Introduction
Osteosarcoma is the most common primary bone
malignancy in children and adolescents, and is characterized by a
highly malignant tendency to destroy the surrounding tissues and
metastasize almost exclusively to the lung, which is the primary
cause of mortality among patients (1,2). In young
patients, osteosarcoma is most often localized in the distal femur
and proximal tibia region (3).
Although osteosarcoma has been treated with neoadjuvant
chemotherapy in combination with surgery for more than three
decades, patients with recurrent or metastatic osteosarcoma have an
extremely poor prognosis, with a long-term survival rate of <10%
(4). To date, the molecular
mechanisms underlying the initiation, development and metastasis of
osteosarcoma are not fully elucidated, and it is essential to
identify novel therapeutic targets and develop therapeutic
strategies against osteosarcoma.
MicroRNAs (miRs) belong to a group of small
noncoding, single-stranded RNA fragments measuring 18–25
nucleotides in length, which are critical regulators in
tumorigenesis and cancer progression (5). Previously, miRs have been demonstrated
to suppress translation or directly cleave target mRNA through
complementary sequence pairing to the 3′-untranslated region (UTR)
or coding region of target mRNA (6).
These data indicate that miRs may be used as diagnostic biomarkers
and may function either as oncogenes or tumor suppressors based on
the effects of their target mRNAs (7,8). It has
been reported in osteosarcoma that multiple miRs, including miR-29,
miR-125b, miR-143 and miR-199a-3p, are involved in tumor growth,
progression and metastasis (9–12). A
previous study revealed that restoration of miR-506 in malignant
transformed human bronchial epithelial cells suppressed cell
proliferation (13). In addition,
overexpression of miR-506 inhibited transforming growth
factor-β-induced epithelial-mesenchymal transition and suppressed
the adhesion, invasion and migration of human breast cancer cells
(14). Furthermore, overexpression of
miR-506 in established hydroxycamptothecin-resistant colon cancer
cells conferred resistance to hydroxycamptothecin by inhibiting the
expression of peroxisome proliferator-activated receptor α
(15). Although miR-506 has been
subjected to extensive study in recent years, its role in the
initiation and progression of osteosarcoma, and the molecular
mechanisms by which miR-506 exerts its effects, are poorly
understood.
Astrocyte elevated gene-1 (AEG-1), also known as
lysine-rich carcinoembryonic antigen-related cell adhesion molecule
1 or metadherin, was initially characterized as a human
immunodeficiency virus-1- and tumor necrosis factor-α-inducible
gene in primary human fetal astrocytes (16,17).
Although AEG-1 is ubiquitously expressed in numerous cell types,
the expression level of AEG-1 is higher in certain solid tumors,
including breast and prostate cancer, malignant glioma,
hepatocellular carcinoma and melanoma, compared to normal
counterpart tissues (18). In
addition, patients with elevated AEG-1 levels have shorter overall
survival times compared with patients with lower AEG-1 levels
(19). Previously, certain studies
have demonstrated that AEG-1 is significantly associated with
chemoresistance and progression of osteosarcoma, and AEG-1 has been
suggested to act as a useful biomarker for the prediction of
osteosarcoma progression and prognosis (20–22).
Therefore, targeted downregulation of AEG-1 may be an effective
treatment strategy against osteosarcoma.
The aim of the present study was to investigate the
role of miR-506 in the pathogenesis of osteosarcoma. The present
results revealed that the expression of AEG-1 was significantly
increased, while the level of miR-506 was significantly decreased,
in human osteosarcoma tissues and cells. Overexpression of miR-506
and knockdown of AEG-1 attenuated proliferation and promoted
apoptosis of osteosarcoma in vitro. Furthermore, miR-506
overexpression was demonstrated to inhibit osteosarcoma cell growth
in vivo. Therefore, the present study provides evidence that
miR-506 suppresses osteosarcoma development by targeting AEG-1,
partly via regulating the Wnt/β-catenin signaling pathway.
Materials and methods
Clinical specimens and cell
culture
A total of 19 pairs of primary osteosarcoma tissues
and matched adjacent non-cancerous bone tissues were obtained from
the First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China) to identify the expression level of miR-506 using
quantitative polymerase chain reaction (qPCR). The characteristics
of the patients are listed in Table
I. All diagnoses were determined according to the criteria of
the World Health Organization (23).
Written informed consent was obtained from the patients. The study
was approved by the Local Research Ethics Committee of the First
Affiliated Hospital of Zhengzhou University (Zhengzhou, China).
 | Table I.Clinicopathological characteristics
of osteosarcoma patients |
Table I.
Clinicopathological characteristics
of osteosarcoma patients
|
Characteristics | Patients, n |
|---|
| Age, years |
|
|
≤18 | 16 |
|
>18 | 3 |
| Gender |
|
|
Male | 10 |
|
Female | 9 |
| Histology |
|
|
Osteoblastic | 13 |
|
Chondroblastic | 5 |
|
Other | 1 |
| Metastasis |
|
|
Yes | 13 |
| No | 6 |
|
Tumor-node-metastasis stages |
|
| I +
II | 7 |
| III +
IV | 12 |
Human normal osteoblastic hFOB 1.19 and human
osteosarcoma MG63 cell lines were obtained from the American Type
Culture Collection (Manassas, VA, USA) and were cultured in
Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine
serum and 1% penicillin/streptomycin (Invitrogen™; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified
atmosphere containing 5% CO2.
qPCR
Total RNA was extracted from frozen tissues and
osteosarcoma cells using TRIzol Reagent (Invitrogen™), according to
the manufacturer's protocol. In total, 1 µl DNase (Qiagen, Inc.,
Valencia, CA, USA) was used. The reverse transcriptase of RNA was
performed with the miScript II RT kit (Qiagen, Inc.), according to
the manufacturer's protocol. The expression level of miR-506 was
quantified by qPCR using TaqMan microRNA Assays (Applied
Biosystems™; Thermo Fisher Scientific, Inc.). Specific primer sets
were designed using Primer Premier version 5.0 software (PREMIER
Biosoft, Palo Alto, CA, USA) and synthesized by Sangon Biotech Co.,
Ltd. (Shanghai, China) as follows: miR-506, forward
5′-GACATGCATAAGGCACCCTTC-3′ and reverse 5′-GTGCAGGGTCCGAGGT-3′;
AEG-1, forward 5′-AAATAGCCAGCCTATCAAGACTC-3′ and reverse
5′-TTCAGACTTGGTCTGTGAAGGAG-3′; β-catenin, forward
5′-GCTGATTTGATGGAGTTGGA-3′ and reverse
5′-TCAGCTACTTGTTCTTGAGTGAA-3′; and β-actin, forward
5′-TGGACTTCGAGCAGGAAATGG-3′ and reverse
5′-ACGTCGCACTTCATGATCGAG-3′. qPCR was performed on a ABI 7900
Sequence Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using the following cycling conditions:
Denaturation, 95°C for 15 min, followed by 40 cycles of 94°C for 15
sec, 55°C for 30 sec and 72°C for 30 sec. The relative expression
of mRNA was calculated and normalized using the ∆Cq method
(24) relative to β-actin. Each test
was performed in triplicate.
