Introduction
Worldwide, hepatocellular cancer (HCC) is the sixth
most common malignant tumor and the third leading cause of
cancer-associated mortality (1,2). One of
the most well-known characteristics of the tumor microenvironment
identified in HCC is tumor hypoxia (3). HCC originates from cirrhosis induced by
chronic liver injury; chronic injury causes fibrogenesis, which
destroys the normal liver blood system and leads to a shortage of
blood circulation, resulting in hypoxia. In addition, the high
proliferation of tumor cells induces local hypoxia within the
microenvironment of HCC (4).
The cellular response to hypoxia is mediated by the
hypoxia-inducible factors (HIFs) family of transcription factors
(5). Hypoxia induced factor-1 (HIF-1)
is the major transcription factor of this family, and is composed
of two subunits: Oxygen-sensitive HIF-1α and aryl hydrocarbon
receptor nuclear translocator (HIF-1β). Under low oxygen tension,
HIF-1α upregulates various hypoxia inducible genes through
dimerization with HIF-1β, and binds to the hypoxia-responsive
subunit in the promoter of target genes (6).
Hypoxia and inflammation are closely associated and
are important in a variety of pathological situations. Hypoxia
elicits tissue inflammation; during acute organ ischemia, including
intestinal or hepatic ischemia, the ischemic organ becomes severely
inflamed (7). However, alterations in
the signaling pathway involved in this pathological process remain
unknown.
Toll-like receptor 4 (TLR4) is a mammalian pattern
recognition receptor, which recognizes lipopolysaccharide (LPS) as
a ligand (8). TLR4 is closely
involved in the development and progression of various inflammatory
diseases, such as inflammatory bowel disease and atherosclerosis
(9–11). A previous study has demonstrated that
downstream signaling of TLR4 leads to an accumulation of HIF-1α,
which is important for TLR4-dependent expression of proinflammatory
cytokines (12). Certain studies have
also demonstrated that bacteria or LPS-induced HIF-1α accumulation
is TLR4 dependent in immune cells (12,13). In
human myeloid monocytic leukemia THP-1 cells, LPS-induced TLR4
signaling triggered crosstalk between HIF-1α and apoptosis
signal-regulating kinase 1 (ASK1) (14). ASK1 contributes to the stabilization
of HIF-1α possibly via the activation of p38 mitogen-activated
protein kinase (MAPK) (14). ASK1 and
p38 are important downstream signaling molecules of the TLR4
signaling pathway (15).
The present study demonstrated the effects of
silencing HIF-1α expression in human hepatocellular carcinoma HepG2
cells, and demonstrated that HIF-1α silencing leads to the
suppression of tumor cell growth, invasion and migration via the
TLR4 signaling pathway.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), penicillin/streptomycin, Basement Membrane
Matrix and phosphate-buffered saline (PBS) were obtained from
Invitrogen™ (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Resatorvid (TAK-242) was purchased from MedChem Express (Monmouth
Junction, NJ, USA). LPS derived from Escherichia coli J5
(catalog no., L5014) was obtained from Sigma-Aldrich (St. Louis,
MO, USA) and ECL® Plus Western Blotting Detection System
was from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). Antibodies
against HIF-1α, epidermal growth factor (EGF), hepatocyte growth
factor (HGF), vascular endothelial growth factor (VEGF), fibroblast
growth factor 2 (FGF2), TLR4, caspase-3, p-ASK1, p-p38 and myeloid
differentiation primary response gene 88 (MyD88) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). The Cell
Counting kit-8 (CCK-8) was from Dojindo Molecular Technologies,
Inc. (Kumamoto, Japan). Reagents for quantitative polymerase chain
reaction (qPCR) analysis, including reverse transcriptase kit from
Promega (Madison, WI, USA) and Thunderbird SYBR qPCR Mix from
Toyobo, Co., Ltd., (Osaka, Japan). Nuclear extraction kits were
purchased from Takara Biotechnology Co., Ltd. (Dalian, China).
Cell culture
HepG2 cells were purchased from American Type
Culture Collection (Manassas, VA, USA) and cultured at 37°C with 5%
CO2 in DMEM supplemented with 10% FBS, 4.5 g/l glucose,
2 mM L-glutamine (Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin.
Short hairpin RNA (shRNA) and
transfection
shRNA targeting the human HIF-1α gene and scramble
control sequences were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). The targeted sequence of sh-HIF-1α was
5′-CCCTAATTGAGTCAAACTTGAGCTTTCAAGTTTGACTCAATTAGGGAAAA-3′, and the
targeted sequence of the negative control shNC was
5′-AGGGAAAACCCTAATTGAGTCAAACTTGAGCTTTCAAGTTTGACTCAATT-3′. Human
HIF-1α shRNA was inserted into the recombinant plasmid pGPU6
(Shanghai GenePharma Co., Ltd., Shanghai, China). For HIF-1α gene
silencing, HepG2 cells were transfected with pGPU6-shHIF-1α or shNC
plasmids using Lipofectamine 2000 (Invitrogen; Thermo Fisher,
Scientific, Inc.). The efficiency of transfection was evaluated by
western blotting with antibodies against HIF-1α.
Following transfection, the cells were maintained at
normoxic conditions (95% air, 5% CO2) for 24 h, and the
cells were subsequently placed in Krebs-Ringer Bicarbonate buffer
(115 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM
KH2PO4, 1.2 mM MgSO4, 24 mM
NaHCO3, 10 mM HEPES; pH 7.4), followed by culturing at
95% N2 and 5% CO2 in a hypoxic environment
for 24 h. In total, 12 h prior to hypoxia, cells were exposed to
LPS (1 µg/ml) or TAK-242 (1 µM). The LPS and TAK-242 remained in
the culture medium and the cells were washed three times with PBS
and then placed into DMEM medium with 10% FBS.
