Introduction
Follicular dendritic cells (FDCs), Langerhans cells
and interdigitating dendritic cells are non-lymphoid,
non-phagocytic cells classified generically as accessory cells of
the lymphoid system. The function of non-phagocytic cells is the
capture and presentation of antigens and immune complexes (1). Lymphoid follicles are present in
extra-nodal lymphoid and lymph node tissues. As described by Monda
et al, FDC sarcomas (FDSCs) were extremely rare in 1986
(2). In recent years, the incidence
rates for FDSC has been gradually increasing. Recently, there have
been a large number of reviews published on this neoplasm (3–5).
Waldeyer's lymphoid tissue (tonsil) in the head and neck region is
the most common extranodal site (40%), followed by the nasopharynx
(20%), parapharyngeal space (12%), maxillary alveolar ridge (4%),
and hard and soft palate (4%) (6).
The current study presents an additional case of FDCS of the spleen
in a 64-year-old woman, and emphasizes the diagnostic difficulties
in this specific population with a review of the pertinent
literature.
Case report
In July 2013, an abdominal mass was found in a
64-year-old woman (who did not present obvious symptoms at that
time) at a health center in Suzhou, China. The patient was then
hospitalized in the Affiliated Wujiang Hospital of Nantong
University (Suzhou, Jiangsu, China) for further examination by
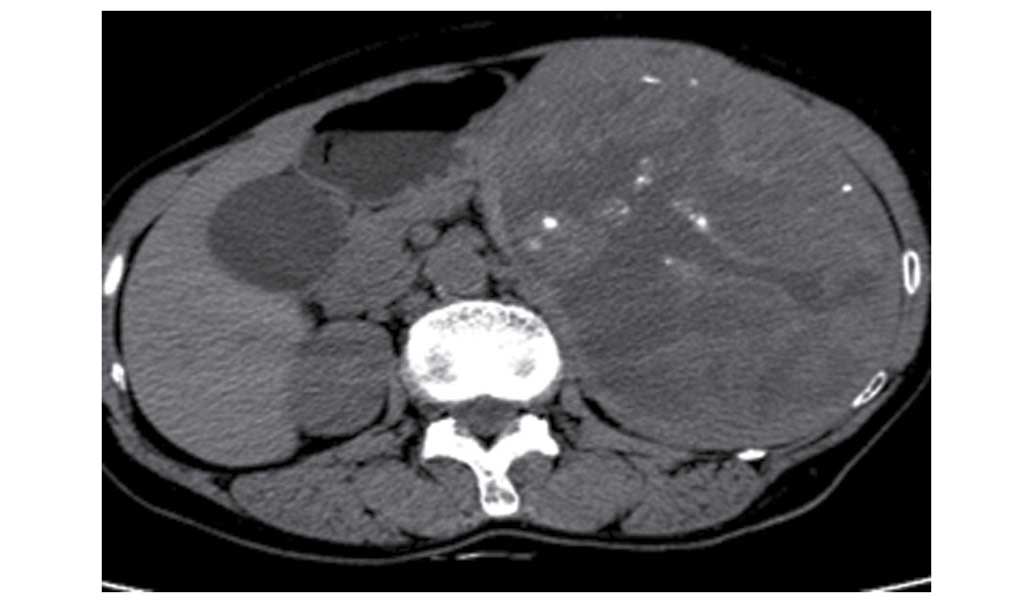
computed tomography (CT). Non-enhanced CT showed that this mass
measured 190×124 mm, and was located in the spleen where a massive
shadow was visible. The lesion manifested as a lower hybrid density
