Introduction
Breast cancer is the most common malignancy
affecting the female population, accounting for approximately 1/4
of all cancers (1). China, a country
that has had a low incidence rate of breast cancer in the past, has
been confronted with increasing breast cancer morbidity in recent
years (2,3). Adjuvant chemotherapy, with its great
efficacy in eradicating residual carcinoma, and thereby lowering
the risk of recurrence and metastasis, has become an indispensable
treatment for early-stage breast cancer (4). In China, adjuvant chemotherapy is widely
used, but is completed at a suboptimal rate. Even in Beijing, a
highly developed city in which China's best medical resources are
available, 12.1% of patients who commence adjuvant chemotherapy
receive <4 cycles of chemotherapy (5).
The addition of docetaxel to anthracycline-based
therapy demonstrated superiority to the temporal standard
doxorubicin and cyclophosphamide (AC) regimen in terms of survival
in metastatic breast cancer (MBC) (6), rendering the epirubicin and docetaxel
(ED) regimen among the most active therapeutic regimens for MBC. In
neoadjuvant chemotherapy (NAC), 6 cycles of the ED regimen have
been shown to result in a higher pathological complete response
rate and this was thus considered a standard regimen (7). However, in adjuvant chemotherapy,
epirubicin and cyclophosphamide followed by docetaxel (EC-D) as the
standard regimen for concurrent administration has been proven to
be less effective with the addition of taxanes to anthracyclines
(8–10). Despite this fact, 6 cycles of adjuvant
ED has been applied as a substitute for 8 cycles of EC-D in China.
However, this issue is still under debate as there is a lack of
evidence for similar comparisons between the regimens. The ED
regimen is favored for its shorter course and fewer
hospitalizations, which are presumed to improve the unsatisfactory
completion of chemotherapy.
In this study, to investigate whether the ED regimen
for 6 cycles may be a substitute for the EC-D regimen in adjuvant
chemotherapy, we performed a retrospective analysis to compare its
feasibility, efficacy and safety.
Patients and methods
Patients
Data on patients who received either the ED or EC-D
regimen after curative surgery at Qilu Hospital of Shandong
University (Shandong, China) from January 2009 to January 2014 were
reviewed. Follow-up information, including the completion status of
post-operative adjuvant therapy, treatment-related side-effects
(mainly as regards hematological toxicities and gastrointestinal
reactions of chemotherapy), outcomes (recurrence, metastasis,
invasive contralateral breast cancer and death from any cause), was
collected regularly via telephone contact and the outpatient
department.
The ED regimen was prescribed as 70 mg/m2
epirubicin and 75 mg/m2 docetaxel at a 3-week interval
for 6 cycles. The EC-D regimen was prescribed as 70
mg/m2 epirubicin and 600 mg/m2
cyclophosphamide followed by 75 mg/m2 docetaxel at a
3-week interval for 4 cycles each. Primary prophylaxis with
granulocyte-colony stimulating factor (G-CSF) was not advised
unless neutropenia had previously occurred. G-CSF measurements were
used if the white blood cell (WBC) count was
<3×109/l. Patients who received the ED or EC-D
regimen were designated as the ED or EC-D groups, respectively.
During the follow-up evaluation, we noted that some
patients converted to the EC-D regimen after 1 or 2 cycles of the
ED regimen or the cyclophosphamide, epirubicin and 5-fluorouracil
(CEF) regimen, despite no intolerable treatment-related toxicities.
Only a few patients strictly completed the aforementioned EC-D
regimen. Thus, we placed patients who converted to the EC-D regimen
without presenting with intolerable toxicities in the EC-D group.
Patients who received NAC or were diagnosed with systemic
metastasis at initial presentation were excluded.
Statistical analysis
The primary end-point was disease-free survival
(DFS), which was defined as the time from surgery to local
recurrence, metastasis, or diagnosis of a second primary cancer or
invasive contralateral breast cancer. Patients with incomplete
follow-up or without a documented DFS event were censored at the
date that they were last known to be alive. Overall survival (OS)
was defined as the time from the date of surgery to death from any
cause. Neutropenia was defined as an absolute WBC count
<3×109/l.
The continuous data were compared using the
Student's t-test. Categorical data were analyzed using Fisher's
exact test. The Kaplan-Meier method was used to estimate the DFS
and OS distributions, and the log-rank test was used to detect
differences in these distributions with respect to treatment. Cox
proportional hazards models were used to estimate the effects of
treatment alone and the effects of treatment after adjusting for
some of the baseline co-variates. The Wald test was used to test
for significant co-variates in the proportional hazards models.
Toxicity was graded according to the National Cancer Institute
Common Toxicity Criteria (version 3.0). For all the statistical
tests, a value of P<0.05 was considered to indicate a
statistically significant difference, and all P-values were
two-sided. Data analysis was performed using SPSS version 18.0
(SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
In total, 374 individuals were enrolled in this
study (250 patients in the ED group and 124 patients in the EC-D
group). The clinicopathological characteristics of the patients and
immunohistochemical analysis are illustrated in Table I. The distribution of most
characteristics was well balanced between the two groups apart from
the estrogen receptor (ER)/progesterone receptor (PR) status.
 | Table I.Clinicopathological characteristics of
the patients enrolled in this study. |
Table I.
