA) every day; wild-type
4NQO + H2O2 group (n=20), treatment with 50
µg/ml 4NQO every day and 3% H2O2 smeared on
tongue mucosa three times a week; Prx1 knockout control group
(n=10), treatment with vehicle (distilled water); Prx1 knockout
4NQO group (n=20), treatment with 50 µg/ml 4NQO every day; and Prx1
knockout 4NQO + H2O2 group (n=20), treatment
with 50 µg/ml 4NQO every day and 3% H2O2
smeared on tongue mucosa three times a week. All these treatments
lasted for 16 weeks. The mice were euthanized and the tongues were
resected and immediately stored in liquid nitrogen for future
molecular/cellular analysis, or in formalin for the preparation of
paraffin-embedded tissue blocks.
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) assay
Apoptosis was examined using In Situ Cell
Death Detection kit, POD (Roche Diagnostics, Mannheim, Germany),
according to the manufacturer's protocol. The paraffin-embedded
tissues were baked at 65°C for 1 h, de-waxed using xylene and
gradually dehydrated with 100, 95, 90, 80 and 70% ethanol. The
specimens were washed twice with phosphate-buffered saline (PBS)
for 5 min each wash, treated with proteinase K solution (10 mM
Tris-HCl with 20 µg/ml proteinase K; Merck Millipore, Darmstadt,
Germany), incubated at 37°C for 15 min, and washed twice with PBS
for 5 min each wash. Dry specimens were treated with 50 µl TUNEL
reaction mixture (dilution, 1:5), covered with a cover slip,
hydrated in light-free conditions and incubated at 37°C for 60 min.
The specimens were subsequently washed three times with PBS for 5
min each wash, and dry specimens were treated with 50 µl
converter-POD, covered with a cover slip, hydrated in light-free
conditions, incubated at 37°C for 60 min, and washed three times in
PBS for 5 min each wash. Finally, the specimens were subjected to
incubation with freshly prepared 3,3′-diaminobenzidine (DAB)
solution for 10 min, hematoxylin staining, soaking twice in
anhydrous ethanol for 5 min and xylene for 2 min and mounting with
neutral gum.
Immunohistochemical staining
The paraffin-embedded mouse tongue specimens (4 µm)
were de-paraffinized and hydrated using gradient alcohol, and
rinsed with PBS. Antigen retrieval for Prx1, ASK1, phosphor-ASK1
and p38 was conducted with a citrate buffer (pH=6.0) in a microwave
oven, and for phosphor-p38 with an EDTA buffer. Subsequently, the
sections were blocked with 3% H2O2 at room
temperature for 15 min to remove the endogenous peroxidase and
incubated in 10% goat serum (Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd., Beijing, China) as a blocking solution at
37°C for 30 min. The specimens were incubated with the following
primary antibodies: Polyclonal rabbit anti-Prx1 (dilution, 1:5,000;
#ab41906; Abcam, Cambridge, MA, USA), polyclonal rabbit anti-ASK1
(dilution, 1:200; #bs-1425R; Bioss, Inc., Beijing, China),
monoclonal rabbit anti-phosphor-ASK1 (dilution, 1:400; #GTX50229;
GeneTex, Inc., Irvine, CA, USA), p38 (dilution, 1:800; #bs-0637R;
Bioss, Inc.) and phosphor-p38 (dilution, 1:200; #4631; Cell
Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight. The
specimens were incubated with biotinylated secondary IgG antibody
(from the MaxVision™ HRP-Polymer anti-Mouse IHC kit; Fuzhou Maixin
Biotech Co., Ltd., Fuzhou, China) at 37°C for 30 min, and then
visualized using DAB staining for 2–5 min. The specimens were
subjected to Mayer's hematoxylin staining, dehydration and
mounting. For the negative control, PBS was used in place of a
primary antibody. Hepatocellular carcinoma tissue and small
intestine tissue were used as the positive controls for Prx1 and
p38, respectively, while breast carcinoma tissue was used as the
positive control for ASK1, phosphor-ASK1 and phosphor-p38.
For evaluating the apoptosis level and the
expression of phosphor-p38, the cells with positive staining were
determined by counting the stained cells using Image-Pro Plus
version 7.0 (Media Cybernetics, Inc., Rockville, MD, USA). In
total, ~1,000 cells were counted for each tumor specimen. In order
to evaluate the expression of Prx1, ASK1, p38 and phosphor-ASK1,
the stained cells from three to five representative microscope
fields were counted for each specimen (magnification, ×200) and the
mean optical density (MOD) was calculated for each mouse tongue
tissue using Image-Pro Plus version 7.0 software as follows: MOD =
integrated option density / area.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from mouse tongue tissues
using TRIzol Reagent (Invitrogen™; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's protocol. cDNA
was synthesized by reverse transcribing 2 µg RNA with the
High-Capacity cDNA Reverse Transcription kit (Applied
Biosystems®; Thermo Fisher Scientific, Inc.). In total,
1 µl aliquots of cDNA were used as the templates for qPCR.