Transfection
Control miR (miR-control;
5′-UGUGCGACGCGGCUGGAUGCG-3′), hsa-miR-506 mimic (miR-506 mimic;
5′-UAAGGCACCCUUCUGAGUAGA-3′), control anti-miR (anti-miR-control;
5′-CACUACGCAGAACCGGAAUAU-3′), anti-miR-506 mimic
(5′-UCUACUCAGAAGGGUGCCUUA-3′) and small interfering RNA (si)
targeting AEG-1 coding sequences (si-AEG-1; forward,
5′-GACACUGGAGAUGCUAAUAUU-3′ and reverse
5′-UAUUAGCAUCUCCAGUGUCUU-3′) were chemically synthesized by
Genepharma, Co., Ltd. (Shanghai, China). Cells (5×105
cells/well) were transfected with the miRs and si using
Lipofectamine® 2000 (Invitrogen™), according to the
manufacturer's protocol.
Western blot analysis
Cells were lysed using RIPA Lysis and Extraction
Buffer (Thermo Fisher Scientific, Inc.), and protein concentration
was measured using BCA Protein Assay kit (Pierce™; Thermo Fisher
Scientific, Inc.). Western blot analysis was conducted as described
previously (1). Briefly, following a
48 h transfection, the proteins were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and subsequently
transferred to nitrocellulose membranes (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The membranes were blocked with 10%
skimmed milk (Sigma-Aldrich, St. Louis, MO, USA) at room
temperature for 2 h and incubated overnight at 4°C with rabbit
polyclonal anti-AEG-1 (catalog no., 40-6500; 1:500; Invitrogen™),
mouse monoclonal anti-β-catenin (catalog no., 610154; 1:500; BD
Transduction Laboratories™; BD Biosciences, Franklin Lakes, NJ,
USA), rabbit polyclonal anti-c-myc (catalog no., sc-764; 1:200;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), mouse monoclonal
anti-cyclin D1 (catalog no., sc-450; 1:100; Santa Cruz
Biotechnology, Inc.), and rabbit polyclonal anti-β-actin (catalog
no., 4967; 1:2,000; Cell Signaling Technology, Inc., Danvers, MA,
USA) antibodies. After washing, the membranes were incubated for 2
h at room temperature with horseradish peroxidase-conjugated
secondary immunoglobulin G goat anti-mouse (catalog no, sc-2005;
1:10,000) or goat anti-rabbit (catalog no, sc-2004; 1:10,000)
antibodies (Santa Cruz Biotechnology, Inc.). The proteins were
visualized using ImageQuant LAS4000 (GE Healthcare Life Sciences,
Chalfont, UK).
MTT assay
For cell viability assays, MG63 cells were
transfected with miR-506 mimic or si-AEG-1. Following transfection
for 48 h, MG63 cells were seeded in a 96-well plate at a density of
4×103 cells per well. After incubation for 24, 48, 72
and 96 h at 37°C in a humidified atmosphere containing 5%
CO2, 10 µl MTT [5 mg/ml in phosphate-buffered saline
(PBS); Sigma-Aldrich] was added to each well and the plates were
incubated for a further 4 h. After removal of the medium, each cell
was treated with 150 µl dimethyl sulfoxide to dissolve the formazan
crystals. Optical density values were determined using a microplate
reader (Model 680 Microplate Reader; Bio-Rad Laboratories, Inc.) at
a wavelength of 490 nm.
MG63 cells transfected with si-β-catenin (sense,
5′-CAGUUGUGGUUAAGCUCUUdTdT-3′ and antisense,
3′-dTdTGUCAACACCAAUUCGAGAA-5′; Genepharma, Co., Ltd) or treated
with 0, 5 and 10 µM CGP049090 (Sigma-Aldrich) were seeded in a
96-well plate and incubated for 48 h at 37°C in a humidified
atmosphere containing 5% CO2. The subsequent MTT was as
aforementioned.
Colony formation assay
Subsequent to transfection for 48 h, MG63 cells were
seeded in a 6-well plate at a density of 500 cells per well and
cultured for 10 days. Colony formation was viewed by staining the
cells with 2% Giemsa solution (Merck Millipore, Darmstadt, Germany)
for 10 min following fixation with 10% methanol for 5 min.
Flow cytometry
Cell apoptosis was evaluated using Annexin
V/fluorescein isothiocyanate (FITC) Apoptosis Detection kit (BD
Biosciences) and propidum iodide (PI; Sigma-Aldrich), according to
the manufacturer's protocol. Briefly, cells were harvested using
0.25% trypsin 48 h after transfection or treatment with CGP049090,
washed twice with cold PBS, and re-suspended in binding buffer.
Subsequently, cells were incubated with 5 µl Annexin V/FITC and 5
µl PI for 15 min at room temperature in the dark. A flow cytometer
(BD Biosciences) was used to detect apoptosis in MG63 cells.
Luciferase reporter assays
MG63 cells were seeded in 24-well plates 24 h prior
to transfection. Subsequently, the cells were transiently
co-transfected with 0.3 µg wild type or mutant reporter plasmid
(Agilent Technologies, Santa Clara, CA, USA) and 50 nM miR-506
mimic or miR-control using Lipofectamine 2000. Firefly and Renilla
luciferase activities were measured 48 h subsequent to transfection
using the Dual Luciferase Assay (Promega, Madison, WI, USA),
according to the manufacturer's protocol. Firefly luciferase
activity was normalized to Renilla, and the value of firefly
luciferase activity/Renilla luciferase activity was analyzed. Three
independent experiments were performed in triplicate.
Tumor formation in nude mice
A total of 8, 4–6-week-old, male BALB/c nude mice
(nu/nu; 20–25 g) were obtained from Vital River Laboratories Co.,
Ltd. (Beijing, China). The animals were housed under specific
pathogen-free conditions and fed with chow and sterile water ad
libitum in a 12 h light/dark cycle at 23 ± 2°C. In total,
2×106 MG63 cells stably overexpressing miR-506 mimic or
miR-control were subcutaneously injected into 4 to 6-week-old nude
mice (n=8 per group). Tumors were measured with calipers to
estimate the tumor volume between day 7 and 28 following injection
according to the following formula: Tumor volume = 0.5 × length ×
width2. The mice were sacrificed a total of 28 days
subsequent to inoculation, and tumour weights were measured. All
animal procedures were performed with the approval of the Local
Medical Experimental Animal Care Commission of the First Affiliated
Hospital of Zhengzhou University.