Clinicopathological characteristics of
the patients enrolled in this study.
|
| ED (n=250) | EC-D (n=124) |
|
|---|
|
|
|
|
|
|---|
| Variables | No. (%) | No. (%) | P-value |
|---|
| Age, years | 47.45±8.65 | 48.57±10.10 | 0.345 |
| Age group |
|
| 0.821 |
|
<50 | 157 (62.8) | 76
(61.3) |
|
| ≥50 | 93
(37.2) | 48
(38.7) |
|
| Menopausal
status |
|
| 0.422 |
|
Pre-menopausal | 165 (66.0) | 76
(61.3) |
|
|
Post-menopausal | 85
(34.0) | 48
(38.7) |
|
| Tumor size |
|
| 0.438 |
| ≤2
cm | 123 (54.0) | 56
(47.9) |
|
| 2<T≤5
cm | 99
(43.4) | 56
(47.9) |
|
| T>5
cm | 6
(2.6) | 5
(4.2) |
|
| Not
available |
22 |
7 |
|
| Pathologic type |
|
| 0.962 |
| IDC | 232 (92.8) | 116 (93.5) |
|
| ILC | 4
(1.6) | 2
(1.6) |
|
| IMPC | 6
(2.4) | 2
(1.6) |
|
|
Others | 8
(3.2) | 4
(3.2) |
|
| Tumor grade |
|
| 0.667 |
| Low | 2
(0.9) | 0 (0) |
|
|
Intermediate | 149 (67.7) | 76
(66.1) |
|
| High | 69
(31.4) | 39
(33.9) |
|
| Not
available |
30 |
9 |
|
| Counts of
positive |
|
| 0.466 |
| lymph nodes |
|
|
|
| 0 | 124 (49.6) | 64
(51.6) |
|
| 1–3 | 74
(29.6) | 42
(33.9) |
|
| 4–9 | 36
(14.4) | 14
(11.3) |
|
| ≥10 | 16 (6.4) | 4
(3.2) |
|
| Surgery |
|
| 0.358 |
| Modified
radical | 240 (96.0) | 119 (96.0) |
|
|
mastectomy |
|
|
|
| Radical
mastectomy | 4
(1.6) | 2
(1.6) |
|
| Breast
conserving | 4
(1.6) | 0
(0.0) |
|
|
surgery |
|
|
|
|
Nipple-sparing | 2
(0.8) | 3
(2.4) |
|
|
mastectomy |
|
|
|
| ER status |
|
| 0.046 |
|
Positive | 178 (71.2) | 75
(60.5) |
|
|
Negative | 72
(28.8) | 49
(39.5) |
|
| PR status |
|
| 0.035 |
|
Positive | 161 (64.4) | 67
(54.0 |
|
|
Negative | 89
(35.6) | 57
(46.0) |
|
| HER2 |
|
| 0.155 |
|
Positive | 55
(24.7) | 37
(32.5) |
|
|
Negative | 168 (75.3) | 77
(67.5) |
|
| Not
available |
27 |
10 |
|
The overall median follow-up time was 38.6 months
(range, 13–72 months), with follow-up times of 39.4 and 38.1 months
for the ED and EC-D groups, respectively. The EC-D group consisted
of 43 patients who strictly adhered to the EC-D regimen, 77
patients who converted to EC-D therapy after receiving 1 cycle of
the ED regimen (ED*1, EC*3, D*4), 3 patients who converted to the
EC-D regimen after 2 cycles of the ED regimen (1 patient received
ED*2, EC*3, D*3, and the remaining 2 patients received ED*2, EC*2,
D*4) and 1 patient who converted from the CEF to the EC-D regimen
during the second cycle (CEF*1, EC*3, D*4). However, the ED group
did not consist of patients who converted to ED therapy from other
regimens. Four human epidermal growth factor receptor 2
(HER2)-positive patients were treated with herceptin for 1 year in
the ED group, and 15 patients in the EC-D group received 1 year of
herceptin treatment.
Completion status
Approximately 10 and 5.6% of the patients failed to
complete the chemotherapy program in the ED and EC-D groups,
respectively. The reasons for therapeutic termination are listed in
Table II. There was no significant
difference in the completion status between the treatment groups
(90% for ED vs. 94.4% for EC-D, P=0.174). The percentage of
patients who quit the program due to severe toxicities did not
differ significantly between the 2 groups (5.6% for ED vs. 3.2% for
EC-D, P=0.443).
 | Table II.Patients who failed to complete
adjuvant therapy out of the total number of patients in each
group. |
Table II.
