Introduction
Erythropoietin (Epo) is a low-molecular weight
glycoprotein produced in the kidney by neural peritubular
fibroblasts and by the outer medulla (1,2), depending
on the amount of oxygen available (3). The Epo hormone facilitates the creation
and maturation of erythroid precursors, and stimulates
erythropoiesis upon binding to its receptor, known as
erythropoietin receptor (EpoR) (4).
Recombinant human Epo (rhEpo) is currently available for the
treatment of anemia resulted from chronic liver diseases (5) or chronic renal failure (6), and is a good alternative to blood
transfusion in cancer-related anemia (7), a frequent symptom of cancer (8) due to chemotherapy (9).
The presence of EpoR has been confirmed in numerous
tumor cells, with or without the stimulatory effect of Epo on these
cells (10–16). While Epo/EpoR signaling in
hematopoietic cells is associated with increased cell proliferation
and/or survival, the Epo/EpoR signaling pathway in tumor cells does
not always lead to increased cell proliferation, but may alter the
sensitivity of cancer cells to therapy (17). The role of Epo and EpoR on growth,
survival and therapeutic response in human cancer cells has been
previously reviewed by Szenajch et al (17) and Debeljak et al (18).
In this regard, although certain studies did not
confirm a direct stimulatory effect of Epo on tumor cells, there is
sufficient evidence of this effect on endothelial cell
proliferation and/or tumor angiogenesis. Epo induced angiogenesis
in murine hepatic tumors (19),
accelerated the growth of EpoR-negative Lewis lung carcinoma cells
by promoting tumor angiogenesis in vivo (20), stimulated neovascularization in
colorectal liver metastases (21),
affected glioma vascular endothelial cells and promoted
angiogenesis in a paracrine manner (22). Furthermore, Epo can also act directly
on glioma stem (23) and breast
cancer stem-like cells (24) by
activating specific pathways resulting in growth, survival and
enhanced tumor progression. Beside the Epo effects on cancer and
endothelial cells, there is another direct effect of Epo on cancer
stem and/or tumor-initiating cells that could explain the enhanced
tumor progression and poor survival observed in certain cancer
patients treated with Epo (18).
Indeed, Epo-induced signaling in cancer, endothelial
and cancer stem cells was associated with the activation of Janus
Kinase (JAK)2, JAK3, signal transducer and activator of
transcription (STAT)3 and STAT5 (but not with JAK1 or STAT1)
(16), Akt phosphorylation (15), extracellular signal-regulated kinase
(Erk) phosphorylation (25), human
telomerase reverse transcriptase (hTERT) gene transcription by
JAK2/STAT5/c-Myc, and hTERT protein phosphorylation by
phosphatidylinositol-4,5-bisphosphate 3-kinase/Akt (26). Previously, Swift et al
(27) and Elliott et al
(28) demonstrated that EpoR is
present at no/low level in 66 different tumor cell lines by western
blotting with the specific and sensitive anti-EpoR antibody A82
(which is currently not commercially available). In addition, EpoR
was undetectable using the A82 antibody in normal and cancerous
tissues, and it was undetectable/low in selected breast tumor cell
lines (28). According to Elliott
et al (28), there is a
discrepancy in the detection of EpoR due to the widespread
distribution of non-specific antibodies against EpoR.
Far-western blotting is a method for analyzing
protein-protein interactions, and could serve as a parallel assay
to standard western blot analysis (29). In far-western blotting, the protein
samples of interest are subjected to electrophoresis and next
immobilized onto a membrane (29).
During the transfer, the sodium dodecyl sulfate (SDS) is
eliminated, and proteins could re-adopt their three-dimensional
structure comprising the interaction site important for probing and
binding to non-antibody proteins (29). In contrast to western blot analysis,
which detects target proteins with specific antibodies, far-western
blotting identifies proteins based on the presence or absence of a
binding site to a particular protein probe (30).
To avoid the problem of using ‘nonspecific’
anti-EpoR antibodies, and to solve the problem of EpoR detection in
general, the present authors propose the use of far-western
blotting. In this technique, Epo is applied as a second antigen
and/or interacting molecule to EpoR, and the anti-Epo antibody
allows to bypass the direct detection of EpoR. In addition, this
technique enables to determine the EpoR functionality, or at least
the capability of Epo to interact with an EpoR of different origin.
The present study demonstrated the ability of far-western blotting
to detect EpoR on human ovarian adenocarcinoma cells (A2780) and
normal human umbilical vein endothelial cells (HUVECs).