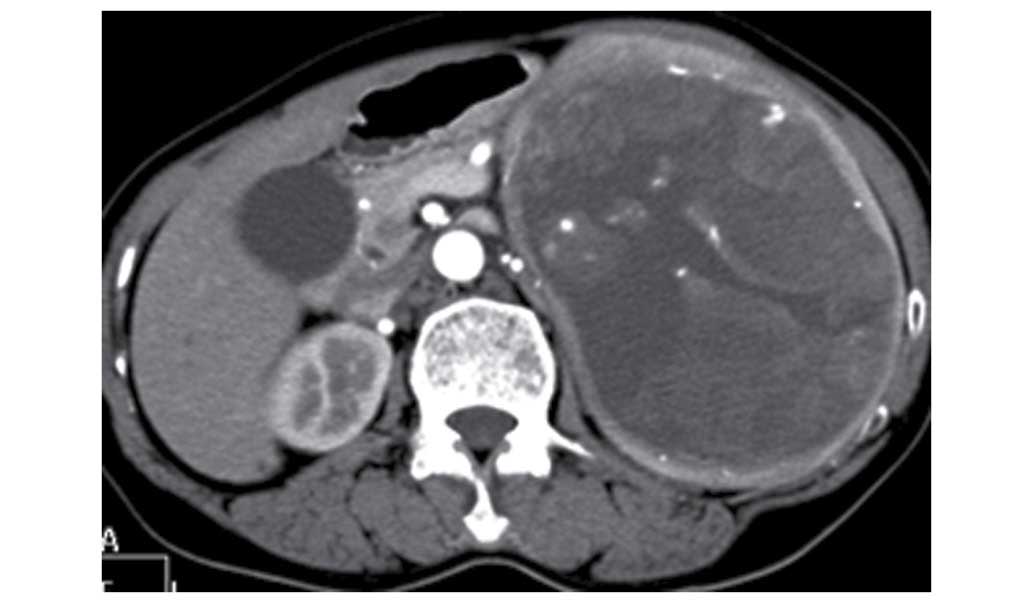
shadow and calcification, with clear borders (Fig. 1). Moderate inhomogenous enhancement
was found at the arterial phase (Fig.
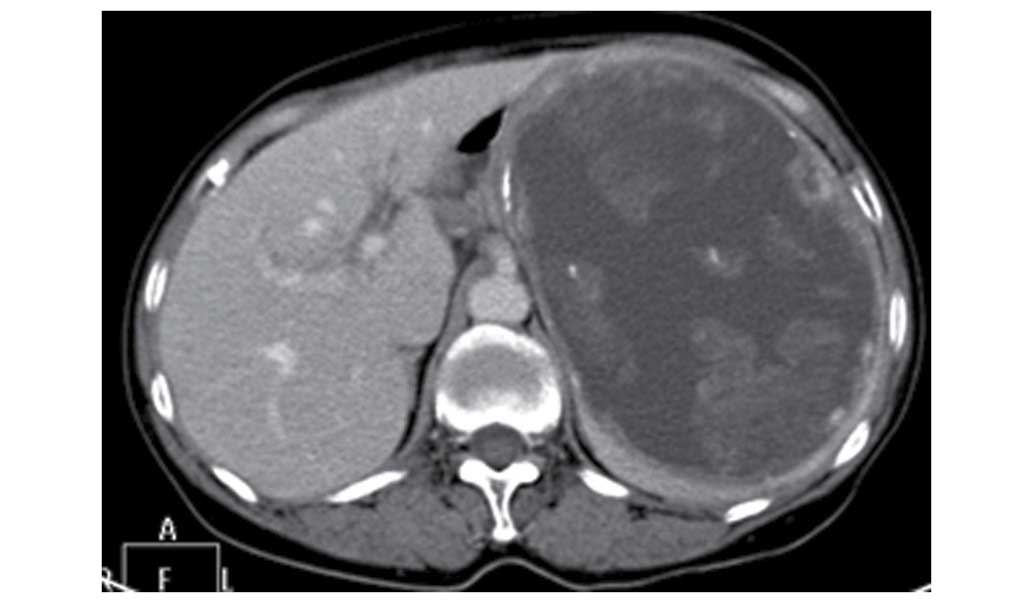
2) and inhomogenous continuous enhancement was found at the
venous phase (Fig. 3) following
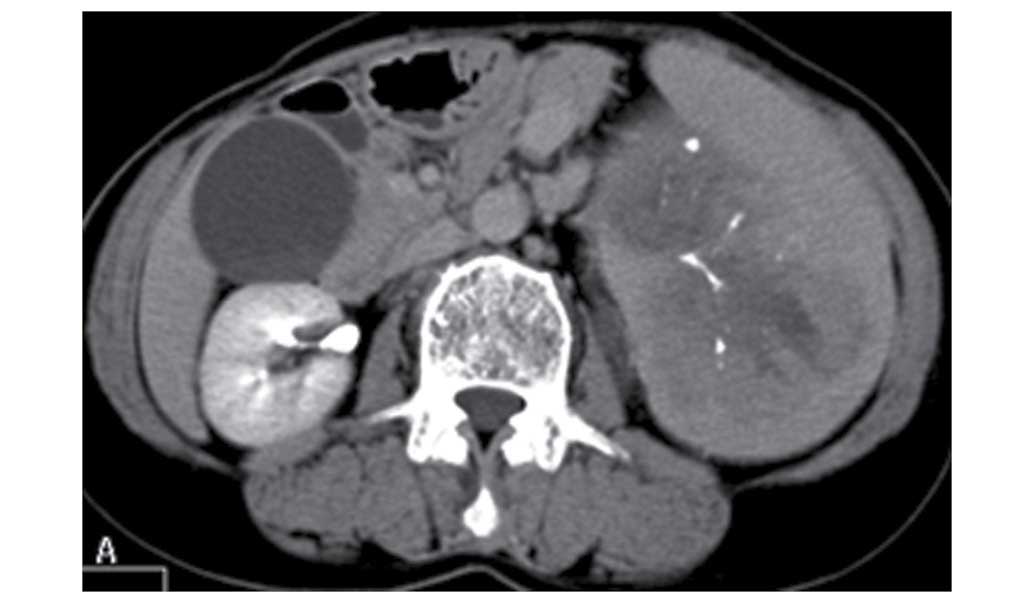
gadolinium administration, and a delayed CT scan showed that the
lesion was significantly enhanced (Fig.
4).
A sample of the splenic mass was obtained at the
time of the diagnostic evaluation and was formalin-fixed,
paraffin-embedded, cut into 3-µm sections, and stained with
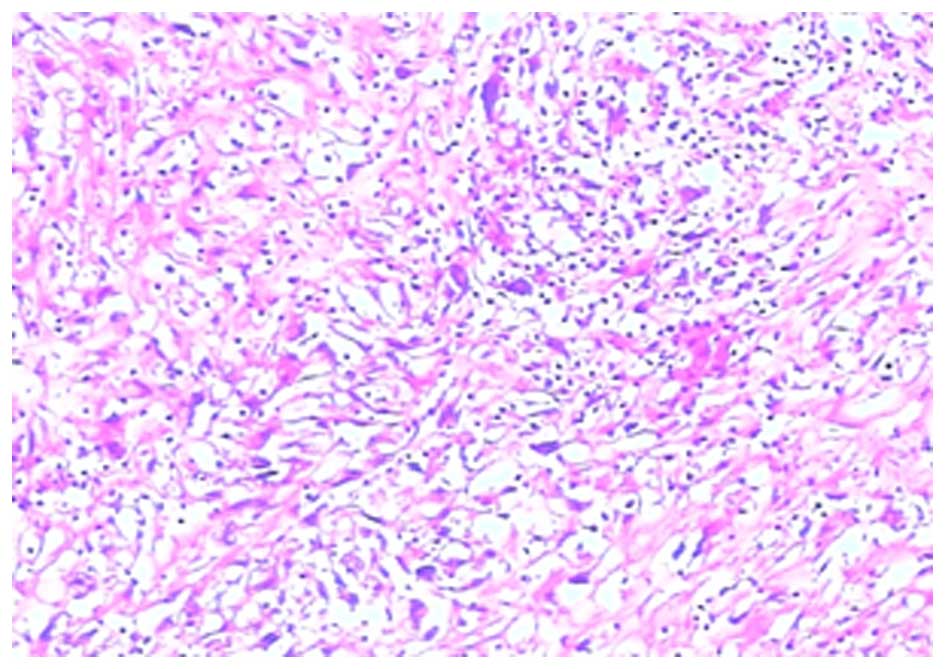
hematoxylin and eosin. The histological examination demonstrated
that the diagnosis was splenic FDCS (Fig.