Patients who failed to complete
adjuvant therapy out of the total number of patients in each
group.
|
| ED (n=250) | (n=124) |
|
|---|
|
|
|
|
|
|---|
| Reasons for not
completing | No. (%) | No. (%) | P-value |
|---|
| Severe
toxicity | 14 (5.6) | 4 (3.2 | 0.443 |
| Cardiac
symptoms | 3 | 2 |
|
|
Gastrointestinal
reactions | 2 | 0 |
|
|
Myelosuppression | 0 | 2 |
|
| Other
toxicity | 9 | 0 |
|
| Other
reasonsa | 11 (4.4) | 3 (2.4) | 0.403 |
| Total | 25 (10.0) | 7 (5.6) | 0.174 |
DFS and OS
The number of metastatic events and deaths in both
groups are summarized in Table III.
In total, 2 breast cancer-related deaths and 4 recurrences were
observed in the patients who failed to complete ED chemotherapy.
All 5 recurrences occurred in patients who completed the EC-D
therapy.
 | Table III.Summary of outcome information out of
the total number of patients. |
Table III.
Summary of outcome information out of
the total number of patients.
|
| ED (n=250) | EC-D (n=124) |
|
|---|
|
|
|
|
|
|---|
| Parameter |
Completeda | Failedb |
Completeda | Failedb | Totalc |
|---|
| Metastasis (no. of
patients) |
|
|
|
|
|
| Neck
lymph nodes | 2 | 0 | 1 | 0 | 3 |
|
Bone | 0 | 2 | 2 | 0 | 4 |
|
Viscera | 6 | 2 | 2 | 0 | 10 |
| NA | 2 | 0 | 0 | 0 | 2 |
|
Total | 10 | 4 | 5 | 0 | 19 |
| Death (no. of
patients) |
|
|
|
|
|
| Disease
progression | 5 | 2 | 0 | 0 | 7 |
| Without
recurrence | 1 | 0 | 0 | 0 | 1 |
|
Total | 6 | 2 | 0 | 0 | 8 |
Among all the patients who completed the therapy
(n=342), there were 10 DFS events in the ED group and 5 in the EC-D
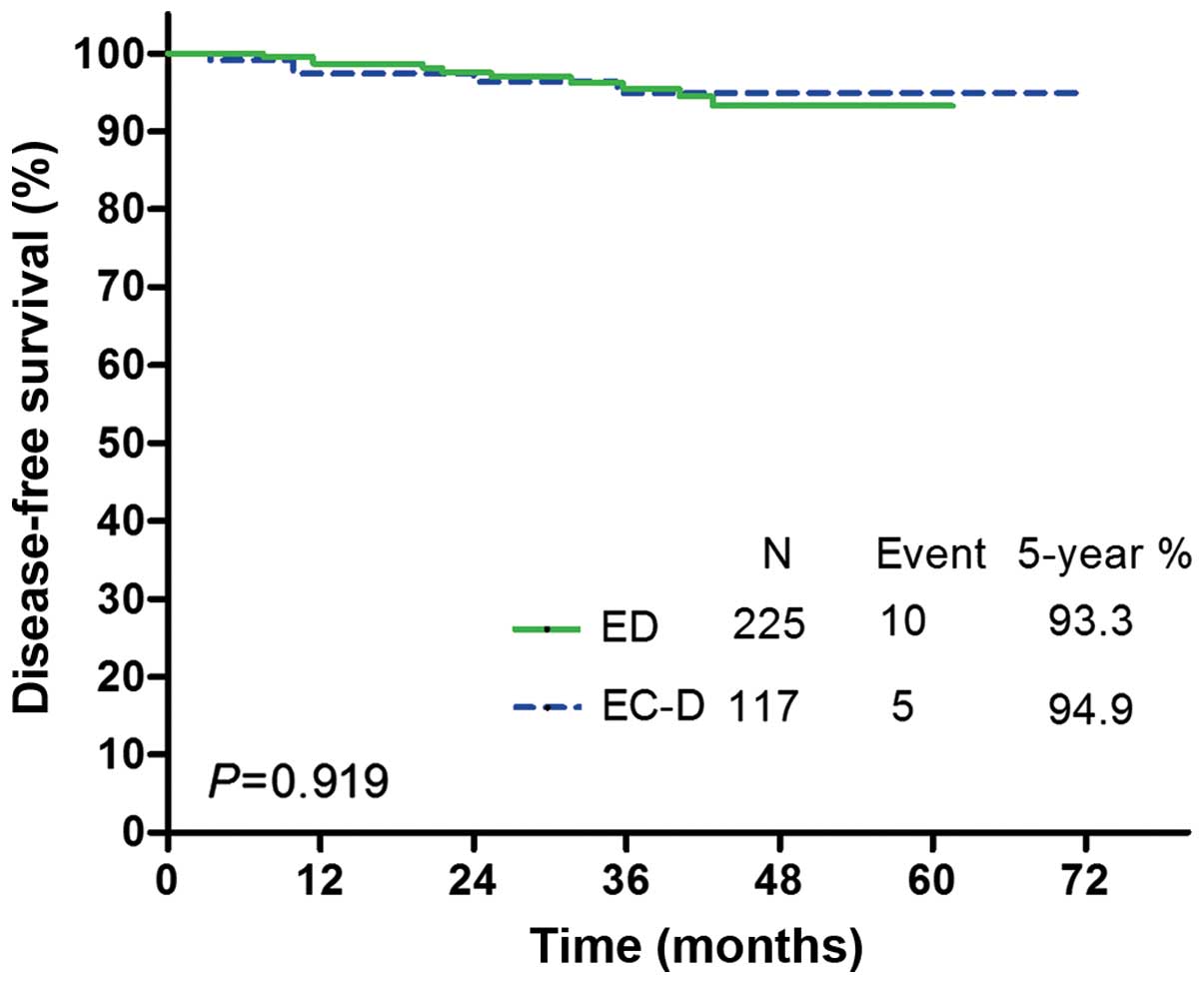
group. DFS Kaplan-Meier curves for each treatment group are
illustrated in Fig. 1. No significant
differences in DFS were observed between the two treatments [hazard
ratio (HR) for EC-D vs. ED, 0.947, 95% confidence interval (CI):
0.327–2.744; P=0.919; Table IV].