Material and methods
Cell culture conditions
A2780 cells were obtained from the American Tissue
Culture Collection (Manassas, VA, USA). Cells were grown in
RPMI-1640 medium with L-glutamine (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The medium was supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and
antibiotic/antimycotic solution (100 U/ml penicillin, 100 µg/ml
streptomycin and 0.25 µg/ml amphotericin B; Invitrogen; Thermo
Fisher Scientific, Inc.). The cells were maintained under standard
tissue culture conditions at 37°C and 5% CO2/95% air
atmosphere. The number of cells was determined using a ZF Coulter
counter (Beckman Coulter, Inc., Brea, CA, USA), and the total cell
viability was analyzed by staining with 0.15% eosin, followed by
light microscopy.
HUVECs were isolated, cultured and characterized as
previously described (31,32). Cells were cultured on gelatin-coated
dishes in cM199 (M199 medium supplemented with 10% heat-inactivated
human serum (PAA; GE Healthcare Life Sciences, Chalfont, UK), 10%
heat-inactivated newborn calf serum (Cambrex Corporation, East
Rutherford, NJ, USA), 150 µg/ml crude endothelial cell growth
factor (ECGF), 5 IU/ml heparin, 100 IU/ml penicillin and 100 µg/ml
streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C under a 5%
CO2/95% air atmosphere. Endothelial cell cultures were
washed with medium without ECGF and heparin 24 h prior to the
experiments.
Far-western blotting
A2780 cells and HUVECs were washed twice with
ice-cold phosphate-buffered saline (PBS), scraped into lysis buffer
[Tris-HCl (pH 7.4), 0.1% SDS, 10% glycerol and 100X protease
inhibitor cocktail (Sigma-Aldrich)] and incubated for 45 min. Then,
lysates were homogenized by sonication on ice for 30 sec at 30 V
(SONOPULS HD 2070; BANDELIN electronic GmbH & Co. KG, Berlin,
Germany). After sonication, lysates were centrifuged at 10,000 × g
for 10 min at 4°C, and the supernatants were transferred into
1.5-ml microcentrifuge tubes and quantified according to the Lowry
protein assay protocol (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Lysates (100 µg each) were then subjected to 12%
SDS-polyacrylamide gel electrophoresis (PAGE) and blotted onto a
polyvinylidene fluoride (PVDF) membrane with transfer buffer [3.6 g
Tris, 18 g glycine and 10% methanol (pH 7.4)]. The PVDF membrane
was blocked with 3% non-fat milk, followed by 1-h incubation with
15 µg rhEpo (40,000 IU/ml; EPREX®; Janssen Biologics B.V., Leiden,
The Netherlands) in 1X Tris-buffered saline (TBS) with 0.5% milk
(pH 7.2–7.4). Then, the membrane was washed and incubated overnight
with primary anti-Epo antibody (catalog no., MAB2871; 1:1,000;
R&D Systems, Inc., Minneapolis, MN, USA) in 1% non-fat milk (pH
7.2–7.4). The following day, the membrane was washed two times for
4 min in TBS with 0.05% Tween 20 (TBST; pH 7.4), and incubated for
1 h with secondary horseradish peroxidase (HRP)-conjugated antibody
[goat anti-mouse immunoglobulin (Ig)G F(ab')2 fragment; 1:2,000;
catalog no., 31436; Pierce; Thermo Fisher Scientific, Inc.] at room
temperature (RT). The antibody reactivity was visualized with
Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific,
Inc.). Bioluminescent signals were detected with ChemiDoc™ XRS+ and
Image Lab 3.0 software (Bio-Rad Laboratories, Inc.) or X-ray films
(Roberts Technology Group, Inc., Chalfont, PA, USA). Traditional
western blotting for the detection of EpoR with primary anti-Epo
(1:1,000; catalog no., MAB2871) and primary goat anti-EpoR
antibodies (1:1,000; catalog no., AF-322-PB; R&D Systems, Inc.)
served as controls for far-western blotting detection.
To confirm the affinity between EpoR and rhEpo
observed using far-western blotting, a second protein interaction
assay where Epo was subjected to 12% SDS-PAGE and blotted onto a
PVDF membrane was performed. The PVDF membrane with rhEpo was then
incubated with A2780 or HUVEC cell lysates (220 µg each) for 1 h in
0.5% milk, followed by overnight incubation with primary anti-EpoR
antibody (1:1,000; catalog no., AF-322-PB) in 1% milk. The
following day, the membrane was washed two times for 4 min in TBST
(pH 7.4), and then incubated for 1 h with secondary HRP-conjugated
antibody (rabbit anti-goat IgG F(ab')2 fragment; Pierce; Thermo
Fisher Scientific, Inc.) at RT. The antibody reactivity was
visualized as mentioned above.