Sequences for all target gene primers were synthesized by Sangon
Biotech (Shanghai, China) as follows: Prx1, forward:
5′-AATGCAAAAATTGGGTATCCTGC-3′ and reverse
5′-CGTGGGACACACAAAAGTAAAGT-3′; ASK1, forward:
5′-AAGTCCCAACCCATAGAAATTCCT-3′ and reverse
5′-AGCCAGTCGGTAAGTTCAGAATCTT-3′; p38, forward
5′-GAGCTGAAGATTCTGGATTTTGG-3′ and reverse
5′-TAGCCACGTAGCCGGTCATT-3′; glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), forward 5′-AGGTCGGTGTGAACGGATTTG-3′ and
reverse 5′-TGTAGACCATGAGTTGAGGTCA-3′. The cycling conditions for
RT-PCR were as follows: 25°C for 10 min, 37°C for 120 min and 85°C
for 5 min. The UltraSYBR Mixture (With ROX) (ComWin Biotech Co.,
Ltd., Beijing, China) was used for qPCR, and the cycling conditions
were as follows: 95°C for 10 min, 95°C for 15 sec and 60°C for 15
sec for 40 cycles. For data analysis, the 2−ΔΔCq method
(25) was used for the normalization
of the genes of interest against GAPDH. The experiments were
conducted three times.
Statistical analysis
Statistically significant differences were analyzed
by χ2, two-tailed Student's t-test and Kruskal-Wallis
one-way analysis of variance test. Bonferroni was used as a
post-hoc test. SPSS version 17.0 software (SPSS, Inc., Chicago, IL,
USA) was used for analysis. P<0.05 was considered to indicate a
statistically significant difference. P<0.017 was considered to
indicate a statistically significant difference in the Bonferroni
test.
Results
Tongue precancerous lesion model
established in Prx1 knockout mice
4NQO was used to induce the development of tongue
precancerous lesions in Prx1 knockout and wild-type mice. No tongue
precancerous lesions were observed in the control mice at the end
of the 16th week, while in Prx1 knockout and wild-type mice treated
with 4NQO or 4NQO + H2O2 the tongues of the
mice exhibited white, thick, rough and visible white patches as
well as surface toughness. Histological observation revealed
epithelial dysplasia with varying degrees and OSCC on the tongues,
indicating that the model of tongue precancerous lesions in Prx1
knockout mice was successfully established. There was a significant
decrease in the degree of moderate or severe epithelial dysplasia
(P=0.016), and mild epithelial dysplasia was clearly elevated
(P=0.011), in Prx1 knockout mice treated with 4NQO +
H2O2 compared with wild-type mice treated
with 4NQO + H2O2 (Fig. 1; Table
I). The application of 3% H2O2 alone (3
times/week) did not induce epithelial dysplasia of tongue mucosa
over 16 weeks (data not shown). These results indicated that Prx1
and H2O2 play a coordination role in
promoting the progression of tongue precancerous lesions.
 | Table I.Incidence and type of mouse tongue
precancerous lesions in six experimental mouse models. |
Table I.
Incidence and type of mouse tongue
precancerous lesions in six experimental mouse models.
| Group | n | Normal mucosal, n
(%) | Mild dysplasia, n
(%) | Moderate-severe
dysplasia, n (%) | OSCC, n (%) |
|---|
| Total | 100 |
|
|
|
|
| Wild-type
control | 10 | 10
(100) | 0 (0) | 0 (0) | 0 (0) |
| Prx1 knockout
control | 10 | 10
(100) | 0 (0) | 0 (0) | 0 (0) |
| Wild-type 4NQO | 20 | 0 (0) | 6
(30) | 14 (70) | 0 (0) |
| Prx1 knockout
4NQO | 20 | 0 (0) | 7
(35) | 12 (50) | 1 (5) |
| Wild-type 4NQO +
H2O2 | 20 | 0 (0) | 1 (5) | 18 (90) | 1 (5) |
| Prx1 knockout 4NQO
+ H2O2 | 20 | 0 (0) | 9
(45)a | 10
(50)a | 1 (5) |
Prx1 is over-expressed in tongue
precancerous lesions
The expression of Prx1 was analyzed by RT-qPCR and
immunohistochemical staining. The mRNA expression of Prx1 was
increased in the wild-type 4NQO group compared with the wild-type
control group (P=0.046). The mRNA expression level of Prx1 was also
increased in the wild-type 4NQO + H2O2 group
compared with the wild-type control group (P=0.009). There was no
statistically significant difference in mRNA expression between the
wild-type 4NQO and 4NQO + H2O2 groups
(Fig. 2A). The protein expression
levels of Prx1 were increased in the 4NQO and 4NQO +
H2O2 groups compared with mice from the
wild-type control group (P=0.035 and P=0.024, respectively). The
expression of Prx1 in the 4NQO + H2O2 group
was increased compared with the 4NQO group, but this was not
statistically significant (P=0.847; Fig.