Cell viability and cell growth
assays
HepG2 cells were exposed to indicated hypoxia
conditions at a density of 1×104 cells/well for 24, 48
and 72 h. The number of viable cells was counted by trypan blue dye
(Beyotime Institute of Biotechnology, Shanghai, China) exclusion
using a hemocytometer.
The viability of the HepG2 cells was measured using
the CCK-8 assay according to the manufacturer's protocol. Briefly,
HepG2 cells were seeded in a 96-well plate (NEST Biotechnology,
Suzhou, China) at 1×104 cells/well, incubated at 37°C
for 24 h, then subjected to a hypoxic environment for 24 h, after
which CCK-8 solution was added for another 4 h incubation. Optical
density was measured using a microplate reader (Multiskan™ MK3;
Thermo Fisher Scientific, Inc.) at 450 nm.
Reverse transcription qPCR
Total RNA was extracted from HepG2 cells using
TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RNA
concentration was determined by UV spectrophotometry (NanoDrop
2000; Thermo Fisher Scientific, Inc.). cDNA was synthesized from 1
µg of RNA (positive control) using reverse transcriptase kit from
Promega. The protocol was repeated using ddH2O instead
of RNA, as the negative control. qPCR was performed using
Thunderbird SYBR qPCR Mix from Toyobo, Co., Ltd. The primer
sequences were designed by PrimerBank (https://pga.mgh.harvard.edu/primerbank) and
synthesized by GeneScript (Nanjing, China) as follows: HIF-1α,
forward 5′-ACTTGGCAACCTTGGATTGGA-3′ and reverse
5′-ATCTCCGTCCCTCAACCTCT-3′ (190 bp); EGF, forward
5′-AGAGGGAGAGGATGCCACAT-3′ and reverse 5′-GGGGTGGAGTAGAGTCAAGA-3′
(206 bp); HGF, forward 5′-ACAGCTTTTTGCCTTCGAGC-3′ and reverse
5′-GCAAGAATTTGTGCCGGTGT-3′ (261 bp); VEGF, forward
5′-TCACCAAGGCCAGCACATAG-3′ and reverse 5′-GAGGCTCCAGGGCATTAGAC-3′
(202 bp); FGF2, forward 5′-TCCACCTATAATTGGTCAAAGTGGT-3′ and reverse
5′-CATCAGTTACCAGCTCCCCC-3′ (121 bp); tumor necrosis factor (TNF)-α,
forward 5′-CTGGGCAGGTCTACTTTGGG-3′ and reverse
5′-CTGGAGGCCCCAGTTTGAAT-3′ (272 bp); interleukin (IL)-6, forward:
5′-TGCAATAACCACCCCTGACC-3′ and reverse 5′-GTGCCCATGCTACATTTGCC-3′
(163 bp). qPCR was performed on a StepOnePlus™ Real-time PCR System
(Applied Biosystems™; Thermo Fisher Scientific, Inc.) with the
following cycle: 95°C for 1 min, followed by 95°C for 15 sec, 58°C
for 30 sec, and 72°C for 30 sec for 40 cycles. Glyceraldehyde
3-phosphate dehydrogenase (GAPDH; forward
5′-GATCCCGCTAACATCAAATG-3′ and reverse 5′-GAGGGAGTTGTCATATTTCTC-3′)
expression was used as an internal control. 2−ΔΔCq was
calculated for every sample and the mRNA expression levels were
indicated with 2−ΔΔCq and normalized to GAPDH (16). The experiments were repeated three
times independently and positive (cDNA from HepG2 cells) and
negative (no cDNA) controls were included.
Tumor cell migration assay
The effects of HIF-1α knockdown on tumor cell
migration was investigated in HepG2 cells grown in serum-free DMEM.
Briefly, HepG2 cells were seeded into a 6-well plate (NEST
Biotechnology) and reach 90% confluence. A single scratch was
created on confluent monolayers using a micropipette tip (1 mm).
Subsequently, wounded monolayers were washed with PBS to remove
cell debris, supplemented with serum-free DMEM and subjected to
hypoxic conditions for 24 h. The median distance of migrating cells
to the wound was determined under an inverted microscope (IX81;
Olympus Corporation, Tokyo, Japan) at 0 and 24 h.
Tumor cell invasion assay
The invasiveness of tumor cells was assessed in
vitro using a Transwell chamber, as previously described
(17). In brief, cells were seeded in
24-well Transwell plates (EMD Millipore, Billerica, MA, USA) in 10%
FBS medium at 1×105 cells/well. After 24 h, the medium
was changed to free-serum DMEM and the cells were subjected to
hypoxia for 24 h, while 10% FBS DMEM was added to the lower
chamber. Non-adherent cells were washed away with PBS and adherent
cells were fixed in ethanol. After staining with 0.1% crystal
violet, pictures were taken using a microscope (IX81; Olympus,
Tokyo, Japan).
Western blot analysis
Total cell lysate was extracted from HepG2 cells
using radioimmunoprecipitation assay lysis buffer (Cell Signaling
Technology, Inc.), according to the manufacturer's protocol,
resolved by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to nitrocellulose membranes. The
membranes were blocked with 5% non-fat dry milk in Tris-buffered
saline (TBS; Solarbio, Beijing, China) for 2 h at room temperature.