Immunoprecipitation with protein A
agarose beads
A total of 80 µl agarose beads (Protein A Agarose;
Innova Biosciences Ltd., Cambridge, UK) were washed twice with 1 ml
ice-cold radioimmunoprecipitation assay (RIPA) buffer
(Sigma-Aldrich) and centrifuged for 30 sec at 8,200 × g at 4°C.
Non-specific binding was blocked by incubation of the agarose beads
with 2% bovine serum albumin for 1 h at RT, followed by two washes
with ice-cold RIPA buffer and centrifugation as mentioned above.
Both protein A agarose beads with 10 µg anti-Epo antibody (MAB
2871) and 22 µg rhEpo with the lysate of A2780 cells or HUVECs (250
µg of proteins each) were then incubated for 2 h on a rotary
agitator (10 rpm, 4°C). After two-times washing, the protein A
agarose beads/anti-Epo antibody complex was added to the rhEpo/cell
lysate complex (EpoR and other proteins) and incubated together for
another 1 h on a rotary agitator (10 rpm, 4°C). Upon washing and
addition of sample buffer, the complex of protein A agarose
beads/anti-Epo/rhEpo/EpoR was boiled for 5 min, centrifuged (10,000
× g at 24°C for 5 min) and subjected to SDS-PAGE (12%). The gel was
washed two times in distilled (d)H2O for 5 min, fixed
(with 40% ethanol, 10% acetic acid and 50% dH2O) for 1
h, washed again twice in dH2O for 10 min, and stained by
overnight incubation in 80% Colloidal Coomassie Blue (0.1%
Coomassie Brilliant Blue G-250, 2% orthophosphoric acid and 10%
ammonium sulfate) and 20% methanol at RT. The following day, the
gel was washed in acetic acid (1%), and the potential EpoR proteins
were detached from the gel and analyzed by matrix-assisted laser
desorption/ionization (MALDI)-time-of-flight (TOF) mass
spectrometry (MS). The detailed protocol of the pull-down assay has
been previously described (33).
Identification of the proteins observed in the gel was performed by
peptide mass fingerprinting, as previously described (34). In short, protein bands were excised
and digested as previously described (35). An aliquot of the digested solution was
mixed with an aliquot of α-cyano-4-hydroxycinnamic acid (Bruker
Corporation, Billerica, MA, USA) in 33% acetonitrile and 0.25%
trifluoroacetic acid. This mixture was then deposited onto a 600-µm
AnchorChip (Bruker Corporation), and allowed to dry. MALDI-MS data
were obtained in an automated analysis loop using an Ultraflex TOF
mass spectrometer (Bruker Corporation). Spectra were acquired in
positive-ion mode at 50 Hz laser frequency, and 100–1,000
individual spectra were averaged. The selected precursor ions were
subjected to fragment ion analysis in tandem TOF mode to obtain the
corresponding MALDI-MS/MS spectra. Automated analysis of MS data
was performed using the FlexAnalysis software version 3.0 (Bruker
Corporation). MALDI-MS and MALDI-MS/MS data were combined through
the BioTools software version 3.2 (Bruker Corporation) to search a
non-redundant protein database in the National Center for
Biotechnology Information website (www.ncbi.nlm.nih.gov/), using Mascot software (server
version 2.4; Matrix Science, Ltd., London, UK). Each experiment was
repeated three times.
Erk1/2 signaling of EpoR in
HUVECs
Erk1/2 signaling was monitored in HUVECs only, since
the same signaling pathway was previously described in A2780 cells
by Solár et al (12). HUVECs
were seeded into Petri dishes with M199 medium containing serum,
and incubated under the standard conditions described above. HUVECs
were washed with serum-free medium 24 h prior to the experiments,
followed by the addition of 5 IU/ml rhEpo to the experimental
group, and the addition of 20 ng vascular endothelial growth factor
(VEGF) to the positive control group. After 5, 10, 30 or 60 min of
incubation, cells were quickly washed twice with ice-cold PBS, and
lysed as mentioned above. HUVEC lysates (30 µg each) were then
subjected to SDS-PAGE (12%), blotted onto PVDF membranes and
incubated overnight with primary anti-p44/42 mitogen-activated
protein kinase (MAPK; #9102, 1:1,000; Cell Signaling Technology,
Inc., Danvers, MA, USA) or anti-phospho-p44/42 MAPK (#9101,
1:1,000; Cell Signaling Technology, Inc.) antibodies at 4°C. The
membrane was then reprobed with anti-β-actin primary antibody
(AC-15; 1:1,000; catalog no., A5441; Sigma-Aldrich) to detect
β-actin protein, which served as a loading control. The following
day, the membrane was washed twice in TBST (pH 7.4) for 4 min, and
incubated for 1 h with secondary HRP-conjugated antibody (goat
anti-rabbit IgG F(ab')2 fragment; catalog no., 31461; Pierce;
Thermo Fisher Scientific, Inc.) at RT. The antibody reactivity was
visualized as mentioned above.