2B). These results indicate that Prx1 may be important in
promoting cell proliferation in oral precancerous lesions.
Prx1 knockout increases cell apoptosis
in tongue precancerous lesions
The apoptotic rate in the wild-type 4NQO group was
elevated compared with the wild-type control group (P<0.001).
The apoptotic rate in the wild-type 4NQO +
H2O2 group was decreased compared with the
4NQO group (P=0.004). An increased apoptotic rate in the Prx1
knockout 4NQO and Prx1 knockout 4NQO + H2O2
groups was observed compared with the wild-type 4NQO (P=0.009) and
wild-type 4NQO + H2O2 groups (P=0.024),
respectively. These results indicate that Prx1 inhibits apoptosis
in tongue precancerous lesions (Fig. 3A
and B).
Prx1 knockout results in the
downregulation of ASK1
In order to evaluate the effect of Prx1 on the
activation of ASK1 in tongue precancerous lesions, the expression
of total ASK1 and phosphor-ASK1 was observed in Prx1 knockout and
wild-type mice. The present results demonstrated that the mRNA
expression level of ASK1 was increased in wild-type 4NQO and
wild-type 4NQO + H2O2 groups compared with
the wild-type control group (P=0.001 and P=0.002, respectively;
Fig. 4A). A statistically significant
difference in the mRNA expression level of ASK1 between wild-type
4NQO and 4NQO + H2O2 groups was observed. The
mRNA expression level of ASK1 was increased in Prx1 knockout
control group compared with wild-type control group (P=0.003;
Fig. 4Ba). The mRNA expression level
of ASK1 in Prx1 knockout 4NQO and Prx1 knockout 4NQO +
H2O2 groups was increased compared with the
wild-type group, although this was not statistically significant
(P=0.704 and P=0.24, respectively; Fig.
4Bb and c).
 | Figure 4.Prx1 knockout leads to a
downregulation of ASK1. (A) mRNA expression level of ASK1 was
elevated in mouse tongue premalignant lesions, as determined by
RT-qPCR. (B) RT-qPCR determination of the relative expression of
ASK1 mRNA in Prx1 knockout mice (a) control group, (b) 4NQO group
and (c) 4NQO + H2O2 group. (C) Prx1 had no
clear association with ASK1 in mouse tongue premalignant lesions in
the (a) wild-type control group, (b) wild-type 4NQO group, (c)
wild-type 4NQO + H2O2 group, (d) Prx1
knockout control group, (e) Prx1 knockout 4NQO group and (f) Prx1
knockout 4NQO + H2O2 group (magnification,
×200). (g) ASK1 MOD value. (D) Prx1 positively regulated the
activation of phosphor-ASK1 in mouse tongue premalignant lesions in
the (a) wild-type control group, (b) wild-type 4NQO group, (c)
wild-type 4NQO + H2O2 group, (d) Prx1
knockout control group, (e) Prx1 knockout 4NQO group and (f) Prx1
knockout 4NQO + H2O2 group (magnification,
×200). (g) Phosphor-ASK1 MOD value. *0.01<P<0.05;
**0.000<P<0.01. Prx1, peroxiredoxin 1; 4NQO,
4-nitroquinoline-1-oxide; ASK-1, apoptosis signal-regulating kinase
1; MOD, mean optical density; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
Immunohistochemical analysis revealed that there was
no statistically significant difference in protein expression of
ASK1 between any groups (Fig. 4C).
Compared with the wild-type 4NQO group, the expression of
phosphor-ASK1 was decreased in the Prx1 knockout 4NQO group
(P=0.022). A similar expression pattern was observed in wild-type
and Prx1 knockout 4NQO + H2O2 groups
(P=0.001). There was no significant difference in the
phosphorylation of ASK1 in the wild-type 4NQO and wild-type 4NQO +
H2O2 groups compared with the wild-type
control group (P=0.481 and P=0.104), suggesting that phosphor-ASK1
has a positive association with Prx1 expression (Fig. 4D).
Prx1 knockout suppresses the
expression of p38
In order to evaluate the effect of Prx1 on the
activation of p38 MAPK in tongue precancerous lesions, the
expression of total p38 and phosphor-p38 was detected in Prx1
knockout and wild-type mice. The mRNA expression level of p38 was
increased in the wild-type 4NQO and 4NQO +
H2O2 groups compared with the wild-type
control group (P=0.021 and P=0.001, respectively). The difference
in mRNA expression levels of p38 between wild-type 4NQO and 4NQO +
H2O2 groups was not statistically significant
(P=0.401; Fig. 5A). The mRNA
expression level of p38 was decreased in the Prx1 knockout 4NQO
group compared with the wild-type 4NQO group (P=0.006). The mRNA
expression of p38 was decreased in the Prx1 knockout 4NQO +
H2O2 group, although no statistically
significant difference was observed with the wild-type 4NQO +
H2O2 group (P=0.649; Fig. 5B).