Thereafter, the membranes were incubated with primary antibodies
against HIF-1α (dilution, 1:1,000; rabbit polyclonal; catalog no.,
ab113642), phospho (p)-ASK1 (dilution, 1:1,000; rabbit polyclonal;
catalog no., 3764), p-p38 MAPK (dilution, 1:1,000; rabbit
polyclonal; catalog no., 9211), caspase-3 (dilution, 1:1,000;
rabbit monoclonal; catalog no., 9665) (Cell Signaling Technology,
Inc.), TLR4 (dilution, 1:1,000; rabbit polyclonal; catalog no.,
ab47839), MyD88 (dilution, 1:1,000; rabbit polyclonal; catalog no.,
ab2064), EGF (dilution, 1:1,000; rabbit polyclonal; catalog no.,
ab9695), FGF2 (dilution, 1:1,000; rabbit polyclonal; catalog no.,
ab126861), VEGF (dilution, 1:1,000; rabbit polyclonal; catalog no.,
ab46154) and HGF (dilution, 1:1,000; rabbit polyclonal; catalog
no., ab83760) (Abcam, Cambridge, MA, USA) at 4°C overnight. After
being washed three times for 10 min in TBS with 0.05% Tween 20
(Solarbio), the membranes were incubated with horseradish
peroxidase-conjugated anti-rabbit secondary antibody (dilution,
1:5,000; catalog no., 7074; Cell Signaling Technology, Inc.) or
anti-mouse secondary antibody (dilution, 1:5,000; catalog no.,
7076; Cell Signaling Technology, Inc.) for 1 h at room temperature.
Specific bands were detected using the Pierce ECL Western Blotting
Substrate (Thermo Fisher Scientific, Inc.). β-actin (dilution,
1:3,000; rabbit polyclonal; catalog no., 4967; Cell Signaling
Technology, Inc.) was used as an internal control.
Statistical analysis
Results are presented as the mean ± standard error
of the mean. Independent t-tests were performed to compare the
difference of the means between control and experiment groups. All
statistical analysis was performed using SPSS version 12.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
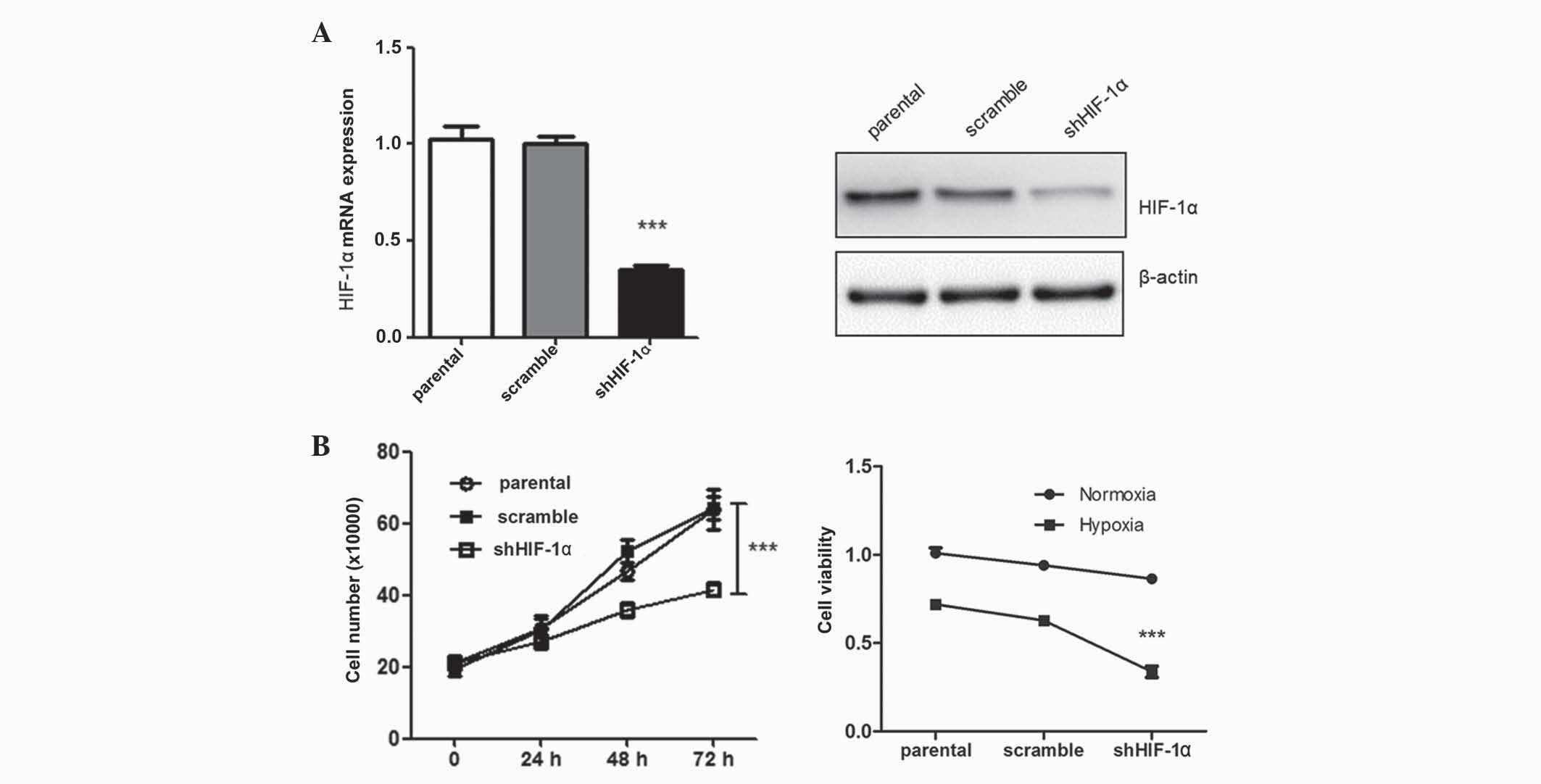
Silencing of HIF-1α suppresses tumor
cell growth
After transfection of HepG2 cells with shHIF-1α,
tumor cell growth was assessed using a CCK-8 assay. In total, 48 h
subsequent to transfection, tumor cell growth was significantly
suppressed compared with the control group (P=0.012) (Fig. 1). The negative effect of HIF-1α
silencing on tumor cell growth was more prominent under hypoxic
conditions. These findings were consistent with previous studies
(6). Silencing of HIF-1α may exhibit
a suppressive tumor effect under hypoxic conditions, and is
possibly mediated by inhibition of several cell proliferation
genes.