When adjusting for age, menopausal status, tumor size, tumor grade,
ER/PR status and nodal status, there were still no significant
differences in DFS between the two treatment groups (HR for EC-D
vs. ED, 0.694, 95% CI: 0.206–2.345; P=0.557; Table IV). When all the patients (n=374)
were analyzed, there were 14 events in the ED group and 5 events in
the EC-D group. The effect of the two treatments on DFS still
exhibited no significant difference between the 2 groups (HR for
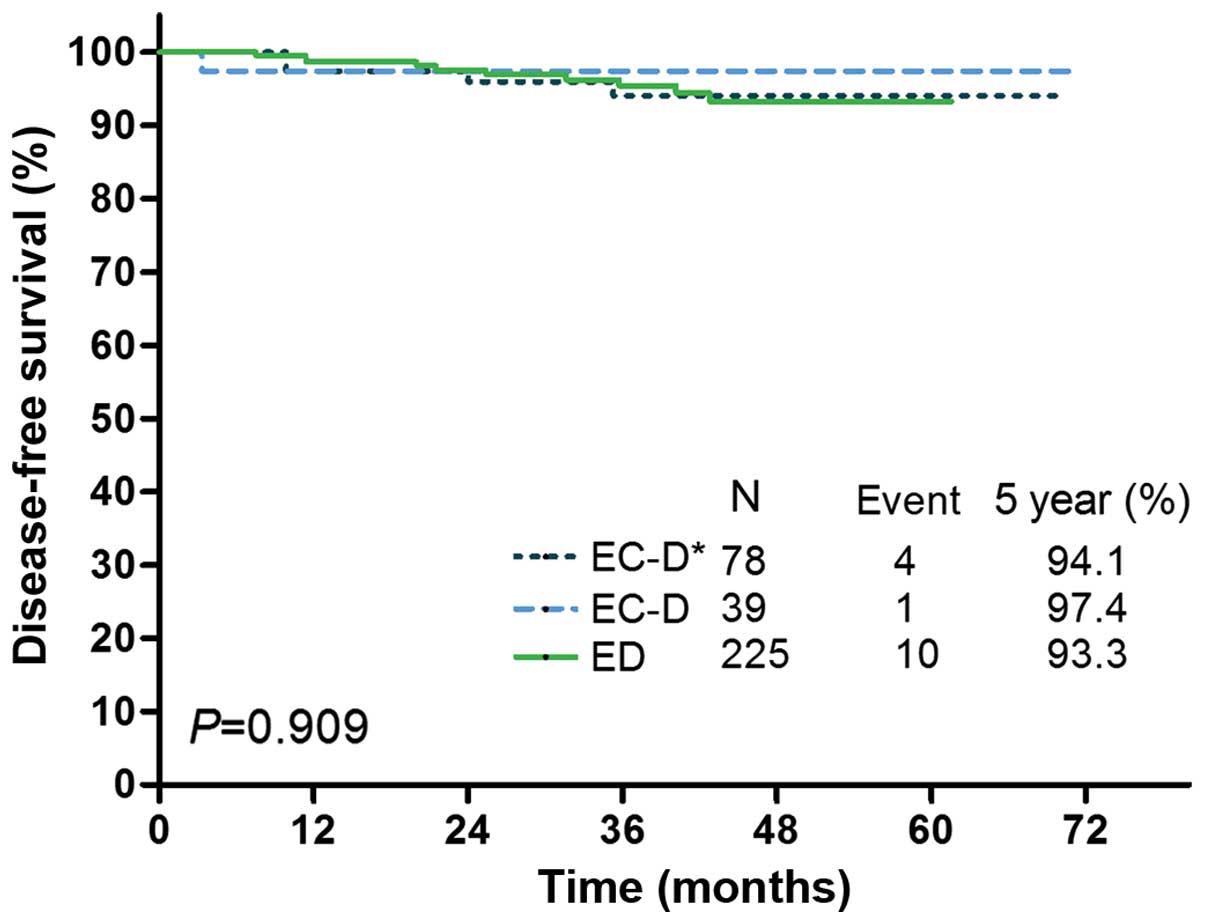
EC-D vs. ED, 0.736, 95% CI: 0.283–1.913; P=0.529; Table IV). The DFS Kaplan Meier curves for
the ED group and the EC-D subgroups with or without a therapy
change are shown in Fig. 2,
demonstrating no significant difference in DFS between the ED group
and the two subgroups of the EC-D group (P=0.909). Fig. 3 illustrates the effect of treatment on
DFS in the subgroups with different baseline characteristics. None
of the interactions between treatment and baseline characteristics
were statistically significant.