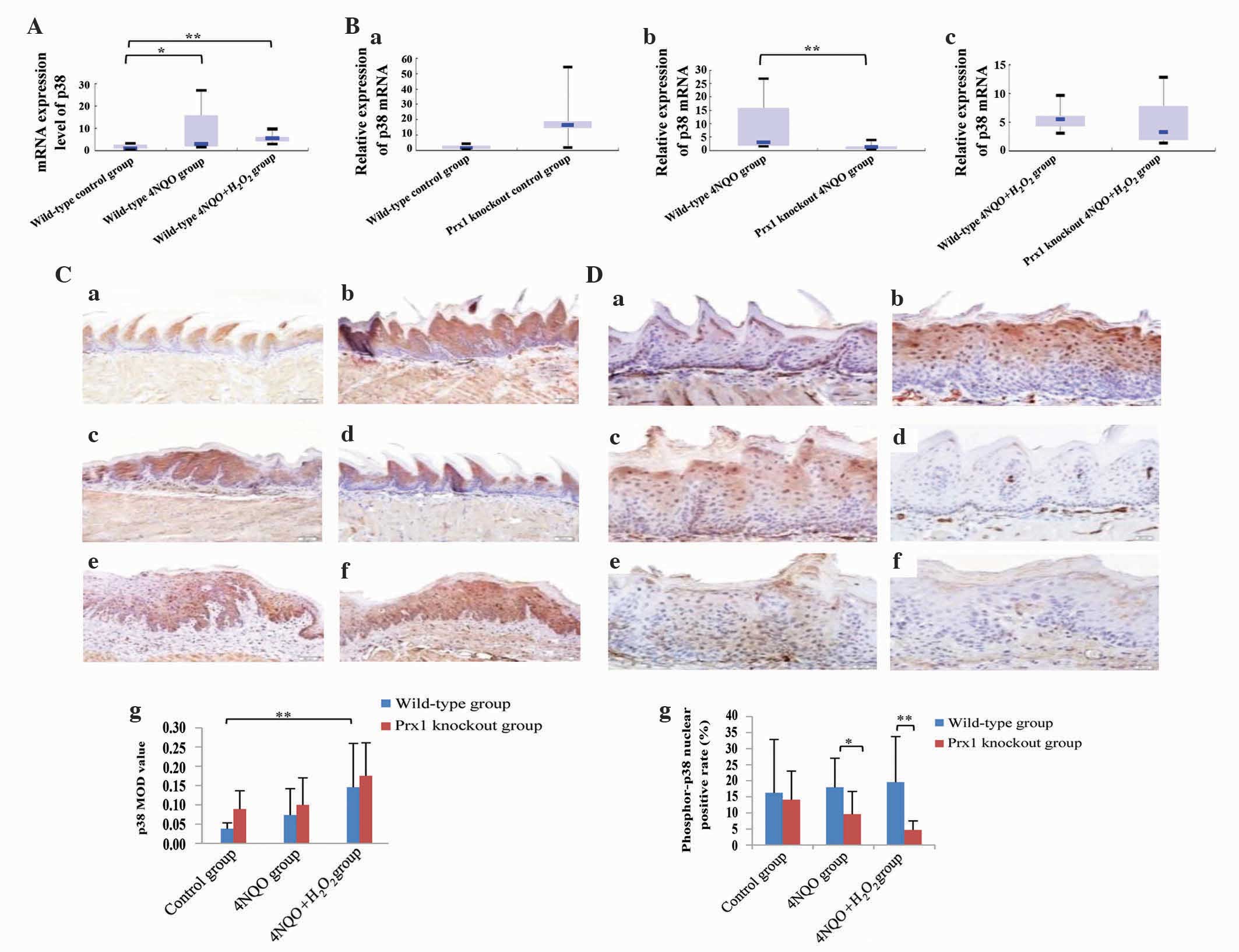 | Figure 5.Prx1 knockout suppresses the
expression of p38. (A) mRNA expression level of p38 was elevated in
mouse tongue premalignant lesions, as determined by RT-qPCR. (B)
RT-qPCR determined the relative expression level of p38 mRNA in
Prx1 knockout mice (a) control group, (b) 4NQO group and (c) 4NQO +
H2O2 group. (C) Prx1 positively regulated the
activation of p38 in mouse tongue premalignant lesions in the (a)
wild-type control group, (b) wild-type 4NQO group, (c) wild-type
4NQO + H2O2 group, (d) Prx1 knockout control
group, (e) Prx1 knockout 4NQO group and (f) Prx1 knockout 4NQO +
H2O2 group (magnification, ×200). (g) p38 MOD
value. (D) Prx1 positively regulated the activation of phosphor-p38
in mouse tongue premalignant lesions in the (a) wild-type control
group, (b) wild-type 4NQO group, (c) wild-type 4NQO +
H2O2 group, (d) Prx1 knockout control group,
(e) Prx1 knockout 4NQO group and (f) Prx1 knockout 4NQO +
H2O2 group (magnification, ×400). (g) Nuclear
positive rate of phosphor-p38. *0.01<P<0.05;
**0.000<P<0.01. Prx1, peroxiredoxin 1; 4NQO,
4-nitroquinoline-1-oxide; MOD, mean optical density; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction. |
The protein expression of p38 was clearly increased
in the wild-type 4NQO + H2O2 group compared
with the wild-type control group (P=0.002; Fig. 5C). The level of phosphor-p38 was
decreased in the Prx1 knockout 4NQO group compared with the
wild-type 4NQO group (P=0.022). The same expression pattern was
observed in Prx1 knockout and wild-type 4NQO +
H2O2 groups (P=0.001). The expression level
of phosphor-p38 was not significantly different in the wild-type
4NQO and 4NQO + H2O2 groups compared with the
wild-type control groups (P=0.606 and P=0.333, respectively),
indicating that phosphor-p38 had a clear positive association with
the expression of Prx1 (Fig. 5D).
Discussion
OLK is the most common oral precancerous lesion,
which may undergo a carcinomatous change to OSCC (26). At present, little is known concerning
the pathogenesis of OLK. Previous studies have demonstrated that
cell apoptosis is suppressed by Prx1 via the ASK1-mediated
signaling pathway in human embryonic kidney 293 and cervical cancer
HeLa cells (23). In a previous study
by the present authors, an increased level of apoptosis was
observed in OLK tissues, and Prx1 knockdown significantly enhanced
the level of apoptosis in dysplastic oral keratinocyte cells (data
not shown). However, even though there are numerous in vitro
