Introduction
Ovarian cancer is the third most prevalent
malignancy of the female reproductive system (1,2). Of all
the types of ovarian cancer, epithelial ovarian cancer has the
poorest prognosis and is the primary cause of cancer-associated
mortality in adult women (1,2). Therefore, understanding the mechanisms
that underlie the development and progression of ovarian cancer is
the focus of numerous, intensive studies. Dysregulation of multiple
signaling pathways has been implicated in the initiation,
progression and metastasis of ovarian cancer, including the
mitogen-activated protein kinase and phosphoinositide
3-kinase/AKT/mammalian target of rapamycin signaling pathways
(2).
The Hippo pathway is an emerging signaling pathway
that is crucial for tissue homeostasis, organ size control, cell
differentiation and the development of various types of human
cancer, including ovarian cancer (3,4). The core
components of the mammalian Hippo pathway consist of the upstream
kinases Mst1/2, large tumor suppressor kinase 1 (LATS1/2) and their
respective scaffold proteins, WW45 and MOB1. Activation of the
Hippo tumor suppressor pathway increases the phosphorylation level
of the transcription coactivator yes-associated protein 1
(YAP)/transcriptional coactivator with PDZ binding motif (TAZ),
which results in the cytoplasmic retention of YAP/TAZ and protein
degradation. As YAP/TAZ promotes cell proliferation and survival
through the activation of downstream transcription factors, most
notably the TEA domain (TEAD) transcription factor family members,
activation of the Hippo pathway results in inhibition of
TEAD-dependent transcription. Dysregulation of the Hippo pathway
has been observed in human cancer, including ovarian cancer
(5–9).
The nuclear expression of YAP has been identified to promote
ovarian cancer tumorigenesis and functions as a poor prognostic
indicator for the disease (7).
However, TAZ, a paralog of YAP in mammalian cells, has not yet been
investigated in ovarian cancer.
The aim of the present study was to investigate the
dysregulation and biological function of TAZ in ovarian cancer. The
study identified that TAZ mRNA and protein are overexpressed in
ovarian cancer, and a meta-analysis of an ovarian cancer database
indicated that high TAZ mRNA expression correlated with poor
prognosis in patients with ovarian cancer. In addition,
TAZ-knockdown resulted in decreased proliferation and migration of
ovarian cancer cells, and verteporfin, a compound that disrupts the
interaction between YAP/TAZ and TEAD, decreased the viability of
the ovarian cancer cells and almost abolished cell migration. Taken
together, the results of the present study indicate that the
overexpression of TAZ, a potential mechanism for the activation of
YAP/TAZ downstream gene expression, may promote ovarian cancer
tumorigenesis and progression, and may serve as a potential
therapeutic drug target.
Materials and methods
Ovarian cancer specimen
collection
All human ovarian cancer and paired normal samples
(n=7) were obtained from the Department of Gynecology, Songgang
People's Hospital, (Shenzhen, China) between December 2011 and
February 2012. The cancer tissues and paired normal tissues were
resected and fast frozen in liquid nitrogen, and then the samples
were transferred into cryogenic tubes and stored at −80°C for
long-term storage. Informed consent was obtained from all patients
and approval was obtained from the Ethics Committee of Songgang
Hospital (Shenzhen, China) for the use of all specimens.
Cell culture and transfection
SKOV-3 ovarian cancer cells were obtained from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in Dulbecco's modified Eagle's medium (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS; Sigma-Aldrich, St. Louis, MO, USA) and 1%
penicillin/streptomycin at 37°C with 5% CO2. Small
interfering RNA (siRNA) transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. The TAZ siRNA primer sequences were as
follows: TAZ-siRNA-1, GACAUGAGAUCCAUCACUA; and TAZ-siRNA-2,
GGACAAACACCCAUGAACA (10). TAZ siRNA
and non-targeting control siRNA (UUCUCCGAACGUGUCACGU). were
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed into cDNA with a PrimeScript® RT-PCR kit
(Takara Biotechnology Co., Ltd., Dalian, China). qPCR was performed
using SYBR® Premix Ex Taq™ (Takara Biotechnology Co.,
Ltd.) on an ABI 7500 Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The primers used were as follows:
Forward, ATTCATCGCCTTCCTAGGGT and reverse, GGCTGGGAGATGACCTTCAC for
TAZ; forward, GTCATCCAACGGGAATGCA and reverse,
TGATCGGTTACCGTGATCAAAA for GAPDH. The cycling conditions were as
follows: 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec
and 60°C for 34 sec. ddH20 was used as a negative
control. The 2−ΔΔCq method was used for quantification
(11).