 | Figure 3.Hazard ratios and 95% CIs for the
different subgroups (patients who completed chemotherapy) (Forest
plot analysis). Data were unavailable for some patients and the
unavailable counts for ‘Tumor size’, ‘Molecular subtype’, ‘HER2
status’, and ‘Histological grade’ are 27, 16, 34 and 35,
respectively. ED, epirubicin and docetaxel; EC-D, epirubicin and
cyclophosphamide followed by docetaxel; HR, hazard ratio; ER,
estrogen receptor; PR, progesterone receptor; HER2, human epidermal
growth factor receptor 2; CI, confidence interval. |
 | Table IV.Univariate and adjusted HRs. |
Table IV.
Univariate and adjusted HRs.
|
| EC-D vs. ED |
|---|
|
|
|
|---|
| Patients | HRa | 95% CI | P-value |
|---|
| Completed
chemotherapy, n=342 |
|
|
|
|
DFS | 0.947 | 0.327–2.744 | 0.919 |
|
Adjusted DFSb | 0.694 | 0.206–2.345 | 0.557 |
| OS | 0.216 | 0.040–1.126 | 0.069 |
|
Adjusted OSb | 0.000 | 0.000–1.56E268 | 0.968 |
| All, n=374 |
|
|
|
|
DFS | 0.736 | 0.283–1.913 | 0.529 |
|
Adjusted DFSb | 0.554 | 0.171–1.789 | 0.323 |
| OS | 0.221 | 0.051–0.959 | 0.044 |
|
Adjusted OSb | 0.000 | 0.000–2.21E259 | 0.967 |
There were 6 deaths among the patients who completed
ED chemotherapy. In the EC-D group, no patients died (Table III). There was no significant
difference in OS between the treatment groups (HR for EC-D vs. ED,
0.216, 95% CI: 0.040–1.126; P=0.069; Table IV). After adjustment, there was no
significant difference in the OS of these groups (P=0.967). When
all the patients were analyzed (n=374), the OS of the ED group was
inferior to that of the EC-D group (HR for EC-D vs. ED, 0.221, 95%
CI: 0.051–0.959; P=0.044; Table IV).
However, after adjustment, the effects of the 2 treatments on OS
did not differ significantly (P=0.968).
Toxicity
There was no significant difference in the incidence
of neutropenia between the two treatment groups (81.2% with ED vs.
78.9% with EC-D, P=0.660; Table V).
The utilization rate of G-CSF was similar between the 2 groups
(76.9% with ED vs. 75.2% with EC-D, P=0.850; Table V). To investigate whether this
negative result was due to the confounding factor of patients who
changed their therapy in the EC-D group, we compared the incidence
and usage of G-CSF between the 2 subgroups (with/without changing
chemotherapy) of the EC-D group. We found that the incidence of
neutropenia and the usage of G-CSF were both similar between the 2
subgroups (P=1.000 for both comparisons, Table VI). Gastrointestinal reactions (e.g.,
vomiting, diarrhea and constipation) were more severe within the
EC-D group than the ED group, and the difference nearly achieved
statistical significance (grade 3/4 gastrointestinal reactions:
29.2% with ED vs. 42.0% with EC-D, P=0.058; Table V). There were no treatment-related
deaths or cases of congestive heart failure or myelodysplastic
syndromes/acute myeloid leukemia in the two treatment groups.
 | Table V.Toxicity in the patients treated with
both regimens. |
Table V.
Toxicity in the patients treated with
both regimens.