Statistical analysis
All data are presented as the mean ± standard
deviation, and analyzed using SPSS version 19.0 software (IBM SPSS,
Armonk, NY, USA). The significance of the observed differences
between groups was calculated using Student's t-test or one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Level of miR-506 is inversely
associated with AEG-1 protein expression in osteosarcoma
AEG-1 is ubiquitously expressed in numerous cell
types and is overexpressed in certain solid tumors (25). To determine whether AEG-1 is
overexpressed in osteosarcoma, the expression level of AEG-1 in
human osteosarcoma tissues and osteosarcoma MG63 cell line was
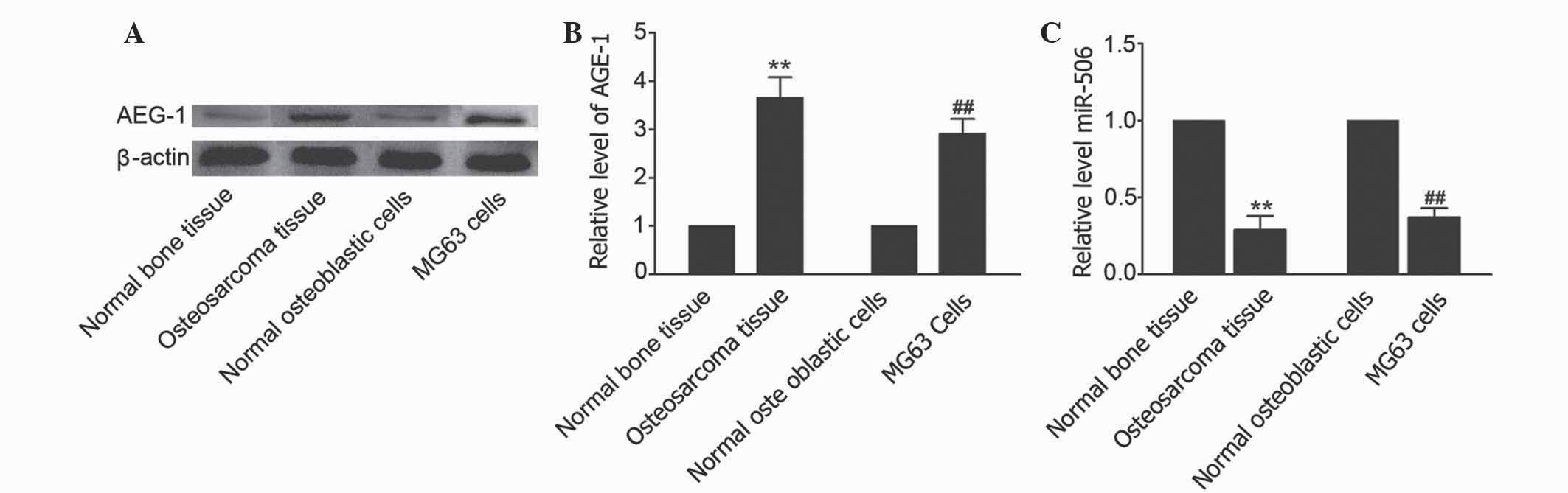
measured using western blot analysis. As shown in Fig. 1A and B, an elevated level of AEG-1 was
observed in osteosarcoma tissues compared with matched adjacent
non-cancerous tissues (P=0.0063). In addition, the level of AEG-1
was increased in MG63 cells compared with human normal osteoblastic
hFOB 1.19 cells. TargetScan (www.targetscan.org/vert_71/) revealed that miR-506 is
predicted to target the AEG-1-associated gene. To investigate the
effects of miR-506 on osteosarcoma, the level of miR-506 was
identified in osteosarcoma tissues and osteosarcoma MG63 cell line.
The results showed that the level of miR-506 in osteosarcoma
tissues and cells was decreased compared with matched adjacent
non-cancerous tissues and hFOB 1.19 cells, respectively (P=0.0090
and P=0.0086, respectively; Fig. 1C).
Therefore, the present study hypothesized that miR-506 may
participate in the regulation of osteosarcoma by targeting
AEG-1.
Upregulation of miR-506 suppresses
proliferation of osteosarcoma cells
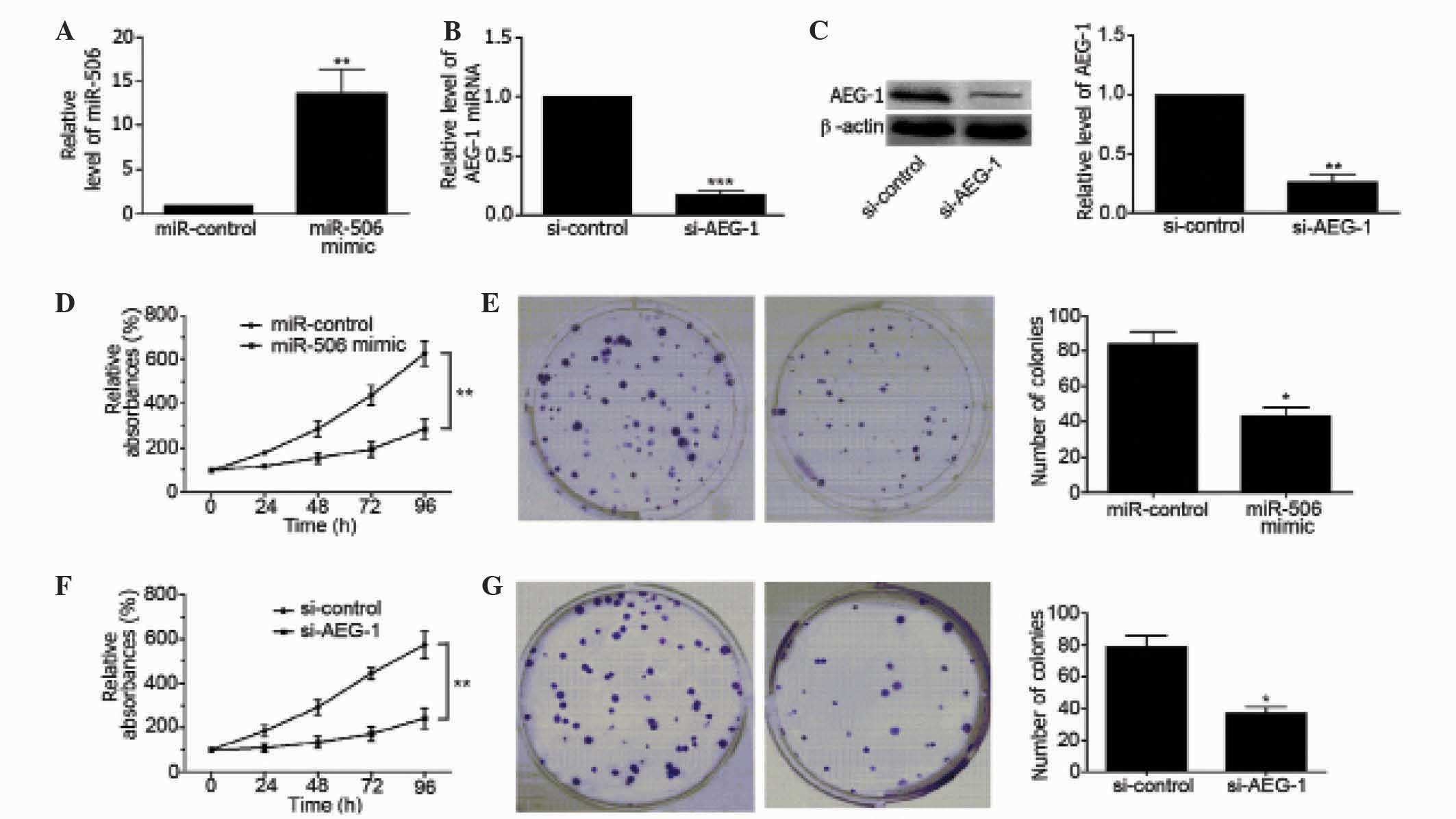
To clarify the regulatory effects of miR-506 on
osteosarcoma, MG63 cells were transfected with miR-506 mimics. As
shown in Fig. 2A, miR-506 was
overexpressed in MG63 cells (P=0.0094), as determined by qPCR. In
addition, the mRNA and protein level of AEG-1 was downregulated in
MG63 cells by transfection with si-AEG-1 (P=0.0003 and P=0.0013,
respectively; Fig. 2B and C).