5).
The tissue sections were evaluated
immunohistochemically using an avidin-biotin-peroxidase complex
method and an automated immunostainer. All tissue sections were
subjected to heat-induced antigen retrieval prior to incubation
with mouse monoclonal anti-human antibodies against cluster of
differentiation (CD)3 (sc-20047), CD20 (sc-393894), CD34
(sc-74499), CD68 (sc-20060), vimentin (sc-373717), neuron-specific
enolase (NSE) (sc-376585), Ki-67 (sc-23900), CD15 (sc-19648), CD23
(sc-18910), CD30 (sc-46683) and epithelial membrane antigen (EMA)
(sc-52329), which were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA) and were used at a dilution 1:200. The tumor
cells positively expressed CD3, CD20, CD34, CD68, vimentin and NSE.
Ki-67 was expressed in 20% of the tumor cell nuclei, and those
tumor cells were negative for CD15, CD23, CD30 and EMA. In October
2013, in order to confirm the diagnosis established by the
Affiliated Wujiang Hospital of Nantong University, the patient
visited the Department of Pathology of the Affiliated Tumor
Hospital of Fudan University (Shanghai, China) with the
pathological sections and paraffin blocks for consultation. The
result of the consultation was that the pathological diagnosis of
the tumor accorded with splenic FDCS, which was consistent with the
diagnosis made by the Affiliated Wujiang Hospital of Nantong
University. Once the definitive diagnosis had been established, the
patient received surgery in the Affiliated Wujiang Hospital of
Nantong University. The patient had a good remission and not
received chemotherapy or radiotherapy. At the time of writing, the
patient was in a healthy condition. Written consent was obtained
from the patient for publication of the present study.
Discussion
FDCS often occurs between the ages of 30 and 50, and
there is no gender predilection (7).
In the majority of cases, enlarged cervical lymph nodes are
present, which are painless and slow growing (8). The patient in the present study only
presented with an abdominal mass and did not present with any other
obvious symptoms. The manifestations of the patient are similar to
the cases reported in the literature (9,10).
Follicular dendritic cells are essential in the lymph nodes, where
their primary role is directed towards antigen presentation and
antigen-dependent B-cell maturation. Hence, the lymph nodes are the
location in which this rare neoplasm most commonly occurs. However,
the tumors can also colonize the inherent lymphoid tissues or be
acquired by other organs that thereby serve as extranodal locations
of occurrence (11). The histological
features of FDCS tend to be stereotypical, with tumors composed of
spindle or oval-shaped cells that show a storiform or whorled
growth model, and are arranged in sheets, nets and fascicles
focally (12). Epithelioid cells and
multinucleated giant cells are visible in the lesions (13). These typical characteristics of
pathology were in agreement with the results of the present case.
Histological findings may increase the suspicion of FDCS, but it is
essential to combine immunohistochemical staining for follicular
cell differentiation in order to avoid misdiagnosis (14). CD21, CD23 and CD35 are the most widely
used markers to demonstrate FDCS differentiation (15). Vimentin, EMA, S-100 protein, CD68,
desmoplakin, muscle-specific actin and leukocyte common antigen
show broadly variable positivity in FDCS. In fact, the only markers
permitting the diagnosis of FDCS are CD21 and CD35 because they are
only expressed by FDCS. Studies have shown that the pathogenesis of
FDCS is associated with the Epstein-Barr (EB) virus, however, a
review of the literature showed that EB virus RNA sequences were
detected in the organs and tissues throughout the body, with the
exception of the liver and spleen, in the majority of FDCS cases
(16).