|
| ED (n=250) | EC-D (n=124) |
|
|---|
|
|
|
|
|
|---|
| Event | No. (%) | No. (%) | P-value |
|---|
|
Neutropeniaa |
|
| 0.660 |
|
Yes | 186 (81.2) | 86
(78.9) |
|
| No | 43
(18.8) | 23
(21.1) |
|
| NA |
21 |
15 |
|
| Treatment |
|
| 0.850 |
|
G-CSF | 176 (76.9) | 82
(75.2) |
|
| Oral
drugs | 10
(4.4) | 4
(3.7) |
|
| No
treatment | 43
(18.8) | 23
(21.1) |
|
| NA |
21 |
15 |
|
| Time to start using
G-CSF |
|
| 0.611 |
| Cycle
1–3 | 93
(89.4) | 49
(86.0) |
|
| 4th
cycle or later | 11
(10.6) | 8
(14.0) |
|
| NA |
72 |
25 |
|
| Treatment-related
death |
|
|
|
| Yes | 0
(0) | 0
(0) |
|
| No | 250 (100) | 124 (100) |
|
| Congestive heart
failure |
|
|
|
| Yes | 0
(0) | 0
(0) |
|
| No | 250 (100) | 124 (100) |
|
| Myelodysplastic
syndrome/acute myeloid leukemia |
| Yes | 0
(0) | 0
(0) |
|
| No | 250 (100) | 124 (100) |
|
| GI reactions |
|
| 0.058 |
| No
symptoms | 46
(19.7) | 16
(14.3) |
|
| Grade
1/2 | 119 (51.1) | 49
(43.8) |
|
| Grade
3/4 | 68
(29.2) | 47
(42.0) |
|
| NA |
17 |
12 |
|
 | Table VI.Toxicity in the subgroups in the EC-D
group. |
Table VI.
Toxicity in the subgroups in the EC-D
group.
|
| EC-D (n=43) | EC-Da (n=81) |
|
|---|
|
|
|
|
|
|---|
| Event | No. (%) | No. (%) | P-value |
|---|
|
Neutropeniab |
|
| 1.000 |
|
Yes | 30
(78.9) | 56 (78.9) |
|
| No | 8
(21.1) | 15 (21.1) |
|
| NA |
5 |
10 |
|
| Treatment |
|
| 1.000 |
|
G-CSF | 29
(76.3) | 53 (74.6) |
|
| Oral
drugs | 1
(2.6) | 3 (4.2) |
|
| No
treatment |
8 (21.1) | 15 (21.1) |
|
| NA |
5 |
10 |
|
| Time to start using
G-CSF |
|
| 0.239 |
| Cycle
1–3 | 17
(77.3) | 32 (91.4) |
|
| 4th
cycle or later | 5
(22.7) | 3 (8.6) |
|
| NA |
21 |
46 |
|
Discussion
This study retrospectively compared the feasibility,
survival and common toxicities of the ED and EC-D regimens as
adjuvant chemotherapy for patients with operable breast cancer. In
total, >90% of the patients completed their chemotherapy in both
groups, indicating that both regimens were feasible.
Although the addition of adjuvant taxanes has
demonstrated an improvement in survival (11), the concurrent use of taxanes and
anthracyclines with a shorter course has proven to be a less
effective method when compared with sequential administration in
adjuvant therapy (8,11,12). In a
meta-analysis with all available phase III randomized trials
comparing the sequential and concurrent use of taxanes and
anthracyclines in adjuvant therapy, Shao et al (8) demonstrated that sequential
administration had a more favorable outcome than concurrent
treatment. However, this meta-analysis consisted of only 3
randomized trials, including the NSABP B-30, BIG 02–98 and
BCIRG-005 trials. Among these trials, the sequential arm with a
higher cumulative dose of anthracyclines and taxanes demonstrated
superiority in survival (10,13), whereas the sequential arm with a
relatively lower cumulative dose of the two drugs showed no
improvement in therapeutic effects (9). As docetaxel has a dose-response effect
(14), the superiority of sequential
regimens could, to some extent, be ascribed to the higher
cumulative dose of docetaxel. Since the number of included trials
was small and none of the included trials were scheduled similar to
our study, this meta-analysis was considered too underpowered to be
used as a reference. In our study, with a higher cumulative dose of
cytotoxic agents in the concurrent group, the DFS was similar
between the two treatment groups. Although the OS in the ED group
was inferior to that in the EC-D group, the difference was not
statistically significant after adjusting for baseline
characteristics or excluding patients who failed to complete the
therapy. Moreover, the ongoing phase III randomized trial
(ClinicalTrials.gov no. NCT01134523)
conducted by Yuan et al (15)
comparing the effect of the ET (75/175 mg/m2
epirubicin/paclitaxel for 6 cycles at a 3-week interval) and EC-T
(90/600 mg/m2 epirubicin/cyclophosphamide at a 3-week
interval followed by 175 mg/m2 paclitaxel at a 2-week
interval for 4 cycles each) regimens as adjuvant therapy for
patients with early breast cancer with positive lymph nodes has
reported primary results. They found that after a median follow-up
of 35.5 months, the DFS of the two arms was similar (log-rank,
P=0.719) and that the incidence of treatment-related toxicity did
not differ significantly (15).
Moreover, Hong et al (16)
reported that there was no significant difference in treatment
response between AD (50/75 mg/m2 adriamycin/docetaxel at
a 3-week interval for 4 cycles each) and AC-T (50/500
mg/m2 adriamycin/cyclophosphamide followed by 175
mg/m2 paclitaxel at a 3-week interval for 4 cycles each)
as NAC in patients with operable breast cancer (16). Both of the aforementioned studies
showed no inferiority in the short-term therapeutic effects of
concurrent treatment, which was consistent with our results.