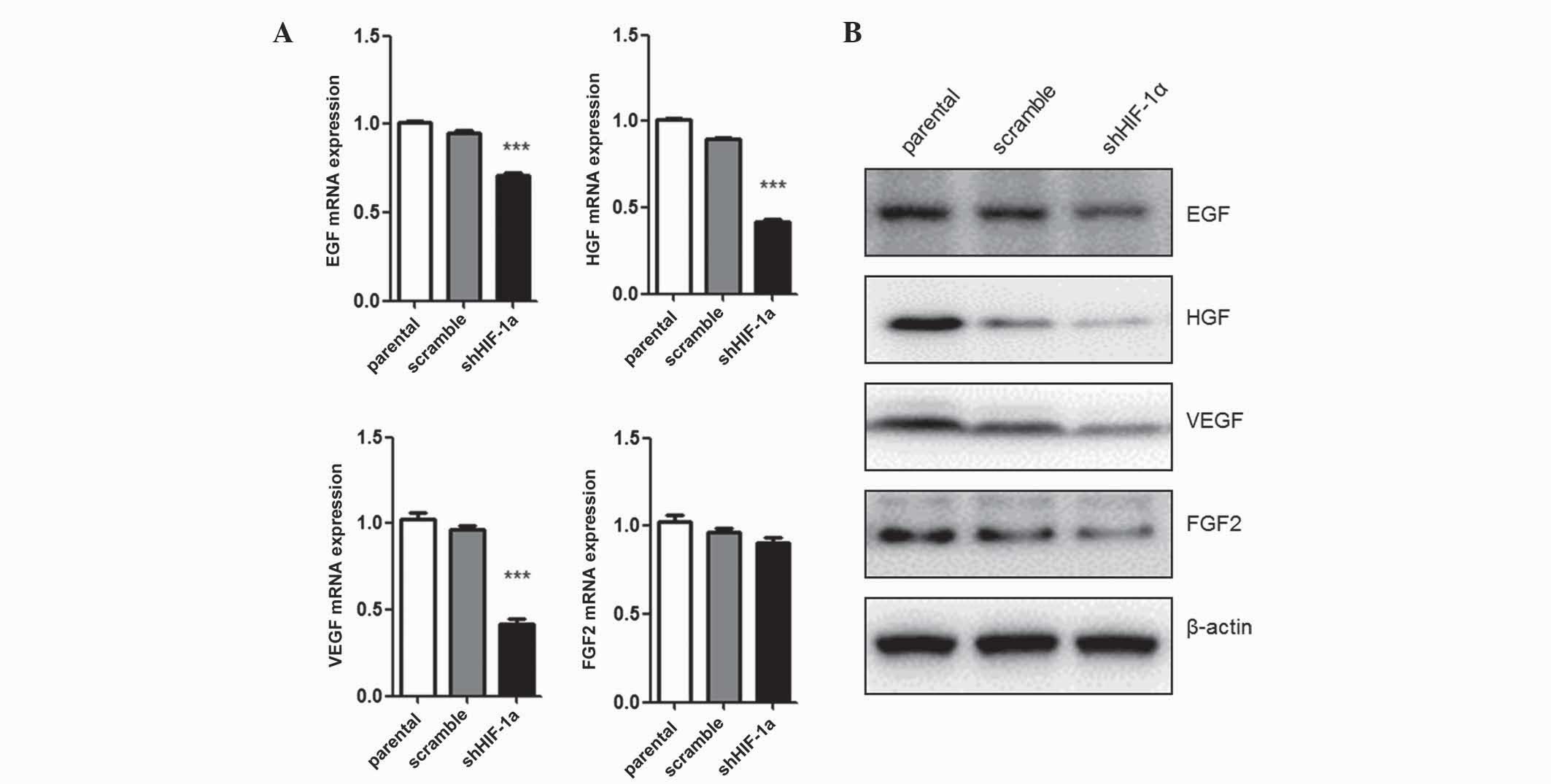
Silencing of HIF-1α affects the
expression of tumor growth-associated genes
Since tumor cell proliferation was inhibited by
HIF-1α silencing, the present study evaluated the mRNA and protein
levels of several growth factors involved in tumor cell growth.
EGF, FGF, VEGF and HGF mRNA expression levels were quantified by
qPCR (Fig. 2A). Under hypoxic
conditions, cells transfected with shHIF-1α exhibited low levels of
EGF (P=0.035), FGF (P=0.027) and VEGF (P=0.016) expression compared
with parental cells. However, the mRNA level of FGF2 expression was
not affected by HIF-1α silencing. The protein expression levels of
these growth factors were detected by western blot analysis, and
demonstrated the same results as the mRNA analysis (Fig. 2B). Collectively, these data suggest
that HIF-1α expression regulates the expression of tumor
growth-associated factors, including EGF, HGF and VEGF, but not
FGF2, under hypoxic conditions.