studies concerning Prx1 and cell apoptosis, there are few in
vivo studies. The present study has for the first time, to the
best of our knowledge, designed carcinogenic experiments in
vivo to observe the effect of Prx1 on cell apoptosis during the
initiation and progression to malignancy of oral mucosa. In order
to confirm the role of Prx1 in oral precancerous lesions in
vivo, the present study established tongue precancerous lesion
mouse models in Prx1 knockout mice and investigated whether Prx1
suppresses apoptosis induced by oxidative stress. The present study
elucidated the possible molecular mechanism during the pathogenesis
and development of oral precancerous lesions.
In eukaryotic cells, four MAPK signal transduction
pathways, including extracellular signal-regulated kinase (ERK)
1/2, JNK, p38 and ERK5, have been identified. ERK1/2, JNK and p38
pathways are typical MAPK signal transduction pathways.
Furthermore, JNK and p38 signaling pathways are associated with
cell apoptosis (27–29). ASK1 is known as a proapoptotic,
stress-activated signaling molecule, and is an ubiquitously
expressed serine-theronine protein kinase that functions as a MAPK
kinase to activate JNK and p38 MAPK signaling cascades (30). Prx1 is the most abundant and
ubiquitously distributed member of the mammalian Prx family. It has
been implicated in regulating cell proliferation, differentiation
and apoptosis (31,32). ASK1 interacts with Prx1 in the
presence of H2O2-induced stress and is
negatively regulated by Prx1 (23).
Nakagawa et al (33) have
demonstrated that the activation of JNK and p38 is attenuated and
hepatocarcinogenesis is increased in ASK1-deficient mice. Yan et
al (34) have identified that
ASK1 activated by arsenic trioxide in leukemic cells may play an
antiapoptotic role, and Park et al (35) have demonstrated that Bacillus
anthracis induces the apoptosis of activated macrophages by
inhibiting the p38 MAPK pathway.
In the present study, the apoptotic rate of cells
increased and the expression of phosphor-ASK1 and phosphor-p38 was
downregulated in tongue precancerous lesions of Prx1 knockout mice.
These results demonstrate that apoptosis suppression by Prx1 may be
associated with the phosphorylation of ASK1 and p38, and that Prx1
has a positive regulatory role in the phosphorylation of ASK1 and
p38. In addition, the present study also detected the transcription
level of Prx1, ASK1 and p38 compared with that in normal
epithelium, and the expression of Prx1, ASK1 and p38 was clearly
increased in precancerous lesions compared with normal epithelium.