Overexpression of miR-506 significantly decreased the viability of
MG63 cells compared with the miR-control-transfected group of cells
(P=0.0038; Fig. 2D) and inhibited the
colony forming ability of the cells (P=0.0157; Fig. 2E). Similarly, downregulation of AEG-1
inhibited the viability of MG63 cells (P=0.0024; Fig. 2F), and inhibited the colony forming
ability of the cells (P=0.0012; Fig.
2G). These findings suggest that miR-506 and AEG-1 are involved
in the regulation of MG63 cell proliferation.
Upregulation of miR-506 inhibits
apoptosis of osteosarcoma cells
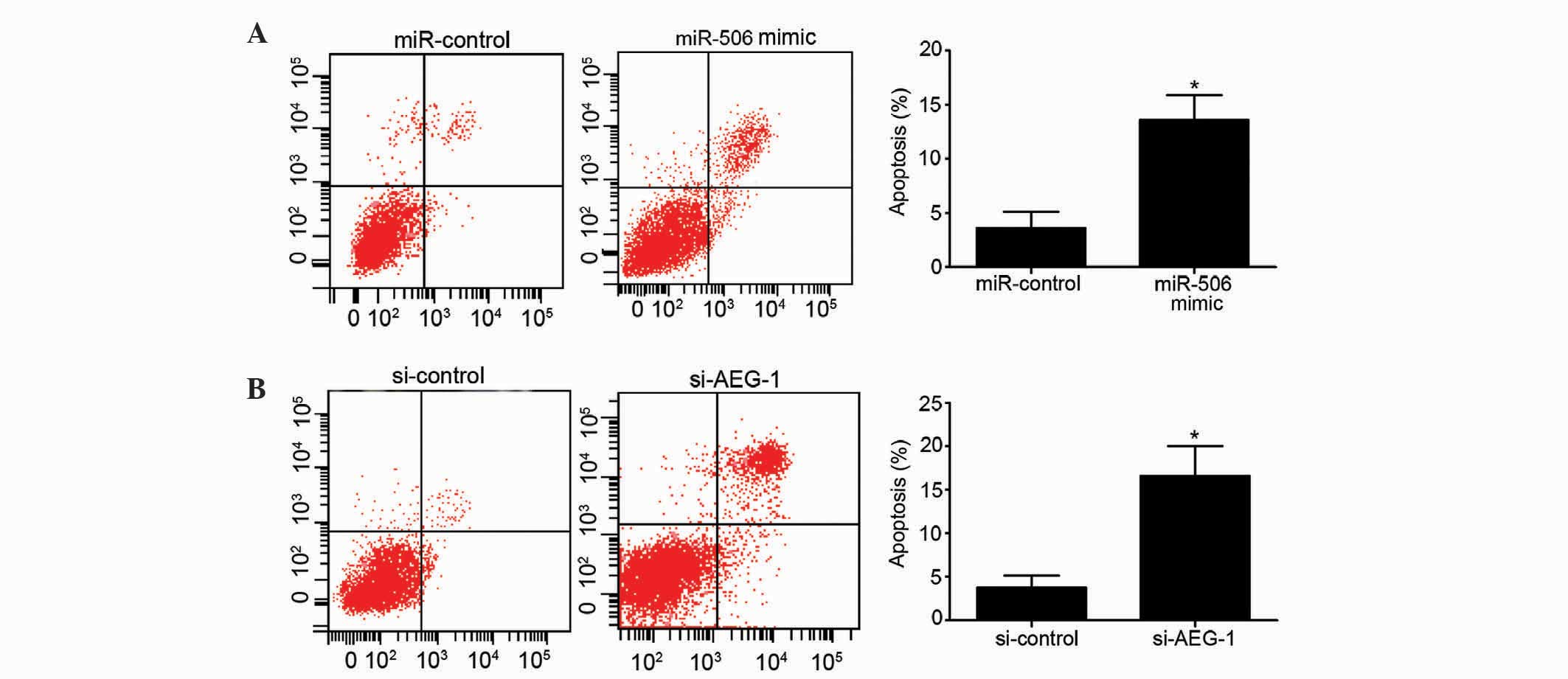
The present study additionally assessed the effects
of miR-506 and AEG-1 on the apoptosis of MG63 cells. Overexpression
of miR-506 significantly increased the apoptotic rate of MG63 cells
compared to the miR-control-transfected group (P=0.0265; Fig. 3A). Similarly, downregulation of AEG-1
induced a higher apoptotic rate of MG63 cells compared with the
si-control group (P=0.0137; Fig. 3B).
Overall, these results indicate that overexpression of miR-506 and
downregulation of AEG-1 have a clear ability to induce MG63 cell
apoptosis.