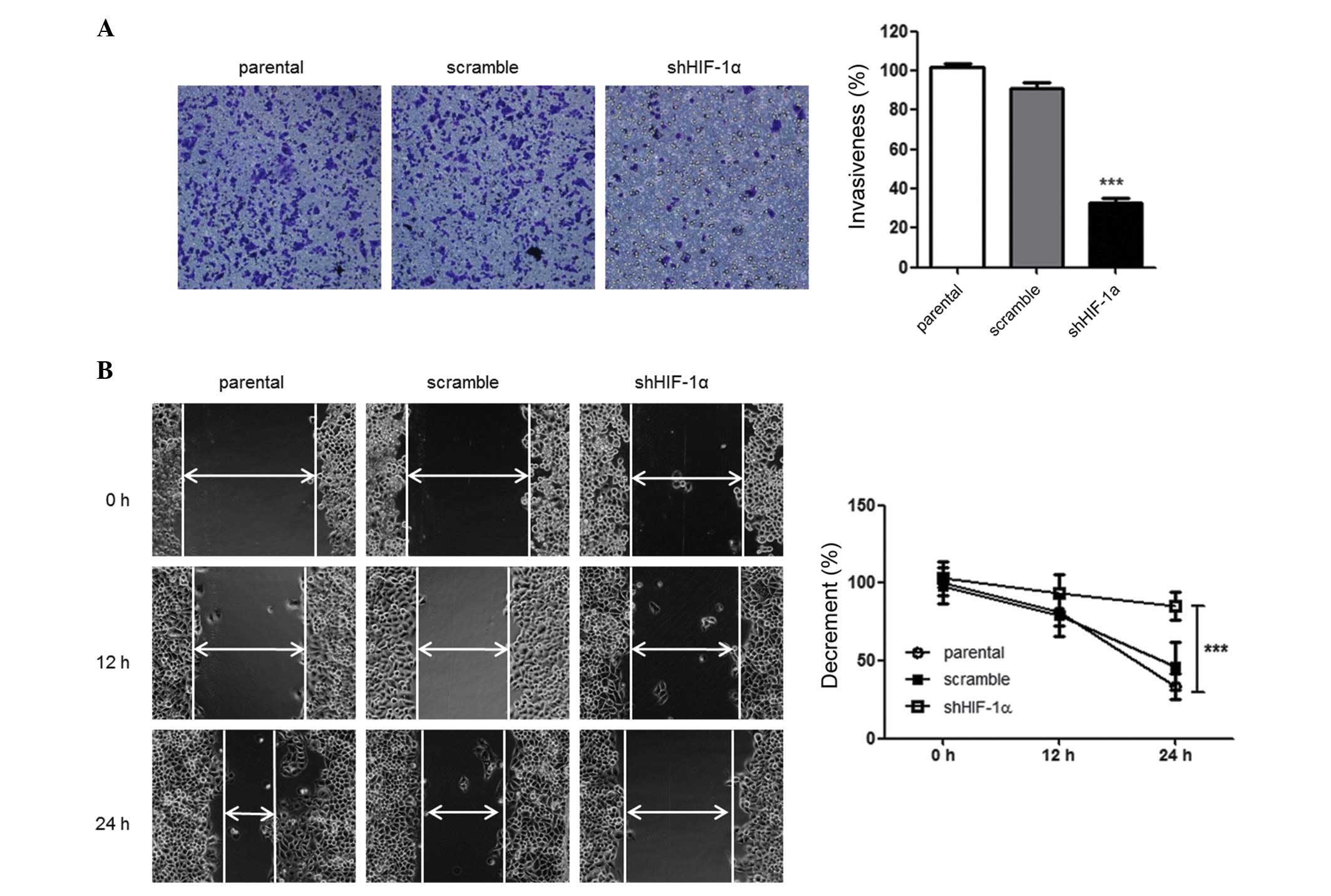
HIF-1α-silencing suppresses tumor cell
invasiveness and motility
Since tumor cells may mobilize and invade into
adjacent and distant regions, the effect of HIF-1α silencing on the
ability of HepG2 cells to migrate and invade was investigated using
wound healing and Transwell assays. The effect of HIF-1α silencing
on the invasiveness of tumor cells was investigated using
Matrigel-coated Transwell chambers. There was a decreased number of
migrated shHIF-1α-tranfected HepG2 cells compared with parental
cells, demonstrating that there was a suppression of the invasive
ability of the tumor cells (P=0.027) (Fig. 3A). Similarly, as compared with the
parental cells, there was a marked decrease in the migration of
shHIF-1α-transfected HepG2 cells in the wound healing assay
(P=0.024) (Fig. 3B). Collectively,
these data suggest that silencing of HIF-1α affects the
invasiveness and migration of HepG2 cells under hypoxic
conditions.
HIF-1α-silencing induces apoptosis in
a TLR4-dependent fashion
Subsequently, the present study evaluated the
alterations in HIF-1α expression following treatment of the HepG2
cells with LPS and TAK-242, which activates or inhibits TLR4
signaling, respectively. The present study demonstrated that LPS
enhanced mRNA and protein accumulation of HIF-1α, and TAK-242
substantially inhibited the expression of HIF-1α compared with the
control group under hypoxic conditions (Fig. 4A).