When Prx1 was knocked-down, the ASK1 transcription level was
significantly increased in the control group, indicating that Prx1
clearly inhibits the transcription of ASK1 in normal mucosa. By
contrast, a knockdown of Prx1 resulted in a significant
downregulation of p38 at a transcriptional level in the
precancerous lesions, suggesting that Prx1 also positively
regulates p38 in precancerous lesions. Overall, Prx1 suppresses
oxidative stress-induced apoptosis in tongue precancerous lesions
by positively regulating ASK1 and p38 expression at a molecular
level.
In the present study, in the Prx1 knockout 4NQO +
H2O2 mice, the degree of moderate to severe
epithelial dysplasia was significantly reduced and mild epithelial
dysplasia was clearly elevated compared with wild-type 4NQO +
H2O2 mice. This suggests that Prx1 enhances
cell proliferation during the pathogenesis of oral precancerous
lesions. Therefore, when oral precancerous lesions are affected by
oxidative stress, Prx1 is important in inhibiting oxidative damage
and apoptosis of cells, and promotes the progression of tongue
precancerous lesions. Lee et al (36) have also demonstrated that Prx1
knockout results in the decrease of cell proliferation, and Prx1 is
associated with tumor size, micro-vassal invasion and Edmonson
tumor grade (37). In addition,
microRNA-510 directly binds to the 3′-untranslated region of Prx1
and blocks its protein expression, leading to a suppression in the
migration of human breast cancer cells (38).
In the present study, the application of
H2O2 alone as an oxidative stressor had no
obvious effect on lesion development. However, more severe lesions
were observed in mice from the wild-type 4NQO +
H2O2 group compared with mice from the
wild-type 4NQO group, indicating that H2O2
application coupled with 4NQO has a positive effect on promoting
the development and progression of lesions.
H2O2 is known as the most common member of
ROS and induces apoptosis in various types of malignances (39,40).
However, in the present study, compared with 4NQO-induced tongue
precancerous lesions, cell apoptosis was moderately reduced in mice
from the 4NQO + H2O2 group. A similar pattern
was observed in Prx1 knockout mice. Previous studies have revealed
that 4NQO treatment leads to the formation of
H2O2 superoxide and hydroxyl radicals, thus
resulting in the production of a substantial amount of 8-OHdG in
DNA and oxidative damage in normal human fibroblasts (41). Tang et al (42) have demonstrated that
H2O2 preconditioning at low concentrations
may protect rat pheochromocytoma PC12 cells from apoptosis induced
by H2O2. In addition, oxidant preconditioning
protects human proximal tubular cells against lethal oxidant injury
(43). In the present study, 3%
H2O2 was applied to mouse tongue mucosa three
times a week during the development of 4NQO-induced mouse tongue
precancerous lesions for 16 weeks. The 3%
H2O2 was revealed to be a mild stimulus
compared with 50 µg/ml 4NQO. The 3% H2O2
treatment may alleviate apoptosis induced by subsequent 4NQO
exposure in tongue mucosa epithelia of the mice. These data
indicate that H2O2 at a low concentration may
inhibit apoptosis. A previous study also revealed that
H2O2 at a low concentration promotes cell
proliferation (44). A low dose of
H2O2 was able to reverse DHM-induced cell
apoptosis of human hepatocellular carcinoma (44). This may indicate that the balance
between ROS production and various antioxidants is vitally
important for cancer cell growth.
The present carcinogenic in vivo experiments
were used to observe the effect of Prx1 on cell apoptosis during
the development and progression to malignancy of mouse tongue
mucosa. The present study concludes that Prx1 may suppress
oxidative stress-induced apoptosis via the ASK1/p38 signaling
pathway in mouse tongue precancerous lesions, and
H2O2 and 4NQO play a coordination role in
promoting the progression of tongue mucosa precancerous lesions. In
addition, H2O2 at a low concentration level
may inhibit apoptosis. The present findings provide novel insights
into Prx1 function and the mechanisms of OLK pathogenesis.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China, Beijing, China (grant nos. 81070836
and 81470752) and Beijing Natural Science Foundation of China,
Beijing, China (grant no. 7152066).
References
|
1
|
van der Waal I: Oral potentially malignant
disorders: Is malignant transformation predictable and preventable?
Med Oral Patol Oral Cir Bucal. 19:e386–e390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amaqasa T, Yamashiro M and Uzawa N: Oral
premalignant lesions: From a clinical perspective. Int J Clin
Oncol. 16:5–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wamakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van der Waal I, Schepman KP, Vander Meij
EH and Smeele LE: Oral leukoplakia: A Clinicopathological review.
Oral Oncol. 33:291–301. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van der Waal I and Axell T: Oral
leukoplakia: A proposal for uniform reporting. Oral Oncol.