AEG-1 is directly targeted by miR-506
in MG63 cells
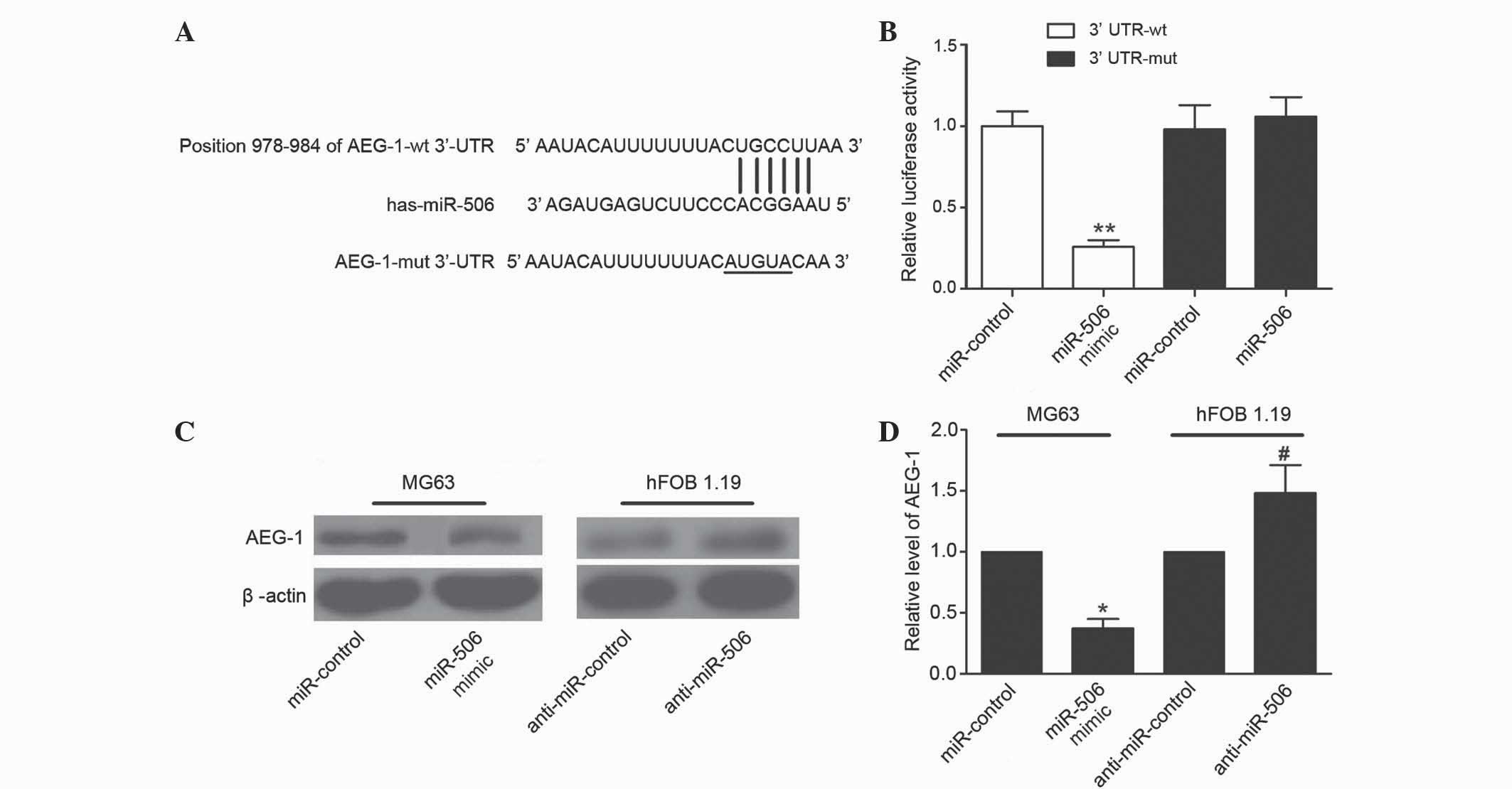
Bioinformatics analysis using TargetScan suggested
that miR-506 was a predicted to target AEG-1, and the ‘seed
sequence’ of miR-506 matched the 3′-UTR of the AEG-1 mRNA (Fig. 4A). To confirm AEG-1 was directly
targeted and regulated by miR-506 in MG63 cells, luciferase
reporter genes were constructed using the AEG-1 3′-UTR and the
mutant counterpart at the miR-506 binding regions, and these were
co-transfected with miR-506 mimics or miR-control into MG63 cells.
Overexpression of miR-506 significantly inhibited the luciferase
activity of AEG-1 with the wild-type 3′-UTR (P=0.0018), but not
with mutant 3′-UTR (Fig. 4B). To
further determine whether miR-506 could functionally affect the
expression of AEG-1, the present study determined if the expression
level of AEG-1 was regulated by miR-506. The results demonstrated
that overexpression of miR-506 suppressed the expression of AEG-1
in MG63 cells, and downregulation of miR-506 increased the level of
AEG-1 in hFOB 1.19 cells (P=0.0168 and P=0.0401, respectively;
Fig. 4C and D). Overall, these
results suggest that the 3′-UTR of AEG-1 is a functional target
site of miR-506 in osteosarcoma cells.
miR-506 inhibits osteosarcoma cell
growth in vivo
To investigate whether miR-506 has a role in a mouse
model of osteosarcoma, MG63 cells stably overexpressing miR-506 or
control-miR were injected subcutaneously into nude mice. The tumor
volume was measured every 7 days. A total of 28 days following
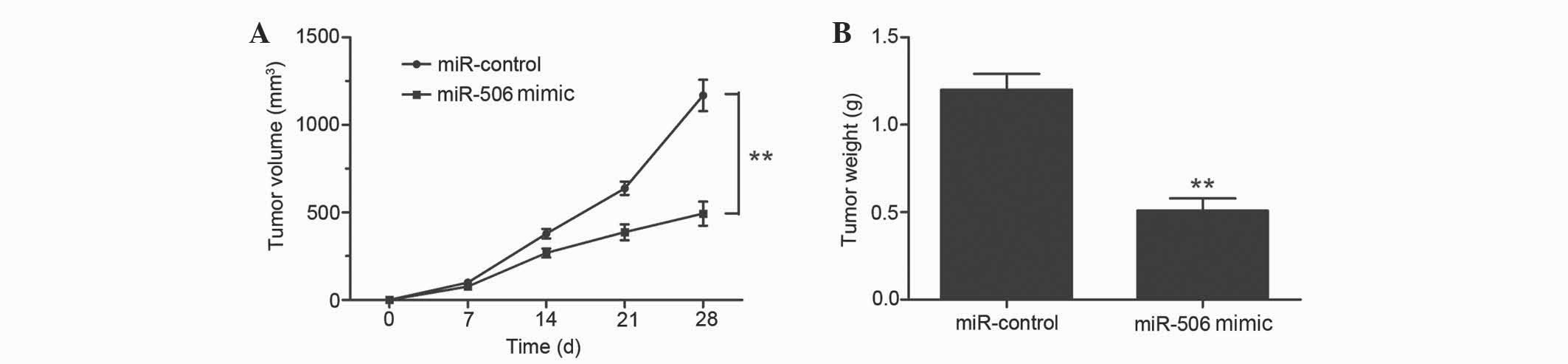
inoculation, mice were sacrificed and tumor weights were measured.
Overexpression of miR-506 significantly inhibited the tumor growth
of MG63 xenografts compared with the negative control group, since
the average volume and weight of the miR-506-overexpressing tumors
were notably decreased (P=0.0023 and P=0.0017, respectively;
Fig. 5A and B). Therefore, miR-506
clearly attenuates osteosarcoma cell growth in vivo.
miR-506 inhibits osteosarcoma
development via regulation of the Wnt/β-catenin signaling
pathway
The Wnt/β-catenin signaling pathway has been widely
implicated in the development of multiple tumors, including
osteosarcoma (26). The present study
detected the expression levels of β-catenin, c-myc, and cyclin D1
in MG63 cells to determine whether miR-506 inhibits osteosarcoma
via the Wnt/β-catenin pathway. As shown in Fig. 6A, upregulation of miR-506 and
downregulation of AEG-1 clearly decreased the expression levels of
β-catenin (P=0.0268 and P=0.0134, respectively), c-myc (P=0.0166
and 0.0129, respectively) and cyclin D1 (P=0.0288 and P=0.0260,
respectively) in MG63 cells. In addition, the Wnt/β-catenin
signaling pathway was inhibited by the present study using
si-β-catenin. The levels of β-catenin mRNA and protein were
significantly decreased in MG63 cells following transfection
(P=0.0011 and P=0.0103, respectively; Fig. 6B and C). Blocking of the Wnt/β-catenin
signaling pathway suppressed proliferation (P=0.0236) and induced
apoptosis (P=0.0046) of MG63 cells (Fig.
6D and E). Furthermore, CGP049090, a small molecule inhibitor
of Wnt/β-catenin, inhibited the expression of β-catenin (5 µM,
P=0.0227; 10 µM, P=0.0086), c-myc (5 µM, P=0.0213; 10 µM, P=0.0017)
and cyclin D1 (5 µM, P=0.0243; 10 µM, P=0.0033) in MG63 cells in a
concentration-dependent manner (Fig.