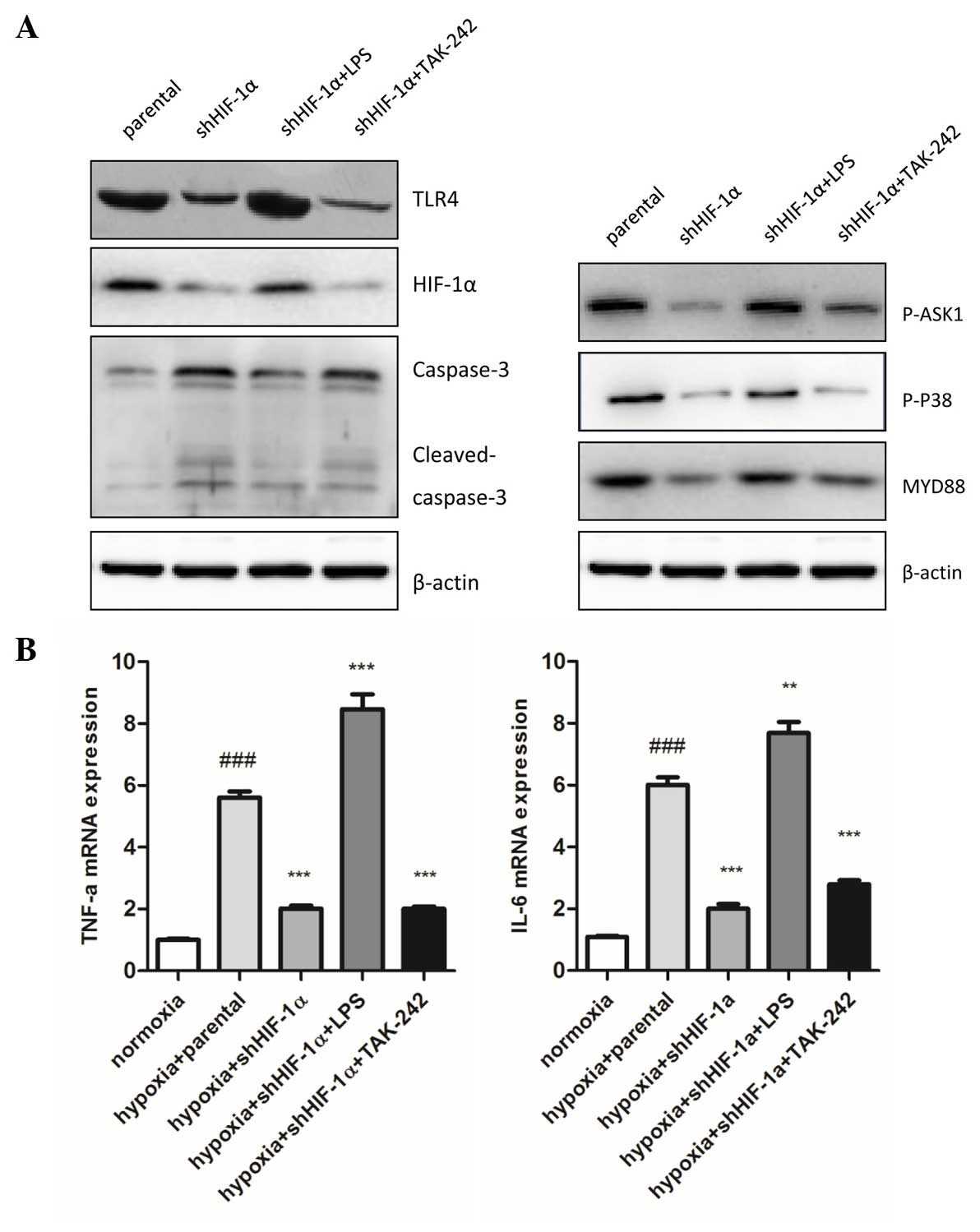 | Figure 4.Involvement of TLR4 signaling pathway
in HIF-1α silencing affects inflammatory and apoptosis under
hypoxic conditions. (A) In total, 30 mg total lysate was harvested
with radioimmunoprecipitation assay buffer to detect TLR4, HIF-1α,
caspase-3, MyD88, p-ASK1 and p-p38 protein expression by western
blot analysis in human hepatocellular carcinoma HepG2 cells. The
expression of TLR4, HIF-1α, MyD88, p-ASK1 NS and p-p38 in shHIF-1α
transfected cells was significantly higher compared with the
parental cells. 1 µg/ml LPS and 1 µM TAK-242 markedly mediated the
expression of these proteins compared with the shHIF-1α transfected
cells. (B) Inflammatory cytokines (TNF-α and IL-6) mRNA expression
levels were detected by quantitative polymerase chain reaction.
Data are presented as the mean ± standard error of the mean of
three independent experiments. ###P<0.001 vs.
normoxia group; ***P<0.001 vs. hypoxia + parental group. HIF-1α,
hypoxia induced factor-1α; sh, short hairpin; TLR4, toll-like
receptor 4; LPS, lipopolysaccharide; p-, phospho-; ASK1, apoptosis
signal-regulating kinase 1; MyD88, myeloid differentiation primary
response gene 88; TNF-α, tumor necrosis factor-α; IL-6,
interleukin-6. |
To understand the role of TLR4 signaling downstream
in TLR-4 mediated HIF-1α accumulation under hypoxic conditions,
MyD88, p-ASK1 and p-p38 protein expression was analyzed using
western blot analysis. The results demonstrated that MyD88, p-ASK1
and p-p38 were significantly decreased in HIF-1α-silenced cells
compared with parental cells. LPS markedly enhanced and TAK-242
markedly reduced the expression of MyD88, p-ASK1 and p-p38
(Fig. 4A).