38:521–526. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Banoczy J, Gintner Z and Dombi C: Tobacco
use and oral leukoplakia. J Dent Educ. 65:322–327. 2001.PubMed/NCBI
|
|
7
|
Reichart PA: Identification of risk groups
for oral precancer and cancer and preventive measures. Clin Oral
Investing. 5:207–213. 2001. View Article : Google Scholar
|
|
8
|
Zhang XL and Reichart PA: A review of
betel quid chewing, oral cancer and precancer in mainland China.
Oral Oncology. 43:424–430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen CL, Chi CW and Liu TY: Hydroxyl
radical formation and oxidative DNA damage induced by area quid in
vivo. J Toxicol Environ Health A. 65:327–336. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Christen AG, Swanson BZ, Glover ED and
Henderson AH: Smokeless tobacco: The folklore and social history of
snuffing, sneezing, dipping, and chewing. J Am Dent Assoc.
105:821–829. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oda D, Nguyen MP, Royack GA and Tong DC:
H2O2 oxidative damage incultured oral
epithelial cells: The effect of short-term vitamin C exposure.
Anticancer Res. 21:2719–2724. 2001.PubMed/NCBI
|
|
12
|
Wu HJ, Chi CW and Liu TY: Effects of PH on
nicotine-induced DNA damage and oxidative stress. J Toxicol Environ
Health A. 68:1511–1523. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bahar G, Feinmesser R, Shpitzer T,
Popovtzer A and Nagler RM: Salivary analysis in oral cancer
patients: DNA and protein oxidation, reactive nitrogen species, and
antioxidant profile. Cancer. 109:54–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rhee SG and Woo HA: Multiple functions of
peroxiredoxins: Peroxidases, sensors and regulatoers of the
intracellular messenger H2O2 and protein
chaperones. Antioxid Redox Signal. 15:781–794. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang SW, Rhee SG, Chang TS, Jeong W and
Choi MH: 2-Cys perosiredoxin function is intracellular signal
transduction: Therapeutic implications. Trends Mol Med. 11:571–578.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yanagawa T, Iwasa S, Ishii T, Tabuchi K,
Yusa H, Onizawa K, Omura K, Harada H, Suzuki H and Yoshida H:
Peroxiredoxin 1 expression in oral cancer: A potential new tumor
marker. Cancer Lett. 156:27–35. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yanagawa T, Omura K, Harada H, Ishii T,
Uwayama J, Nakaso K, Iwasa S, Koyama Y, Onizawa K, Yusa H and
Yoshida H: Peroxiredoxin 1 expression in tongue squamous cell
carcinomas as involved in tumor recurrence. Int J Oral Maxillofac
Surg. 34:915–920. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ge Li-hua, Hou Min, Yang Jing, Chen Tong
and Tang Xiao-fei: Prx1 overexpression in human oral leukoplakia.
Beijing Kou Qiang Yi Xue Za Zhi. 20:135–137. 2012.(In Chinese).
|
|
20
|
Nishitoh H, Saitoh M, Mochida Y, Takeda K,
Nakano H, Rothe M, Miyazono K and Ichijo H: ASK1 is essential for
JNK/SAPK activation by TRAF2. Mol Cell. 2:389–395. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saitoh M, Nishitoh H, Fujii M, Takeda K,
Tobiume K, Sawada Y, Kawabata M, Miyazono K and Ichijo H: Mammalian
thioredoxin is a direct inhibitor of apoptosis signal-regulating
kinase (ASK) 1. EMBO J. 17:2596–2606. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takeda K, Matsuzawa A, Nishitoh H and
Ichijo H: Roles of MAPKKK ASK1 in stress-induce cell death. Cell
Struct Funct. 28:23–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SY, Kim TJ and Lee KY: A novel
function of peroxiredoxin 1 (Prx-1) in apoptosis signal-regulating
kinase 1 (ASK1)-mediated signaling pathway. FEBS Lett.
582:1913–1918. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Min, Liang Hong, Quan Xiong-Zhi,
Miao Cong-cong and Tang Xiao-Fei: Establishment of Prx 1 gene
knockout mice. Beijing Kou Qiang Yi Xue Za Zhi. 20:246–248.
2012.(In Chinese).
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kramer IR, Lucas RB, Pindborg JJ and Sobin
LH: Definition of leukoplakia and related lesions: An aid to
studies on oral precancer. Oral Surg Oral Med Oral Pathol.
46:518–539. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen CL, Lin DF, Chang WT, Huang WC, Teng
CF and Lin YS: Ceramide induces p38 MAPK and JNK activation through
a mechanism involving a thioredoxin-interacting protein-mediated
pathway. Blood. 111:4365–4374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park GB, Kim YS, Lee HK, Song H, Cho DH,
Lee WJ and Hur DY: Endoplasmic reticulum stress-mediated apoptosis
of EBV-transformed B cells by cross-linking of CD70 is dependent
upon generation of reactive oxygen species and activation of p38
MAPK and JNK pathway. J Immunol. 185:7274–7284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Tang J, Cao B, Zhang Z, Li J,
Schimmer AD, He S and Mao X: The natural pesticide dihydrorotenone
induces human plasma cell apoptosis by triggering endoplasmic
reticulum stress and activating p38 signaling pathway. PLoS One.