Results
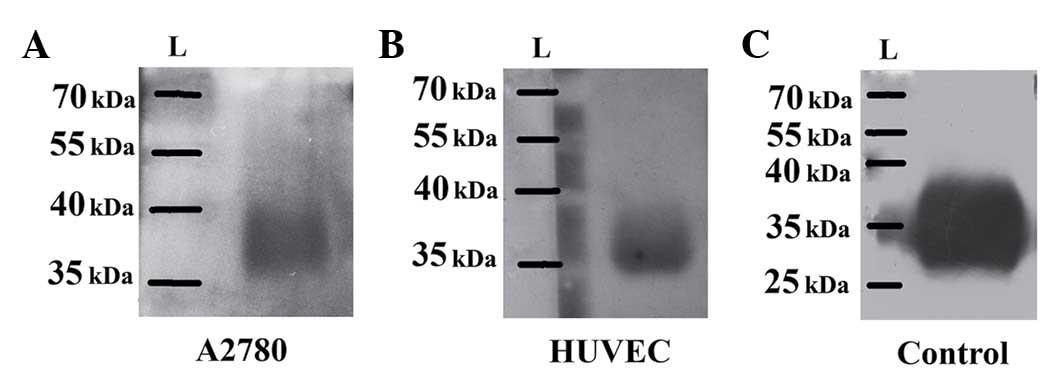
Far-western blotting was demonstrated
to be a simple way to specifically detect EpoR
Far-western blotting avoids the problem of direct
identification of EpoR with a potentially non-specific anti-EpoR
antibody, and also confirms the interaction of rhEpo with EpoR.
Whole proteins of ovarian adenocarcinoma A2780 and normal
endothelial HUVECs (including EpoR) were separated and blotted on
PVDF membranes, thus representing the antigen for the interaction
with rhEpo. The expected EpoR antigen/rhEpo antigen complex was
identified with an anti-Epo antibody, followed by traditional
western blot detection (Fig. 1A). Two
blots without rhEpo served as controls for far-western blot
detection. In this regard, one blot was incubated with anti-Epo
(Fig. 1B) and the second one with
anti-EpoR antibodies (Fig. 1C).
Indeed, far-western blotting revealed the same 57-kDa protein in
the EpoR/rhEpo/anti-Epo-antibody complex blot and in the control
blot with anti-EpoR antibody. In addition, the control blot with
anti-Epo antibody detection did not exhibit any protein of 57 kDa
in size (Fig. 1B).
Far-western blot detection of rhEpo/EpoR interaction
was also confirmed by a second type of interaction assay. For that
purpose, rhEpo was blotted onto PVDF membranes, followed by
incubation with cell lysates (EpoR) and western blot detection
using anti-EpoR antibody. Notably, rhEpo was detected not only by
traditional immunoblotting with an anti-Epo antibody (Fig. 2C), but also through
rhEpo/EpoR/anti-EpoR-antibody complex, as represented in Fig. 2A and B. In this regard, both A2780
cells (Fig. 2A) and HUVECs (Fig. 2B) exhibited a clear interaction of
their EpoR protein with rhEpo.
For further verification of the aforementioned
rhEpo/EpoR interaction, immunoprecipitation with protein A agarose
beads was applied. Independently incubated anti-Epo antibody with
protein A agarose beads and cell lysates with rhEpo were
subsequently mixed and incubated together, and the resulting
protein complex was separated on SDS-PAGE and stained with
Coomassie Blue. While the control groups did not reveal any
interaction between the protein A agarose beads and the proteins in
the cell lysates or the added rhEpo (data not shown), the anti-Epo
antibody captured on the protein A agarose beads did interact with
39-, 57- and >57-kDa proteins (Fig.
3). Only the 57-kDa protein was detached from the gel,
subjected to MALDI-TOF analysis and verified as Epo-interacting
EpoR (P<0.014), thus corresponding to the full-length EpoR. The
details of MS analysis and Mascot search are as follows: number of
peptides matched by MS, 6; number of peptides matched by MS/MS, 2;
score, 73; sequence coverage, 53%; accession no. matched-gi|6137384
(www.ncbi.nlm.nih.gov/protein/gi%7C6137384).