Compared with the hypoxia parental cells, the
inflammatory response and cell apoptosis were markedly attenuated
by silencing HIF-1α, which was demonstrated by the expression of
inflammatory cytokines (TNF-α and IL-6; P=0.036 and P=0.027,
respectively) and cleaved caspase-3 expression, which is an active
apoptosis regulating molecule (Fig. 4A
and B). These results suggest that HIF-1α significantly affects
cell inflammation and apoptosis in hypoxic conditions.
Discussion
Hypoxia is known as one of the basic hallmarks of
solid tumors (18). HIF-1 is
important in the cellular response to tumor hypoxia and imposes the
biggest challenge in oncotherapy (19,20). In
the tumor, HIF-1α is an important inducer of cell proliferation,
metastasis, neovascularization and survival (21,22), and
is regulated by oxygen concentration. Under hypoxic conditions,
HIF-1α is concentrated in the cytoplasm, while in normoxia, HIF-1α
proteins are quickly degraded (23).
TLR4 is one of the most physiologically important TLRs, and
recognizes LPS as a ligand. It induces activation of downstream
signaling networks that initiate innate immune signaling cascades
and pro-inflammatory responses (24).
The present study investigated the functions of HIF-1α on tumors
under hypoxic conditions using shHIF-1α to silence the mRNA and
protein expression of HIF-1α in HepG2 cells. The present study
demonstrated that TLR4 expression was mediated by HIF-1α.
In the present study, HepG2 cells were transfected
with shRNA to HIF-1α and the cells were subjected to hypoxic
conditions. As previously reported, the effect of this inhibition
on tumor growth was observed using CCK-8 assay and a cell viability
assay (6). Subsequently, based on the
observation that tumor growth was inhibited in these cells, the
expression of growth factors, including EGF, HGF, VEGF and FGF2,
which are associated with tumor growth, were assessed using qPCR
and western blot analysis. EGF is a growth factor that stimulates
cell proliferation, growth and differentiation by binding to its
receptor (25). HGF regulates cell
growth and motility by activating a tyrosine kinase signaling
cascade, while VEGF is a signaling protein produced by cells which
stimulates neovasculogenesis and angiogenesis (26). FGF2 is associated with the regulation
of tumor angiogenesis and metastasis (27). The present study revealed that
silencing HIF-1α in HepG2 cells downregulates the mRNA and protein
expression of these four growth factors, leading to anti-tumor
effects, which is consistent with previous findings (28). The influence of hypoxia and HIF-1α
knockdown in HepG2 cells was evaluated using wound healing and
tumor cell invasion assays. The present study demonstrated that
when HIF-1α expression was inhibited cell migration was suppressed
and the invasive abilities of the cells were decreased.
TLR4 downstream signaling involves recruitment of
adapter proteins, including MyD88 (29). MyD88-dependent signaling is associated
with HIF-1α activation and MyD88 is required to stabilize HIF-1α
under normoxic conditions following stimulation with LPS (30). The present results indicate that
MyD88-dependent TLR4 signaling is involved in HIF-1α activation
under hypoxic conditions and affect apoptosis-associated caspase-3
activation. ASK1 and p38 are considered to be involved in the
activation of TLR4. ASK1 is an evolutionarily conserved
MAP3-kinase, and an active form of the kinase interacts with the
TNF receptor associated factor 6 forming a catalytically active
complex, which activates p38 MAPK (15). To investigate the role of ASK1-p38 in
TLR4-mediated HIF-1α signaling under hypoxia, p-ASK1, p-p38 and
HIF-1α levels were assessed in the present study. The present
results revealed that expression of these proteins in the parental
hypoxia group of cells was significantly higher compared with the
shHIF-1α group. Furthermore, LPS and TAK-242 treatment markedly
mediated the expression of p-ASK1, p-p38 and cleaved caspase-3 in
shHIF-1α HepG2 cells compared with the parental/hypoxia and
shHIF-1α groups that were not treated with LPS and TAK-242.
Therefore, the present authors suggest that TLR4 signaling leads to
an accumulation of HIF-1α by ASK1-activated p38 under hypoxic
conditions.
In conclusion, HIF-1α is known to have a critical
role in tumor development, since it promotes energy metabolism,
proinflammatory responses and apoptosis. In the present study,
apoptosis-associated factors and inflammatory cytokine TNF-α amd
IL-6 mRNA release were detected using western blot analysis and
qPCR. The present results revealed that inhibition of HIF-1α
activity leads to a markedly increased activation of caspase-3,
while LPS and TAK-242 treatment mediate that activation.
LPS-activated TLR4 may promote the production of inflammatory
cytokines in HepG2 cells, and HIF-1α silencing and TAK-242
treatment may decrease the release of these cytokines. The present
results support the hypothesis of the present authors that HIF-1α
may mediate TLR4 activation dependent proinflammatory cytokine
release and apoptosis of tumor cells, suggesting that the
HIF-1α/TLR4 signaling cohort may act as a novel therapeutic target
for the treatment of HCC.
References
|
1
|
Ferenci P, Fried M, Labrecque D, Bruix J,
Sherman M, Omata M, Heathcote J, Piratsivuth T, Kew M, Otegbayo JA,
et al: World gastroenterology organisation global guideline.
Hepatocellular carcinoma (hcc): A global perspective. J
Gastrointestin Liver Dis. 19:311–317. 2010.PubMed/NCBI
|
|
2
|
Nordenstedt H, White DL and El-Serag HB:
The changing pattern of epidemiology in hepatocellular carcinoma.