The majority of FDCS tumors have clear boundaries
and are nodular or lobulated, with a size that is dependent on the
location of the tumor. Usually, the tumors are small in volume,
superficial and nodular with expansive growth, while hemorrhage or
necrosis is rare. The tumors located in the abdominal cavity or
abdominal organs are found deeper and are larger, with the presence
of hemorrhage and necrosis, and possible infiltration into the
adjacent parenchymal organs or soft tissues, as reported in the
present case, which was consistent with the literature (17). On magnetic resonance imaging, FDCS may
appear as an isointense expansive mass on T1-weighted scans, while
homogeneous and slightly hyperintense on T2-weighted scans
following gadolinium injection, with uniform moderate enhancement
(18). These features may be useful
in the diagnosis of this rare condition. However, more studies are
required to describe the radiological characteristics of FDCS. As
with any other sarcomas, a resection of the tumor with a wide
margin is generally recommended whenever feasible. In cases with
aggressive disease, such as those with extracapsular invasion, a
high mitotic index or unresectable tumors, radiotherapy could be
considered. Adjuvant chemotherapy, including a cyclophosphamide,
hydroxydaunorubicin, oncovin and prednisone regimen, carboplatin or
Adriamycin, has previously been recommended (19).
In conclusion, FDCS is a rare tumor that has been
recognized only recently. The disease rate is probably
underestimated, particularly when the tumors occur in extranodal
sites. The present study describes an additional case of a woman
with follicular dendritic cell sarcoma in the spleen, and discusses
the main clinical characteristics, treatment options and prognosis
of this neoplasm, particularly emphasizing the diagnostic
difficulties. The aim of the present study is to aid radiologists
and pathologists to further understand this tumor. Although the
histological features of FDCS are rather stereotypical, they are
likely to be misdiagnosed, as monoclonal-specific FDC markers are
not routinely used in the immunohistochemical study of
poorly-differentiated malignant tumors. The use of such markers
appears to be necessary for any poorly-differentiated tumor. At
present, surgical excision is the most effective treatment for the
majority of patients with FDCS. In certain cases, where the tumor
is deeply situated, large or infiltrated into the adjacent tissues,
adjuvant chemotherapy and radiotherapy are the optimal treatments.
Generally, the prognosis of FDCS is favorable (18,20,21).
References
|
1
|
Karligkiotis A, Contis D, Bella M,
Machouchas N, Volpi L, Melis A and Meloni F: Pediatric follicular
dendritic cell sarcoma of the head and neck: A case report and
review of the literature. Int J Pediatr Otorhinolaryngol.
77:1059–1064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monda L, Warnke R and Rosai J: A primary
lymph node malignancy with features suggestive of dendritic
reticulum cell differentiation. Am J Pathol. 122:562–572.
1986.PubMed/NCBI
|
|
3
|
Vaideeswar P, George SM, Kane SV,
Chaturvedi RA and Pandit SP: Extranodal follicular dendritic cell
sarcoma of the tonsil-case report of an epithelioid cell variant
with osteoclastic giant cells. Pathol Res Pract. 205:149–153. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang L, Admirand JH, Moran C, Ford RJ and
Bueso-Ramos CE: Mediastinal follicular dendritic cell sarcoma
involving bone marrow: A case report and review of the literature.
Ann Diagn Pathol. 10:357–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Romero-Guadarrama MB, Reyes-Posada O,
Hernández-González MM and Durán-Padilla MA: Follicular dendritic
cell sarcoma/tumor: 2 cases of a rare tumor of difficult clinical
and pathological diagnosis. Ann Diagn Pathol. 13:257–262. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chera BS, Orlando C, Villaret DB and
Mendenahall WM: Follicular dendritic cell sarcoma of the head and
neck: Case report and literature review. Laryngoscope.