Since EpoR signaling in A2780 cells has been
previously published (12), the
present study provided evidence of the functionality of the
rhEpo/EpoR complex on HUVECs. These cells were starved overnight,
and incubated with rhEpo for 5–60 min or with rhVEGF (positive
control) for 10 min, and then processed for western blot analysis.
rhEpo at a concentration of 5 IU/ml increased the phosphorylation
of Erk1/2 at 30 min, and reached the maximum at 60 min after the
addition of rhEpo. Furthermore, the phosphorylation of Erk1/2 at 60
min following rhEpo addition achieved the same intensity than
phospho-Erk1/2 induced by recombinant VEGF (positive control)
(Fig. 4).
 | Figure 4.Epo induced Erk1/2 signaling in
HUVECs. Western blot analysis of Erk1/2 and phospho-Erk1/2 proteins
in HUVECs at 5, 10, 30 and 60 min after incubation with 5 IU/ml
rhEpo. HUVECs stimulated with 20 ng rhVEGF were used as a positive
control, while HUVECs without rhEpo or rhVEGF stimulation were used
as a negative control. NC, negative control; PC, positive control;
Erk, extracellular signal-regulated kinase; HUVEC, human umbilical
vein endothelial cell; Epo, erythropoietin; rhEpo, recombinant
human erythropoietin; rhVEGF, recombinant human vascular
endothelial growth factor. |
Discussion
Hematopoiesis is an essential body process that
supplies new blood cells (36).
Following the identification of Epo as a main stimulator of this
process, research has focused more recently on its receptor, EpoR
(37). Epo stimulates cells through
its interaction with EpoR on the cell surface (12). EpoR has been identified not only in
hematopoietic cells, but also in multiple non-hematopoietic cells
and tissues, where autocrine, paracrine and endocrine actions have
been proposed for EpoR (38).
Different types of tumors and cell lines have been found to express
EpoR messenger (m)RNA transcripts, which may be translated into
full-length, soluble or other truncated forms of EpoR (39). EpoR expression has been demonstrated
in a panel of 29 tumor cell lines, including 18 adherent cell lines
(40). In this regard, several groups
have reported the presence of EpoR also in ovarian cancer cell
lines (12,27,40–42), but
disagreements, particularly in the proportion of mRNA and the
amount of surface EpoR protein, were reported. Furthermore, Ribatti
et al (43) demonstrated the
presence of EpoR in HUVECs and EA.hy926 hematopoietic cells (a cell
fusion derived of HUVECs and lung carcinoma A549 cells). However,
Sinclair et al (44) claim
that numerous researchers have selected non-specific anti-EpoR
antibodies for their studies, thus producing false-positive results
(45). Therefore, the presence of
EpoR protein in different normal and cancer cell lines is
questionable. To avoid such false positivity and to additionally
demonstrate that EpoR is the Epo-interacting protein, the present
authors suggest to use far-western blotting, as this technique
allows bypassing of the direct detection of EpoR. This method
requires both rhEpo as well as an specific antibody against rhEpo,
which can be verified through detection of blotted rhEpo by
traditional western blotting (used as a positive control in the
present study). Indeed, besides the demonstration of Epo/EpoR
interaction, far-western blotting allowed to confirm in the current
case the presence of EpoR protein in human ovarian adenocarcinoma
A2780 cells and in normal HUVECs. In this regard, Sinclair et
al (44) detected 59-kDa EpoR in
HUVECs using the primary A82 anti-EpoR antibody, but the
concentration of EpoR homodimers per HUVEC was low, and the
rhEpo/EpoR interaction in these cells was absent. Notably, although
the present western blot results with the primary anti-EpoR
antibody AF322-PB (used as far-western blotting control) revealed
57- and 59-kDa EpoR proteins, only the 57-kDa protein was confirmed
by far-western blotting and immunoprecipitation with protein A
agarose beads, followed by MALDI-TOF verification, as the
rhEpo-interacting EpoR protein.
The present authors have previously investigated the
functionality of EpoR in A2780 cells through rhEpo-induced
signaling transduction and its modification after the addition of
soluble EpoR or specific lentiviral-mediated small hairpin (sh)RNA
silencing of EpoR expression (12).