6F). CGP049090 clearly inhibited proliferation (5 µM, P=0.0373;
10 µM, P=0.0088) and induced apoptosis (P=0.0008) of MG63 cells
(Fig. 6G and H). These data
demonstrate that miR-506 suppresses osteosarcoma by regulation of
AEG-1 through inhibition of the Wnt/β-catenin signaling
pathway.
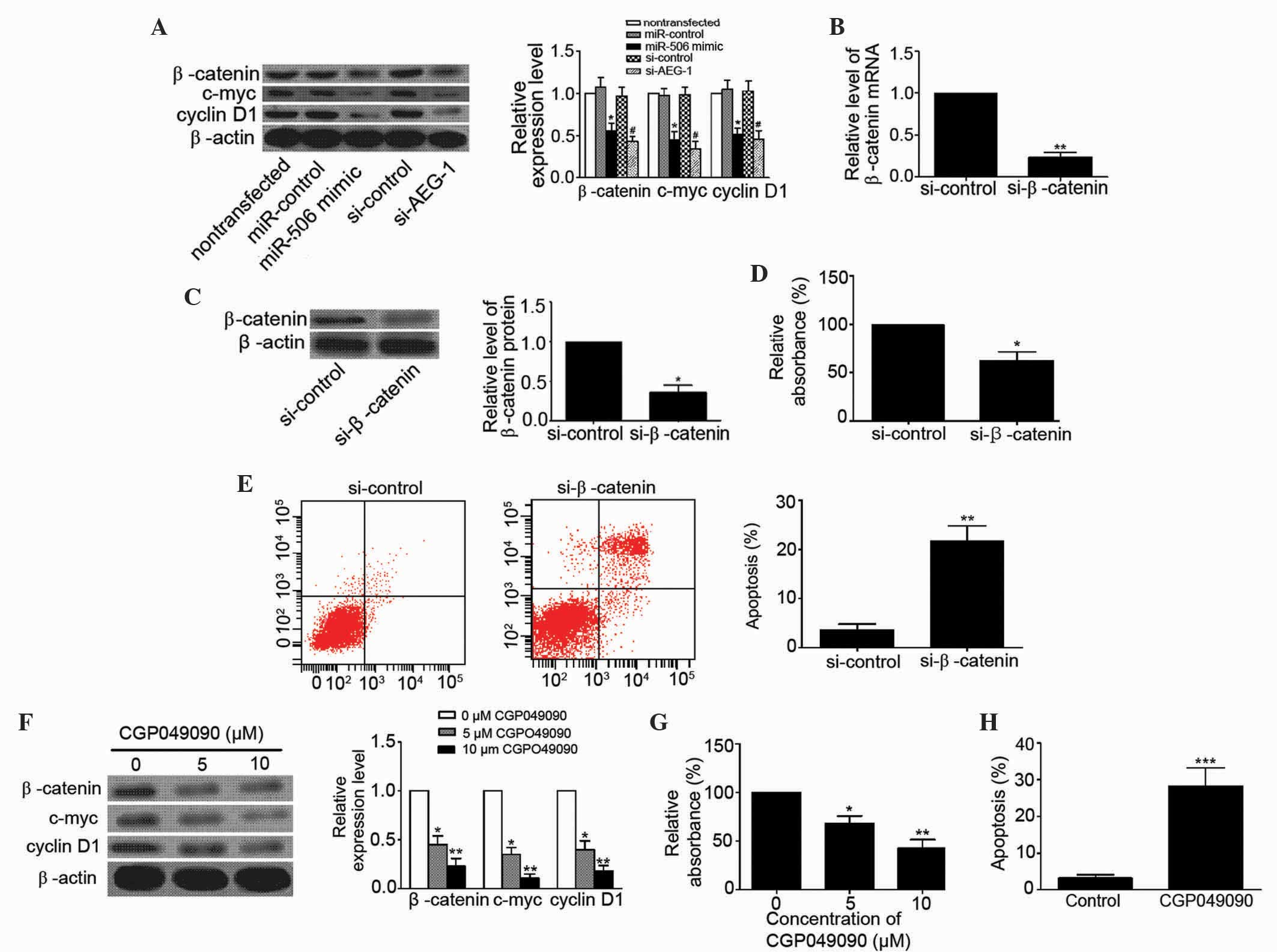 | Figure 6.miR-506 inhibited osteosarcoma by
targeting AEG-1 via the Wnt/β-catenin pathway signaling. (A)
Western blot analysis showed that overexpression of miR-506 and
knockdown of AEG-1 decreased the expression levels of β-catenin (94
kDa), c-myc (49 kDa) and cyclin D1 (34 kDa) in human osteosarcoma
MG63 cells. *P<0.05 vs. miR-control or nontransfected;
#P<0.05 vs. si-control. (B and C) Relative levels of
β-catenin (B) mRNA and (C) protein were significantly decreased in
MG63 cells transfected with si-β-catenin. *P<0.05, **P<0.01
vs. si-control. (D and E) Blocking the Wnt/β-catenin pathway using
si-β-catenin (D) inhibited MG63 cell proliferation and (E) induced
apoptosis of MG63 cells. *P<0.05, **P<0.01 vs. si-control.
(F-H) CGP049090, a small molecule inhibitor of Wnt/β-catenin, (F)
inhibited the expression of β-catenin, c-myc and cyclin D1 in MG63
cells in a concentration-dependent manner, (G) inhibited MG63 cell
proliferation in a concentration-dependent manner and (H) induced
apoptosis of MG63 cells (10µM CGP049090). *P<0.05, **P<0.01,
***P<0.001 vs. control (0 µM CGP049090). miR, microRNA; AEG-1,
astrocyte elevated gene-1; si, small interfering RNA. |
Discussion
To improve osteosarcoma therapy, novel therapeutic
targets require identification, and therapeutic strategies need to
be developed. Recently, the utilization of miRs has provided novel
insights into osteosarcoma therapy. Numerous studies have revealed
that miRs are critical as tumor suppressors or oncogenes in
osteosarcoma. However, their involvements in the underlying
molecular mechanisms remain to be further elucidated. The present
study revealed that the expression level of miR-506 was decreased
in osteosarcoma tissues and cells compared with matched adjacent
non-cancerous bone tissues and normal osteoblastic cells,
respectively. In addition, an overexpression of miR-506 was
revealed to suppress osteosarcoma cell proliferation and enhance
apoptosis in vitro, and inhibit tumor growth in vivo.
The present data suggest that miR-506 is associated with the
development and progression of osteosarcoma, and may be a promising
diagnostic biomarker and therapeutic target for osteosarcoma.
Recently, a study revealed that the miR-29 family are important in
the development and progression of human osteosarcoma; serum levels
of miR-29a and miR-29b were independent prognostic factors for
overall and disease-free survival (9). Other studies have revealed that
detection of serum miR-133b, miR-206, miR-148a, miR-196a and
miR-196b expression has clinical potential as novel diagnostic
biomarkers and are efficient predictors of prognosis in
osteosarcoma patients (27,28). Nevertheless, the carcinogenic
mechanisms of these miRs on osteosarcoma have not been fully
elucidate. Therefore, the present findings provide valuable
information and therapeutic benefits in osteosarcoma
development.