Dig Liver Dis. 42(Suppl 3): S206–S214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Greer SN, Metcalf JL, Wang Y and Ohh M:
The updated biology of hypoxia-inducible factor. EMBO J.
31:2448–2460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu XZ, Xie GR and Chen D: Hypoxia and
hepatocellular carcinoma: The therapeutic target for hepatocellular
carcinoma. J Gastroenterol Hepatol. 22:1178–1182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Semenza GL: Hypoxia-inducible factor 1
(HIF-1) pathway. Sci STKE. 2007:cm82007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1)alpha: Its protein stability
and biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koeppen M, Eckle T and Eltzschig HK: The
hypoxia-inflammation link and potential drug targets. Curr Opin
Anaesthesiol. 24:363–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beutler B: Tlr4: Central component of the
sole mammalian LPS sensor. Curr Opin Immunol. 12:20–26. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dhupar R, Klune JR, Evankovich J, Cardinal
J, Zhang M, Ross M, Murase N, Geller DA, Billiar TR and Tsung A:
Interferon regulatory factor 1 mediates acetylation and release of
high mobility group box 1 from hepatocytes during murine liver
ischemia-reperfusion injury. Shock. 35:293–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cario E and Podolsky DK: Differential
alteration in intestinal epithelial cell expression of Toll-like
receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect
Immun. 68:7010–7017. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edfeldt K, Swedenborg J, Hansson GK and
Yan ZQ: Expression of toll-like receptors in human atherosclerotic
lesions: A possible pathway for plaque activation. Circulation.
105:1158–1161. 2002.PubMed/NCBI
|
|
12
|
Lall H, Coughlan K and Sumbayev VV:
HIF-1alpha protein is an essential factor for protection of myeloid
cells against LPS-induced depletion of ATP and apoptosis that
supports toll-like receptor 4-mediated production of IL-6. Mol
Immunol. 45:3045–3049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peyssonnaux C, Cejudo-Martin P, Doedens A,
Zinkernagel AS, Johnson RS and Nizet V: Cutting edge: Essential
role of hypoxia inducible factor-1alpha in development of
lipopolysaccharide-induced sepsis. J Immunol. 178:7516–7519. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sumbayev VV: LPS-induced toll-like
receptor 4 signalling triggers cross-talk of apoptosis
signal-regulating kinase 1 (ASK1) and HIF-1alpha protein. FEBS
Lett. 582:319–326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsuzawa A, Saegusa K, Noguchi T,
Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K
and Ichijo H: ROS-dependent activation of the TRAF6-ASK1-p38
pathway is selectively required for TLR4-mediated innate immunity.
Nat Immunol. 6:587–592. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Daoud A, Song J, Xiao F and Shang J:
B-9-3, a novel β-carboline derivative exhibits anti-cancer activity
via induction of apoptosis and inhibition of cell migration in
vitro. Eur J Pharmacol. 724:219–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng W, Liu P, Pan W, Singh SR and Wei Y:
Hypoxia and hypoxia inducible factors in tumor metabolism. Cancer
Lett. 356:263–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Semenza GL: HIF-1 mediates metabolic
responses to intratumoral hypoxia and oncogenic mutations. J Clin
Invest. 123:3664–3671. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Onishi H, Morisaki T, Nakao F, Odate S,
Morisaki T and Katano M: Protein-bound polysaccharide decreases
invasiveness and proliferation in pancreatic cancer by inhibition
of hedgehog signaling and HIF-1α pathways under hypoxia. Cancer
Lett. 335:289–298. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pugh CW and Ratcliffe PJ: Regulation of
angiogenesis by hypoxia: Role of the HIF system. Nat Med.
9:677–684. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu H, Xu R, Feng L, Guo W, Cao N, Qian C,
Teng P, Wang L, Wu X, Sun Y, et al: A novel chromone derivative
with anti-inflammatory property via inhibition of ROS-dependent
activation of TRAF6-ASK1-p38 pathway. PloS One. 7:e371682012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grabinski N, Bartkowiak K, Grupp K, Brandt
B, Pantel K and Jücker M: Distinct functional roles of Akt isoforms
for proliferation, survival, migration and EGF-mediated signalling
in lung cancer derived disseminated tumor cells. Cell Signal.
23:1952–1960. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamaguchi R, Yano H, Iemura A, Ogasawara
S, Haramaki M and Kojiro M: Expression of vascular endothelial
growth factor in human hepatocellular carcinoma. Hepatology.
28:68–77. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Soufla G, Sifakis S and Spandidos DA: FGF2
transcript levels are positively correlated with EGF and IGF-1 in
the malignant endometrium. Cancer Lett. 259:146–155. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jensen RL, Ragel BT, Whang K and Gillespie
D: Inhibition of hypoxia inducible factor-1α (HIF-1α) decreases
vascular endothelial growth factor (VEGF) secretion and tumor
growth in malignant gliomas. J Neurooncol. 78:233–247. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Neill LA and Bowie AG: The family of
five: TIR-domain-containing adaptors in toll-like receptor
signalling. Nat Rev Immunol. 7:353–364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Spirig R, Djafarzadeh S, Regueira T, Shaw
SG, von Garnier C, Takala J, Jakob SM, Rieben R and Lepper PM:
Effects of TLR agonists on the hypoxia-regulated transcription
factor HIF-1alpha and dendritic cell maturation under normoxic
conditions. PloS One. 5:e00109832010. View Article : Google Scholar : PubMed/NCBI
|