Both methods resulted in the deprivation of Erk1/2 signaling
induced by rhEpo treatment in A2780 cells. In addition, EpoR
silencing reduced the protein levels of JAK2 and STAT5 in
shRNA-treated cells in comparison with non-targeted (NT)shRNA
control cells. Although the addition of rhEpo did not stimulate
A2780 cell proliferation, downregulation of EpoR expression by
shRNA resulted in reduced cell proliferation when monitored at 48,
72 and 96 h after seeding, compared with NTshRNA control-treated
and normal A2780 cells (12). Despite
the higher levels of EpoR transcripts detected in HUVECs, Sinclair
et al (44) did not find any
significant Erk1/2 or Akt phosphorylation after rhEpo stimulation.
The interaction of rhEpo with EpoR of vascular endothelial cells
was declared by increased intracellular levels of free calcium and
tyrosine phosphorylation of STAT5 (46–48). In
the present study, overnight-starved HUVECs incubated with rhEpo
showed increased Erk1/2 phosphorylation at 30 min after the
addition of rhEpo at a concentration of 5 IU/ml. Indeed, the
phosphorylation of Erk1/2 at 60 min after rhEpo addition achieved
the same intensity than the phospho-Erk1/2 signal induced by
recombinant VEGF protein.
In conclusion, the present study confirmed the
presence and the interaction of rhEpo/EpoR using the far-western
blotting technique in both human ovarian adenocarcinoma A2780 cells
as well as in normal HUVECs. Furthermore, modified
immunoprecipitation of EpoR followed by MALDI-TOF MS analysis
confirmed the 57-kDa protein as the human Epo-interacting EpoR
protein in both cell lines.
Acknowledgements
The present study was supported by the Scientific
Grant Agency of the Ministry of Education, Science, Research and
Sport of the Slovak Republic (Bratislava, Slovak Republic; grant
no. VEGA 1/0394/15) and by internal scientific grants from Pavol
Jozef Šafárik University in Košice (Košice, Slovak Republic; grant
nos. VVGS-2014-190 and VVGS-2014-220).
References
|
1
|
Lacombe C, Da Silva JL, Bruneval P,
Fournier JG, Wendling F, Casadevall N, Camilleri JP, Bariety J,
Varet B and Tambourin P: Peritubular cells are the site of
erythropoietin synthesis in the murine hypoxic kidney. J Clin
Invest. 81:620–623. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koury ST, Bondurant MC, Semenza GL and
Koury MJ: The use of in situ hybridization to study erythropoietin
gene expression in murine kidney and liver. Microsc Res Tech.
25:29–39. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frede S, Freitag P, Geuting L, Konietzny R
and Fandrey J: Oxygen-regulated expression of the erythropoietin
gene in the human renal cell line REPC. Blood. 117:4905–4914. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lodish H, Flygare J and Chou S: From stem
cell to erythroblast: Regulation of red cell production at multiple
levels by multiple hormones. IUBMB Life. 62:492–496. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruno CM, Neri S, Sciacca C, Bertino G, Di
Prima P, Cilio D, Pellicano R, Caruso L and Cristaldi R: Plasma
erythropoietin levels in anaemic and non-anaemic patients with
chronic liver diseases. World J Gastroenterol. 10:1353–1356.
2004.PubMed/NCBI
|
|
6
|
Pinevich AJ and Petersen J: Erythropoietin
therapy in patients with chronic renal failure. West J Med.
157:154–157. 1992.PubMed/NCBI
|
|
7
|
Bohlius J, Weingart O, Trelle S and Engert
A: Cancer-related anemia and recombinant human erythropoietin-an
updated overview. Nat Clin Pract Oncol. 3:152–164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cella D: The functional assessment of
cancer therapy-anemia (FACT-An) Scale: A new tool for the
assessment of outcomes in cancer anemia and fatigue. Semin Hematol.
34(3 Suppl 2): S13–S19. 1997.