AEG-1 is known to be involved in multiple human
cancers (18). Elevated AEG-1
expression is linked to progression of cervical intraepithelial
neoplasia and poor prognosis in cervical cancer (29). Knockdown of AEG-1 induced prostate
cancer cell apoptosis via the activation of forkhead box 3a
(30). In the present study, to
investigate whether the loss function of AEG-1 is connected to the
development of osteosarcoma, the expression of AEG-1 was
downregulated in MG63 cells. The results indicated that knockdown
of AEG-1 inhibited cell proliferation, suppressed colony-forming
ability and induced apoptosis. In summary, the present data
demonstrates that AEG-1 is involved in mediating cell proliferation
and survival. Similarly, a previous study has revealed that AEG-1
regulates the migration and invasion of osteosarcoma U2OS cells
(22). Furthermore, the present study
confirmed that the 3′-UTR of AEG-1 is a functional target site for
miR-506 in MG63 cells. Studies in other types of cancer have
indicated that miR-506 has an antineoplastic function (14,31);
however, the role of miR-506 in tumor cells is not fully
understood. A previous study indicated that downregulation of
miR-506 in ovarian carcinoma facilitated an aggressive phenotype,
whereas overexpression of miR-506 in ovarian cancer cells inhibited
cell proliferation and promoted senescence by directly targeting
the cyclin-dependent kinase 4/6-forkhead box M1 axis (32). miR-506 also represents a novel class
of miR that regulates E-cadherin and vimentin/N-cadherin in the
suppression of epithelial-to-mesenchymal transition and metastasis,
and is associated with a good prognosis in epithelial ovarian
cancer (31). To the best of our
knowledge, the current results present the first evidence that
miR-506 may have therapeutic potential against osteosarcoma.
The canonical Wnt/β-catenin signaling pathway is one
of the fundamental mechanisms that regulates cell proliferation,
polarity and cell fate determination during embryonic development
and homeostatic self-renewal in multiple adult tissues (33). Therefore, mutations in this pathway
are often associated with cancer and other diseases. The
Wnt/β-catenin signaling pathway has been revealed to be excessively
activated in osteosarcoma and contributes to the development and
progression of osteosarcoma (34,35).
Dihydroartemisinin inhibits tumor growth of human osteosarcoma
cells by elevating the catalytic activity of glycogen synthase
kinase 3β (GSK3β), which results in lower protein level and
transcriptional activity of β-catenin (36). Overexpression of bone morphogenetic
protein 9 decreased the expression levels of β-catenin mRNA and
protein, downregulated its downstream proteins c-myc and
osteoprotegerin, and suppressed the phosphorylation level of GSK-3β
(Ser 9) in osteosarcoma cells (37).
In addition, a previous study revealed that celecoxib, a
cyclooxygenase (COX)-2 inhibitor, exerted an inhibitory effect on
the viability of MG63 cells in a time- and dose-dependent manner,
by inhibiting the expression of β-catenin and c-myc, and encoding
cyclin D1 (38). This suggests that
β-catenin is required for MG63 cell survival and the Wnt/β-catenin
pathway is a COX-2-independent target for non-steroidal
anti-inflammatory drugs in osteosarcoma. The present study revealed
that the Wnt/β-catenin signaling pathway was suppressed by
overexpression of miR-506 or downregulation of AEG-1. Additionally,
inhibition of the Wnt/β-catenin signaling pathway by si-β-catenin
or CGP049090 significantly attenuated the viability and evoked
apoptosis of MG63 cells in the present study. Therefore, the
present study suggests that AEG-1 promotes osteosarcoma development
by activating the Wnt/β-catenin pathway, and miR-506 downregulates
the expression of AEG-1, which inhibits the Wnt/β-catenin pathway
and provides therapeutic benefits in osteosarcoma.
To conclude, the present study has demonstrated that
overexpression of miR-506 suppresses proliferation and induces
apoptosis of osteosarcoma cells by targeting AEG-1. In addition,
the present study revealed that miR-506 exhibits antineoplastic
abilities by regulating the Wnt/β-catenin pathway. These findings
provide novel insights for miRs in osteosarcoma.
References
|
1
|
Zhu J, Feng Y, Ke Z, Yang Z, Zhou J, Huang
X and Wang L: Down-regulation of miR-183 promotes migration and
invasion of osteosarcoma by targeting Ezrin. Am J Pathol.
180:2440–2451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R,
Vernon P, Cao L and Tang D: HMGB1 promotes drug resistance in
osteosarcoma. Cancer Res. 72:230–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang J, Liu K, Yu Y, Xie M, Kang R,
Vernon P, Cao L, Tang D and Ni J: Targeting HMGB1-mediated
autophagy as a novel therapeutic strategy for osteosarcoma.
Autophagy. 8:275–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang E, Kim L, Choi JM, Park SE, Rhee EJ,
Lee WY, Oh KW, Park SW, Park DI and Park CY: Ezetimibe stimulates
intestinal glucagon-like peptide 1 secretion via the MEK/ERK
pathway rather than dipeptidyl peptidase 4 inhibition. Metabolism.
64:633–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu M, Lang N, Qiu M, Xu F, Li Q, Tang Q,
Chen J, Chen X, Zhang S, Liu Z, et al: miR-137 targets Cdc42
expression, induces cell cycle G1 arrest and inhibits invasion in
colorectal cancer cells. Int J Cancer. 128:1269–1279. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen L, Wang X, Wang H, Li Y, Yan W, Han
L, Zhang K, Zhang J, Wang Y, Feng Y, et al: miR-137 is frequently
down-regulated in glioblastoma and is a negative regulator of
Cox-2. Eur J Cancer. 48:3104–3111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Croce C: Introduction to the role of
microRNAs in cancer diagnosis, prognosis and treatment. Cancer J.
18:213–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang C, Yao C, Li H, Wang G and He X:
Combined elevation of microRNA-196a and microRNA-196b in sera
predicts unfavorable prognosis in patients with osteosarcomas. Int
J Mol Sci. 15:6544–6555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hong Q, Fang J, Pang Y and Zheng J:
Prognostic value of the microRNA-29 family in patients with primary
osteosarcomas. Med Oncol. 31:372014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu LH, Li H, Li JP, Zhong H, Zhang HC,
Chen J and Xiao T: miR-125b suppresses the proliferation and
migration of osteosarcoma cells through down-regulation of STAT3.
Biochem Bioph Res Commun. 416:31–38. 2011. View Article : Google Scholar
|
|
11
|
Osaki M, Takeshita F, Sugimoto Y, Kosaka
N, Yamamoto Y, Yoshioka Y, Kobayashi E, Yamada T, Kawai A, Inoue T,
et al: MicroRNA-143 regulates human osteosarcoma metastasis by
regulating matrix metalloprotease-13 expression. Mol Ther.