The concurrent administration of anthracyclines and
taxanes hyas been reported to have more severe hematological
toxicities compared to sequential and anthracycline-based regimens
(6,16). The primary prophylactic use of G-CSF
based on National Comprehensive Cancer Network guidelines for a
higher risk of febrile neutropenia (FN) was also recommended
(6,17). However, the results of our study
suggested that when primary prophylactic G-CSF was not routinely
used, the severity of myelosuppression with the ED regimen was
similar to that with the EC-D regimen on the basis of the identical
incidence of neutropenia and the utilization rate of G-CSF, as well
as the small number of patients whose treatment was limited by
severe myelosuppression. This result could be explained by the
timely support of G-CSF and the difference in population
susceptibility between Mongolians and Caucasians. The
above-mentioned ongoing phase III randomized trial based on Chinese
patients comparing ET and EC-T also reported no significant
difference in toxicities, including grade 3/4 neutropenia, in their
primary results (15). However,
another study on Korean patients suggested that the AD regimen was
associated with a significantly higher incidence of FN and grade
3/4 neutropenia compared with the AC-T regimen in the neoadjuvant
setting (16). The study used
docetaxel at a 3-week interval in the AD group and paclitaxel at a
3-week interval in the AC-T group. Docetaxel administered every 3
weeks was proven to be associated with higher grade hematological
toxicity compared with paclitaxel administered at a 3-week interval
by the E1199 trial (18); the higher
incidence of severe toxicity, such as myelosuppression in the AD
group was partially due to different taxanes. Moreover, in the
BCIRG-005 trial, although the incidence of grade 3/4 neutropenia
during the 6 cycles of the TAC arm was significantly higher than
that of the AC-T arm (17.4 vs. 7.7%; P<0.001), the incidence of
neutropenic infection was similar between the two treatments (8.5%
with AC-T vs. 9.7% with AT; P=0.25) (9). Taken together, these studies
demonstrated that the ED regimen was safe and feasible with the
support of G-CSF.
Our study had several limitations. First, it was a
retrospective study with a relatively small sample size, a short
follow-up period and few DFS/OS events, which affected the power in
comparing the efficacy of the two treatments. Moreover, the EC-D
group consisted of a large number of patients who converted to the
EC-D regimen after 1–2 cycles of ED and other regimens, which also
reduced the power of the study. Thus, the similarities in efficacy
between the two regimens could have arisen by chance. However, the
large number of patients who changed from the ED regimen to the
EC-D regimen was a reflection of the controversy between these two
treatments. Due to the limitations of our study, more credible
results are needed from prospective randomized clinical trials.
Thus, the final results of the phase III trial performed by Yuan
et al (15) are eagerly
anticipated. Second, we failed to provide the incidence of grade
3/4 neutropenia and FN, which could directly reflect the severity
of myelosuppression in the treatments. However, we could infer from
the similarities in the incidence of neutropenia, the usage rate of
G-CSF, the start time of the use of G-CSF and the number of
patients who quit due to severe hematological toxicity between the
treatment groups, that the ED regimen was safe with the proper use
of G-CSF.
Despite these limitations, our study, to the best of
our knowledge, was the first to compare these two frequently used
adjuvant chemotherapy regimens in China. In addition, to perform a
more accurate comparison of the efficacy, we investigated whether
patients who underwent a therapy change in the EC-D group affected
the results by analyzing the survival and toxicity events in the
subgroups with or without a therapy change. Considering that the ED
group consisted of more ER/PR-positive patients, we calculated the
DFS and OS after adjusting for ER/PR status, and no interactions
were found between treatment efficacy and the ER/PR status.
In conclusion, according to our results, the
completion status and hematological toxicities were similar between
the ED and EC-D regimens, while the gastrointestinal tolerance of
the ED regimen was better than that of the EC-D regimen. Moreover,
the mid-term DFS and OS of the ED regimen were not inferior to the
EC-D regimen. Thus, the ED regimen for 6 cycles may be an
alternative for the EC-D regimen as postoperative adjuvant
chemotherapy for patients with operable breast cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81272903), the Scientific Research
Foundation of Shandong Province for Outstanding Young Scientist
Award (no. BS2014YY055), the China Postdoctoral Science Foundation
(no. 2015M572050) and the Key Research and Development Program of
Shandong Province (no. 2015GSF118035).
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M, Forman
D, Bray F, Dikshit R, Elser S, Mathers C, Rebelo M and Parkin DM:
GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC
CancerBase No. 11 (Internet). International Agency for Research on
Cancer (Lyon, France). 2013.http://globocan.iarc.frAccessed. May 07–2015
|
|
2
|
Fan L, Zheng Y, Yu KD, Liu GY, Wu J, Lu
JS, Shen KW, Shen ZZ and Shao ZM: Breast cancer in a transitional
society over 18 years: Trends and present status in Shanghai,
China. Breast Cancer Res Treat. 117:409–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Porter P: ‘Westernizing’ women's risks?