|
|
9
|
Demetri GD, Kris M, Wade J, Degos L and
Cella D: Quality-of-life benefit in chemotherapy patients treated
with epoetin alfa is independent of disease response or tumor type:
Results from a prospective community oncology study. Procrit study
group. J Clin Oncol. 16:3412–3425. 1998.PubMed/NCBI
|
|
10
|
Jeong JY, Hoxhaj G, Socha AL, Sytkowski AJ
and Feldman L: An erythropoietin autocrine/paracrine axis modulates
the growth and survival of human prostate cancer cells. Mol Cancer
Res. 7:1150–1157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu P, Zhang N, Wang X, Zhang C, Li T, Ning
X and Gong K: The erythropoietin/erythropoietin receptor signaling
pathway promotes growth and invasion abilities in human renal
carcinoma cells. PLoS One. 7:e451222012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Solár P, Hrčková G, Varinská L, Solárová
Z, Kriška J, Uhrínová I, Kello M, Mojžiš J, Fedoročko P and
Sytkowski AJ: Location and the functionality of erythropoietin
receptor(s) in A2780 cells. Oncol Rep. 28:141–146. 2012.PubMed/NCBI
|
|
13
|
Miyake M, Goodison S, Lawton A, Zhang G,
Gomes-Giacoia E and Rosser CJ: Erythropoietin is a JAK2 and ERK1/2
effector that can promote renal tumor cell proliferation under
hypoxic conditions. J Hematol Oncol. 6:652013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trost N, Stepisnik T, Berne S, Pucer A,
Petan T, Komel R and Debeljak N: Recombinant human erythropoietin
alters gene expression and stimulates proliferation of MCF-7 breast
cancer cells. Radiol Oncol. 47:382–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abhold E, Rahimy E, Wang-Rodriguez J,
Blair KJ, Yu MA, Brumund KT, Weisman RA and Ongkeko WM: Recombinant
human erythropoietin promotes the acquisition of a malignant
phenotype in head and neck squamous cell carcinoma cell lines in
vitro. BMC Res Notes. 4:5532011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lopez TV, Lappin TR, Maxwell P, Shi Z,
Lopez-Marure R, Aguilar C and Rocha-Zavaleta L: Autocrine/paracrine
erythropoietin signalling promotes JAK/STAT-dependent proliferation
of human cervical cancer cells. Int J Cancer. 129:2566–2576. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Szenajch J, Wcislo G, Jeong JY, Szczylik C
and Feldman L: The role of erythropoietin and its receptor in
growth, survival and therapeutic response of human tumor cells From
clinic to bench-a critical review. Biochim Biophys Acta.
1806:82–95. 2010.PubMed/NCBI
|
|
18
|
Debeljak N, Solár P and Sytkowski AJ:
Erythropoietin and cancer: The unintended consequences of anemia
correction. Front Immunol. 5:5632014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamatsu K, Nishimura Y, Suzuki M,
Kanamori S, Maenishi O and Yasuda Y:
Erythropoietin/erythropoietin-receptor system as an angiogenic
factor in chemically induced murine hepatic tumors. Int J Clin
Oncol. 9:184–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okazaki T, Ebihara S, Asada M, Yamanda S,
Niu K and Arai H: Erythropoietin promotes the growth of tumors
lacking its receptor and decreases survival of tumor-bearing mice
by enhancing angiogenesis. Neoplasia. 10:932–939. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rupertus K, Senger S, Menger MD, Schilling
MK and Kollmar O: Darbepoetin-α promotes neovascularization and
cell proliferation in established colorectal liver metastases. J
Surg Res. 176:517–523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nico B, Annese T, Guidolin D, Finato N,
Crivellato E and Ribatti D: Epo is involved in angiogenesis in
human glioma. J Neurooncol. 102:51–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao Y, Lathia JD, Eyler CE, Wu Q, Li Z,
Wang H, McLendon RE, Hjelmeland AB and Rich JN: Erythropoietin
receptor signaling through STAT3 is required for glioma stem cell
maintenance. Genes Cancer. 1:50–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Todaro M, Turdo A, Bartucci M, Iovino F,
Dattilo R, Biffoni M, Stassi G, Federici G, De Maria R and Zeuner
A: Erythropoietin activates cell survival pathways in breast cancer
stem-like cells to protect them from chemotherapy. Cancer Res.
73:6393–6400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pérés EA, Valable S, Guillamo JS, Marteau
L, Bernaudin JF, Roussel S, Lechapt-Zalcman E, Bernaudin M and
Petit E: Targeting the erythropoietin receptor on glioma cells
reduces tumour growth. Exp Cell Res. 317:2321–2332. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akiyama M, Kawano T, Mikami-Terao Y,
Agawa-Ohta M, Yamada O, Ida H and Yamada H: Erythropoietin
activates telomerase through transcriptional and
posttranscriptional regulation in human erythroleukemic JAS-REN-A
cells. Leuk Res. 35:416–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Swift S, Ellison AR, Kassner P, McCaffery
I, Rossi J, Sinclair AM, Begley CG and Elliott S: Absence of
functional EpoR expression in human tumor cell lines. Blood.
115:4254–4263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elliott S, Swift S, Busse L, Scully S, Van
G, Rossi J and Johnson C: Epo receptors are not detectable in
primary human tumor tissue samples. PLoS One. 8:e680832013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jadwin JA, Mayer BJ and Machida K:
Detection and quantification of protein-protein interactions by
far-western blotting. Methods Mol Biol. 1312:379–398. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Elliott S, Busse L, McCaffery I, Rossi J,
Sinclair A, Spahr C, Swift S and Begley CG: Identification of a
sensitive anti-erythropoietin receptor monoclonal antibody allows
detection of low levels of EpoR in cells. J Immunol Methods.