19:1123–1130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duan Z, Choy E, Harmon D, Liu X, Susa M,
Mankin H and Hornicek F: MicroRNA-199a-3p is downregulated in human
osteosarcoma and regulates cell proliferation and migration. Mol
Cancer Ther. 10:1337–1345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Y, Liu H, Li Y, Wu J, Greenlee AR,
Yang C and Jiang Y: The role of miR-506 in transformed 16HBE cells
induced by anti-benzo[a]pyrene-trans-7, 8-dihydrodiol-9,
10-epoxide. Toxicol Lett. 205:320–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arora H, Qureshi R and Park WY: miR-506
regulates epithelial mesenchymal transition in breast cancer cell
lines. PloS One. 8:e642732013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tong JL, Zhang CP, Nie F, Xu XT, Zhu MM,
Xiao SD and Ran ZH: MicroRNA 506 regulates expression of PPAR alpha
in hydroxycamptothecin-resistant human colon cancer cells. FEBS
Lett. 585:3560–3568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He W, He S, Wang Z, Shen H, Fang W, Zhang
Y, Qian W, Lin M, Yuan J, Wang J, et al: Astrocyte elevated gene-1
(AEG-1) induces epithelial-mesenchymal transition in lung cancer
through activating Wnt/β-catenin signaling. BMC Cancer. 15:1072015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Emdad L, Sarkar D, Su ZZ, Randolph A,
Boukerche H, Valerie K and Fisher PB: Activation of the nuclear
factor kappaB pathway by astrocyte elevated gene-1: Implications
for tumor progression and metastasis. Cancer Res. 66:1509–1516.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoo BK, Emdad L, Su ZZ, Villanueva A,
Chiang DY, Mukhopadhyay ND, Mills AS, Waxman S, Fisher RA, Llovet
JM, et al: Astrocyte elevated gene-1 regulates hepatocellular
carcinoma development and progression. J Clin Invest. 119:465–477.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu C, Chen K, Zheng H, Guo X, Jia W, Li M,
Zeng M, Li J and Song L: Overexpression of astrocyte elevated
gene-1 (AEG-1) is associated with esophageal squamous cell
carcinoma (ESCC) progression and pathogenesis. Carcinogenesis.
30:894–901. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu B, Wu Y and Peng D: Astrocyte elevated
gene-1 regulates osteosarcoma cell invasion and chemoresistance via
endothelin-1/endothelin A receptor signaling. Oncol Lett.
5:505–510. 2013.PubMed/NCBI
|
|
21
|
Wang F, Ke Z-F, Sun SJ, Chen WF, Yang SC,
Li SH, Mao XP and Wang LT: Oncogenic roles of astrocyte elevated
gene-1 (AEG-1) in osteosarcoma progression and prognosis. Cancer
Biol Ther. 12:539–548. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang F, Ke ZF, Wang R, Wang YF, Huang LL
and Wang LT: Astrocyte elevated gene-1 (AEG-1) promotes
osteosarcoma cell invasion through the JNK/c-Jun/MMP-2 pathway.
Biochem Bioph Res Commun. 452:933–939. 2014. View Article : Google Scholar
|
|
23
|
Schajowicz F: World Health Organization:
Histological Typing of Bone Tumours (2nd). Springer-Verlag. Berlin:
1993.
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu H, Song X, Liu C, Xie L, Wei L and Sun
R: Knockdown of astrocyte elevated gene-1 inhibits proliferation
and enhancing chemo-sensitivity to cisplatin or doxorubicin in
neuroblastoma cells. J Exp Clin Cancer Res. 28:192009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guan H, Tan P, Xie L, Mi B, Fang Z, Li J,
Yue J, Liao H and Li F: FOXO1 inhibits osteosarcoma oncogenesis via
Wnt/β-catenin pathway suppression. Oncogenesis. 4:e1662015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang C, Yao C, Li H, Wang G and He X:
Serum levels of microRNA-133b and microRNA-206 expression predict
prognosis in patients with osteosarcoma. Int J Clin Exp Pathol.
7:4194–4203. 2014.PubMed/NCBI
|
|
28
|
Ma W, Zhang X, Chai J, Chen P, Ren P and
Gong M: Circulating miR-148a is a significant diagnostic and
prognostic biomarker for patients with osteosarcoma. Tumor Biol.
35:12467–12472. 2014. View Article : Google Scholar
|
|
29
|
Huang K, Li LA, Meng Y, You Y, Fu X and
Song L: High expression of astrocyte elevated gene-1 (AEG-1) is
associated with progression of cervical intraepithelial neoplasia
and unfavorable prognosis in cervical cancer. World J Surg Oncol.
11:2972013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kikuno N, Shiina H, Urakami S, Kawamoto K,
Hirata H, Tanaka Y, Place RF, Pookot D, Majid S, Igawa M and Dahiya
R: Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer
progression through upregulation of FOXO3a activity. Oncogene.
26:7647–7655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Y, Hu L, Zheng H, Bagnoli M, Guo Y,
Rupaimoole R, Rodriguez-Aguayo C, Lopez-Berestein G, Ji P, Chen K,
et al: MiR-506 inhibits multiple targets in the
epithelial-to-mesenchymal transition network and is associated with
good prognosis in epithelial ovarian cancer. J Pathol. 235:25–36.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu G, Sun Y, Ji P, Li X, Cogdell D, Yang
D, Parker Kerrigan BC, Shmulevich I, Chen K, Sood AK, et al:
MiR-506 suppresses proliferation and induces senescence by directly
targeting the CDK4/6-FOXM1 axis in ovarian cancer. J Pathol.
233:308–318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma Y, Ren Y, Han EQ, Li H, Chen D, Jacobs
JJ, Gitelis S, O'Keefe RJ, Konttinen YT, Yin G and Li TF:
Inhibition of the Wnt-β-catenin and Notch signaling pathways
sensitizes osteosarcoma cells to chemotherapy. Biochem Bioph Res
Commun. 431:274–279. 2013. View Article : Google Scholar
|
|
35
|
Hoang BH, Kubo T, Healey JH, Yang R,
Nathan SS, Kolb EA, Mazza B, Meyers PA and Gorlick R: Dickkopf 3
inhibits invasion and motility of Saos-2 osteosarcoma cells by
modulating the Wnt-beta-catenin pathway. Cancer Res. 64:2734–2739.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Y, Wang W, Xu J, Li L, Dong Q, Shi Q,
Zuo G, Zhou L, Weng Y, Tang M, et al: Dihydroartemisinin inhibits
tumor growth of human osteosarcoma cells by suppressing
Wnt/β-catenin signaling. Oncol Rep. 30:1723–1730. 2013.PubMed/NCBI
|
|
37
|
Lv Z, Wang C, Yuan T, Liu Y, Song T, Liu
Y, Chen C, Yang M, Tang Z, Shi Q and Weng Y: Bone morphogenetic
protein 9 regulates tumor growth of osteosarcoma cells through the
Wnt/β-catenin pathway. Oncol Rep. 31:989–994. 2014.PubMed/NCBI
|
|
38
|
Xia JJ, Pei LB, Zhuang JP, Ji Y, Xu GP,
Zhang ZP, Li N and Yan JL: Celecoxib inhibits β-catenin-dependent
survival of the human osteosarcoma MG-63 cell line. J Int Med Res.
38:1294–1304. 2010. View Article : Google Scholar : PubMed/NCBI
|