118:1607–1612. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tisch M, Hengstermann F, Kraft K, von
Hinüber G and Maier H: Follicular dendritic cell sarcoma of the
tonsil: Report of a rare case. Ear Nose Throat J. 82:507–509.
2003.PubMed/NCBI
|
|
8
|
Hu T, Wang X, Yu C, Yan J, Zhang X, Li L,
Li X, Zhang L, Wu J, Ma W, et al: Follicular dendritic cell sarcoma
of the pharyngeal region. Oncol Lett. 5:1467–1476. 2013.PubMed/NCBI
|
|
9
|
Li Z, Jin K, Yu X, Teng X, Zhou H, Wang Y,
Teng L and Cao F: Extranodal follicular dendritic cell sarcoma in
mesentery: A case report. Oncol Lett. 2:649–652. 2011.PubMed/NCBI
|
|
10
|
Starr JS, Attia S, Joseph RW, Menke D,
Casler J and Smallridge RC: Follicular dendritic cell sarcoma
presenting as a thyroid mass. J Clin Oncol. 33:e74–e76. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perez-Ordoñez B and Rosai J: Follicular
dendritic cell tumor: Review of the entity. Semin Diagn Pathol.
15:144–154. 1998.PubMed/NCBI
|
|
12
|
Chan JK, Fletcher CD, Nayler SJ and Cooper
K: Follicular dendritic cell sarcoma. Clinicopathologic analysis of
17 cases suggesting a malignant potential higher than currently
recognized. Cancer. 79:294–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ge R, Liu C, Yin X, Chen J, Zhou X, Huang
C, Yu W and Shen X: Clinicopathologic characteristics of
inflammatory pseudotumor-like follicular dendritic cell sarcoma.
Int J Clin Exp Pathol. 7:2421–2429. 2014.PubMed/NCBI
|
|
14
|
Biddle DA, Ro JY, Yoon GS, Yong YW, Ayala
AG, Ordonez NG and Ro J: Extranodal follicular dendritic cell
sarcoma of the head and neck region: Three new cases, with a review
of the literature. Mod Pathol. 15:50–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soriano AO, Thompson MA, Admirand JH,
Fayad LE, Rodriguez AM, Romaguera JE, Hagemeister FB and Pro B:
Follicular dendritic cell sarcoma: A report of 14 cases and a
review of the literature. Am J Hematol. 82:725–728. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shia J, Chen W, Tang LH, Carlson DL, Qin
J, Guillem JG, Nobrega J, Wong WD and Klimstra DS: Extranodal
follicular dendritic cell sarcoma: Clinical, pathologic, and
histogenetic characteristics of an underrecognized disease entity.
Virchows Arch. 449:148–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Encabo RS, McHugh J, Carrau RL, Kassam A
and Heron D: Follicular dendritic cell sarcoma of the nasopharynx.
Am J Otolaryngol. 29:262–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clement P, Saint-Blancard P, Minvielle F,
Le Page P and Kossowski M: Follicular dendritic cell sarcoma of the
tonsil: A case report. Am J Otolaryngol. 27:207–210. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fonseca R, Yamakawa M, Nakamura S, van
Heerde P, Miettinen M, Shek TW, Myhre Jensen O, Rousselet MC and
Tefferi A: Follicular dendritic cell sarcoma and interdigitating
reticulum cell sarcoma: A review. Am J Hematol. 59:161–167. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakashima T, Kuratomi Y, Shiratsuchi H,
Yamamoto H, Yasumatsu R, Yamamoto T and Komiyama S: Follicular
dendritic cell sarcoma of the neck; a case report and literature
review. Auris Nasus Larynx. 29:401–403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Domínguez-Malagón H, Cano-Valdez AM,
Mosqueda-Taylor A and Hes O: Follicular dendritic cell sarcoma of
the pharyngeal region: Histologic, cytologic, immunohistochemical,
and ultrastructural study of three cases. Ann Diagn Pathol.
8:325–332. 2004. View Article : Google Scholar : PubMed/NCBI
|