352:126–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Van Hinsbergh VW, Sprengers ED and
Kooistra T: Effect of thrombin on the production of plasminogen
activators and PA inhibitor-1 by human foreskin microvascular
endothelial cells. Thromb Haemost. 57:148–153. 1987.PubMed/NCBI
|
|
32
|
Defilippi P, van Hinsbergh V, Bertolotto
A, Rossino P, Silengo L and Tarone G: Differential distribution and
modulation of expression of alpha 1/beta 1 integrin on human
endothelial cells. J Cell Biol. 114:855–863. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mlynarcik P, Bencurova E, Madar M, Mucha
R, Pulzova L, Hresko S and Bhide M: Development of simple and rapid
elution methods for proteins from various affinity beads for their
direct MALDI-TOF downstream application. J Proteomics.
75:4529–4535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bhide MR, Escudero R, Camafeita E, Gil H,
Jado I and Anda P: Complement factor H binding by different Lyme
disease and relapsing fever Borrelia in animals and human. BMC Res
Notes. 2:1342009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shevchenko A, Tomas H, Havlis J, Olsen JV
and Mann M: In-gel digestion for mass spectrometric
characterization of proteins and proteomes. Nat Protoc.
1:2856–2860. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Till JE and McCulloch E: A direct
measurement of the radiation sensitivity of normal mouse bone
marrow cells. Radiat Res. 14:213–222. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bonsdorf E and Jalavisto E: A humoral
mechanism in anoxic erythrocytosis. Acta Physiol Scand. 16:150–170.
1948. View Article : Google Scholar
|
|
38
|
Arcasoy MO: The non-haematopoietic
biological effects of erythropoietin. Br J Haematol. 141:14–31.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arcasoy MO, Jiang X and Haroon ZA:
Expression of erythropoietin receptor splice variants in human
cancer. Biochem Biophys Res Commun. 307:999–1007. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Miller CP, Lowe KA, Valliant-Saunders K,
Kaiser JF, Mattern D, Urban N, Henke M and Blau CA: Evaluating
erythropoietin-associated tumor progression using archival tissues
from a phase III clinical trial. Stem Cells. 27:2353–2361. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jeong JY, Feldman L, Solar P, Szenajch J
and Sytkowski AJ: Characterization of erythropoietin receptor and
erythropoietin expression and function in human ovarian cancer
cells. Int J Cancer. 122:274–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Paragh G, Kumar SM, Rakosy Z, Choi SC, Xu
X and Acs G: RNA interference-mediated inhibition of erythropoietin
receptor expression suppresses tumor growth and invasiveness in
A2780 human ovarian carcinoma cells. Am J Pathol. 174:1504–1514.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ribatti D, Presta M, Vacca A, Ria R,
Giuliani R, Dell'Era P, Nico B, Roncali L and Dammacco F: Human
erythropoietin induces a pro-angiogenic phenotype in cultured
endothelial cells and stimulates neovascularization in vivo. Blood.
93:2627–2636. 1999.PubMed/NCBI
|
|
44
|
Sinclair AM, Coxon A, McCaffery I, Kaufman
S, Paweletz K, Liu L, Busse L, Swift S, Elliott S and Begley CG:
Functional erythropoietin receptor is undetectable in endothelial,
cardiac, neuronal, and renal cells. Blood. 115:4264–4272. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Laugsch M, Metzen E, Svensson T, Depping R
and Jelkmann W: Lack of functional erythropoietin receptors of
cancer cell lines. Int J Cancer. 122:1005–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vogel V, Kramer HJ, Bäcker A,
Meyer-Lehnert H, Jelkmann W and Fandrey J: Effects of
erythropoietin on endothelin-1 synthesis and the cellular calcium
messenger system in vascular endothelial cells. Am J Hypertens.
10:289–296. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Haller H, Christel C, Dannenberg L, Thiele
P, Lindschau C and Luft FC: Signal transduction of erythropoietin
in endothelial cells. Kidney Int. 50:481–488. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Janmaat ML, Heerkens JL, de Bruin AM,
Klous A, de Waard V and de Vries CJ: Erythropoietin accelerates
smooth muscle cell-rich vascular lesion formation in mice through
endothelial cell activation involving enhanced PDGF-BB release.
Blood. 115:1453–1460. 2010. View Article : Google Scholar : PubMed/NCBI
|