8:e699112013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ichijo H, Nishida E, Irie K, ten Dijke P,
Saitoh M, Moriquchi T, Takaqi M, Matsumoto K, Miyazono K and Gotoh
Y: Induction of apoptosis by ASK1, a mammalian MAPKKK that
activates SAPK/JMK and p38 signaling pathway. Science. 275:90–94.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Turner-Ivey B, Manevich Y, Schulte J,
Kistner-Griffin E, Jezierska-Drutel A, Liu Y and Neumann CA: Role
for Prdx1 as a specific sensor in redox-regulated senescence in
breast cancer. Oncogene. 32:5302–5314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim YJ, Lee WS, Ip C, Chae HZ, Park EM and
Park YM: Prx1 supppresses radiation-induced c-Jun NH2-terminal
kinase signaling in lung cancer cells through interaction with the
glutathione S-transferase Pi/c-Jun NH2-termianl kinase complex.
Cancer Res. 66:7136–7142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakagawa H, Hirata Y, Takeda K, Hayakawa
Y, Sato T, Kinoshita H, Sakamoto K, Nakata W, Hikiba Y, Omata M, et
al: Apoptosis signal-regulating kinase 1 inhibits
hepatocarcinogenesis by controlling the tumor-suppressing function
of stress-activated mitogen-activated protein kinase. Hepatology.
54:185–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan W, Arai A, Aoki M, Ichijo H and Miura
O: ASK1 is activated by arsenic trioxide in leukemic cells through
accumulation of reactive oxygen species and may play a negative
role in induction of apoptosis. Biochem Biophys Res Commun.
355:1038–1044. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park JM, Greten FR, Li ZW and Karin M:
Macrophage apoptosis by anthrax lethal factor through p38 MAP
kinase inhibition. Science. 297:2048–2051. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee YJ, Song DS, Yoo JS, Hyung KE, Lee MJ,
Moon YH, Lee IH, Go BS, Park SY and Hwang KW: Protective functionsm
of peroxiredoxin-1 against cytokine-induced MIN6 pancreatic β-cell
line death. Can J Physiol Pharmacol. 91:1037–1043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun QK, Zhu JY, Wang W, Lv Y, Zhou HC, Yu
JH, Xu GL, Ma JL, Zhong W and Jia WD: Diagnostic and prognostic
significance of peroxiredoxin 1 expression in human hepatocellular
carcinoma. Med Oncol. 31:7862014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo QJ, Mills JN, Bandurraga SG, Nogueira
LM, Mason NJ, Camp ER, Larue AC, Turner DP and Findlay VJ:
MicroRNA-510 promotes cell and tumor growth by targeting
peroxiredoxin1 in breast cancer. Breast Cancer Res. 15:R702013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lennicke C, Rahn J, Lichtenfels R,
Wessjohann LA and Seliger B: Hydrogen peroxide-production, fate and
role in redox signaling of tumor cells. Cell Commun Signal.
13:392015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Min SK, Lee SK, Park JS, Lee J, Paeng JY,
Lee SI, Lee HJ, Kim Y, Pae HO, Lee SK and Kim EC: Endoplasmic
reticulum stress is involved inn hydrogen peroxide induced
apoptosis in immortalized and malignant human oral keratinocytes. J
Oral Pathol Med. 37:490–498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Arima Y, Nishiqori C, Takeuchi T, Oka S,
Morimoto K, Utani A and Miyachi Y: 4-Nitroquinoline 1-oxide forms
8-hydroxydeoxyguanosine in human fibroblasts through reactive
oxygen species. Toxicol Sci. 91:382–392. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tang XQ, Chen J, Tang EH, Feng JQ and Chen
PX: Hydrogen peroxide preconditioning protects PC12 cells against
apoptosis induced by oxidative stress. Sheng Li Xue Bao.
57:211–216. 2005.PubMed/NCBI
|
|
43
|
Lee HT, Xu H, Ota-setlik A and Emala CW:
Oxidant preconditioning protects human proximal tubular cells
against lethal oxidant injury via p38 MAPK and heme oxygenase-1. Am
J Nephrol. 23:324–333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin B, Tan X, Liang J, Wu S, Liu J, Zhang
Q and Zhu R: A reduction in reactive oxygen species contributes to
dihydromyricetin-induced apoptosis in human hepatocellular
carcinoma cells. Sci Rep. 4:70412014. View Article : Google Scholar : PubMed/NCBI
|



