Breast cancer in lower-income countries. N Engl J Med. 358:213–216.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan XM, Wang N, Ouyang T, Yang L, Song
MY, Lin BY, Xie YT, Li JF, Pan KF, You WC, et al: Current status of
diagnosis and treatment of primary breast cancer in beijing, 2008.
Chin J Cancer Res. 23:38–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nabholtz JM, Falkson C, Campos D, Szanto
J, Martin M, Chan S, Pienkowski T, Zaluski J, Pinter T, Krzakowski
M, et al: TAX 306 Study Group: Docetaxel and doxorubicin compared
with doxorubicin and cyclophosphamide as first-line chemotherapy
for metastatic breast cancer: Results of a randomized, multicenter,
phase III trial. J Clin Oncol. 21:968–975. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steger GG, Galid A, Gnant M, Mlineritsch
B, Lang A, Tausch C, Rudas M, Greil R, Wenzel C, Singer CF, et al:
ABCSG-14: Pathologic complete response with six compared with three
cycles of neoadjuvant epirubicin plus docetaxel and granulocyte
colony-stimulating factor in operable breast cancer: Results of
ABCSG-14. J Clin Oncol. 25:2012–2018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shao N, Wang S, Yao C, Xu X, Zhang Y,
Zhang Y and Lin Y: Sequential versus concurrent anthracyclines and
taxanes as adjuvant chemotherapy of early breast cancer: A
meta-analysis of phase III randomized control trials. Breast.
21:389–393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eiermann W, Pienkowski T, Crown J, Sadeghi
S, Martin M, Chan A, Saleh M, Sehdev S, Provencher L, Semiglazov V,
et al: Phase III study of doxorubicin/cyclophosphamide with
concomitant versus sequential docetaxel as adjuvant treatment in
patients with human epidermal growth factor receptor 2-normal,
node-positive breast cancer: BCIRG-005 trial. J Clin Oncol.
29:3877–3884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Francis P, Crown J, Di Leo A, Buyse M,
Balil A, Andersson M, Nordenskjöld B, Lang I, Jakesz R, Vorobiof D,
et al: BIG 02–98 Collaborative Group: Adjuvant chemotherapy with
sequential or concurrent anthracycline and docetaxel: Breast
International Group 02–98 randomized trial. J Natl Cancer Inst.
100:121–133. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Laurentiis M, Cancello G, D'Agostino D,
Giuliano M, Giordano A, Montagna E, Lauria R, Forestieri V,
Esposito A, Silvestro L, et al: Taxane-based combinations as
adjuvant chemotherapy of early breast cancer: A meta-analysis of
randomized trials. J Clin Oncol. 26:44–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldstein LJ, O'Neill A, Sparano JA, Perez
EA, Shulman LN, Martino S and Davidson NE: Concurrent doxorubicin
plus docetaxel is not more effective than concurrent doxorubicin
plus cyclophosphamide in operable breast cancer with 0 to 3
positive axillary nodes: North American Breast Cancer Intergroup
Trial E 2197. J Clin Oncol. 26:4092–4099. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Swain SM, Jeong JH, Geyer CE Jr,
Costantino JP, Pajon ER, Fehrenbacher L, Atkins JN, Polikoff J,
Vogel VG, Erban JK, et al: Longer therapy, iatrogenic amenorrhea,
and survival in early breast cancer. N Engl J Med. 362:2053–2065.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harvey V, Mouridsen H, Semiglazov V,
Jakobsen E, Voznyi E, Robinson BA, Groult V, Murawsky M and Cold S:
Phase III trial comparing three doses of docetaxel for second-line
treatment of advanced breast cancer. J Clin Oncol. 24:4963–4970.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan P, Wang J, Ma F, Fan Y, Luo Y, Cai R,
Zhang P, Li Q and Xu B: Comparison of six cycles of epirubicin and
paclitaxel (ET) versus four cycles of epirubicin and
cyclophosphamide, followed by four cycles of paclitaxel (EC-T) as
adjuvant therapy for operable breast cancer in women with positive
axillary nodes. J Clin Oncol. 32(Suppl; abstr 1042): 5s2014.
|
|
16
|
Hong WS, Jeon JY, Kang SY, Jung YS, Kim
JY, Ahn MS, Kang DK, Kim TH, Yim HE, An YS, et al: Comparison of
neoadjuvant adriamycin and docetaxel versus adriamycin,
cyclophosphamide followed by paclitaxel in patients with operable
breast cancer. J Korean Surg Soc. 85:7–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smith TJ, Khatcheressian J, Lyman GH, Ozer
H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J,
Cross SJ, et al: 2006 update of recommendations for the use of
white blood cell growth factors: An evidence-based clinical
practice guideline. J Clin Oncol. 24:3187–3205. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sparano JA, Wang M, Martino S, Jones V,
Perez EA, Saphner T, Wolff AC, Sledge GW Jr, Wood WC and Davidson
NE: Weekly paclitaxel in the adjuvant treatment of breast cancer. N
Engl J Med. 358:1663–1671. 2008. View Article : Google Scholar : PubMed/NCBI
|