Western blot analysis
Cancer tissues and cultured SKOV-3 cells were
harvested and lysed with 1% NP-40 lysis buffer [50 mM Tris-HCl, pH
7.8; 150 mM NaCl; 1% NP-40 with protease inhibitor cocktail (P8340;
Sigma-Aldrich); phenylmethylsulfonyl fluoride; 50 mM NaF; and 1 mM
Na3VO4]. Protein (50 µg per sample) was
separated by 10% SDS-PAGE and transferred to a nitrocellulose
membrane. The membrane was blocked with 5% milk and incubated at
4°C overnight with the following primary antibodies: Monoclonal
rabbit anti-YAP/TAZ (#8418), monoclonal rabbit anti-zonula
occludens-1 (ZO-1; #8193) (Cell Signaling Technology, Inc.,
Danvers, MA, USA), monoclonal rabbit anti-vimentin (#2707-1;
Epitomics, Burlingame, CA, USA), monoclonal mouse E-cadherin
(#610181; BD Transduction Laboratories; BD Biosciences, Franklin
Lakes, NJ, USA), monoclonal mouse N-cadherin (#610920; BD
Transduction Laboratories; BD Biosciences) and monoclonal
mouse-β-actin (#A1978; Sigma-Aldrich). All antibodies used in this
study were diluted at a 1:1,000 ratio unless otherwise stated.
Subsequently, the membrane was incubated with secondary antibodies
horeradish peroxidase (HRP)-labeled goat anti-rabbit immunoglobulin
(Ig)G(H+L) (#A0208; Beyotime Institute of Biotechnology, Haimen,
China) and HRP-labeled goat anti-mouse IgG(H+L) (#A0216; Beyotime
Institute of Biotechnology), and the proteins were visualized using
enhanced chemiluminescent reagents (EMD Millipore, Billerica, MA,
USA).
Migration assay
Transwell systems (24-well insert; Corning
Incorporated, Corning, NY, USA) were used to analyze cell migratory
ability. siRNA-transfected SKOV-3 cells were suspended in media
containing 1% FBS, seeded at a density of 1.0×105
cells/well in the upper chamber and incubated at 37°C with 5%
CO2. Cells on the upper membrane surface were removed 12
or 24 h later using a cotton bud, whilst cells on the lower
membrane were fixed in 4% formaldehyde, stained with crystal violet
and counted under a phase-contrast microscope.
Cell proliferation and viability
assay
siRNA-transfected SKOV-3 cells were seeded in a
96-well plate (2,000 cells/well), and the number of cells was
measured daily using a Cell Counting Kit-8 (CCK-8) assay (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) for 5 days. For the
verteporfin (#SML0534; Sigma-Aldrich) treatment experiments, 6,000
SKOV-3 cells were seeded per well and treated with the indicated
concentrations of verteporfin (0.1, 0.3, 1.0, 3.0 and 9.0 µM) for 1
day, incubated at 37°C with 5% CO2. Cell viability was
measured by CCK-8 assay (Dojindo Molecular Technologies, Inc.),
according to the manufacturer's protocol.
Statistical analysis
Kaplan-Meier plots of the overall and
progression-free survival time of patients with ovarian cancer
stratified by TAZ mRNA expression level were constructed using
KMplot (http://kmplot.com/analysis). Survival
data were analyzed using the log-rank test; all other data were
analyzed using Student's t-test. Results are presented as the mean
± standard deviation. Statistical analysis was performed using SPSS
19.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference. For the cell
proliferation and Transwell assays, each experiment was repeated
twice. Typical results of one experiment are shown. For the cell
proliferation assay, standard deviation was calculated based on the
use of 6 repeated wells per group. For the Transwell assay,
standard deviation was calculated based on 3 repeated Transwells
per group.
Results
TAZ expression is upregulated in
ovarian cancer
TAZ is a paralog of YAP in mammalian cells (3,4) and
dysregulation of YAP has been reported in ovarian cancer; however,
the function of TAZ in ovarian cancer has not yet been
investigated. To understand the dysregulation of TAZ in ovarian
cancer, the expression of TAZ mRNA was analyzed in 7 ovarian cancer
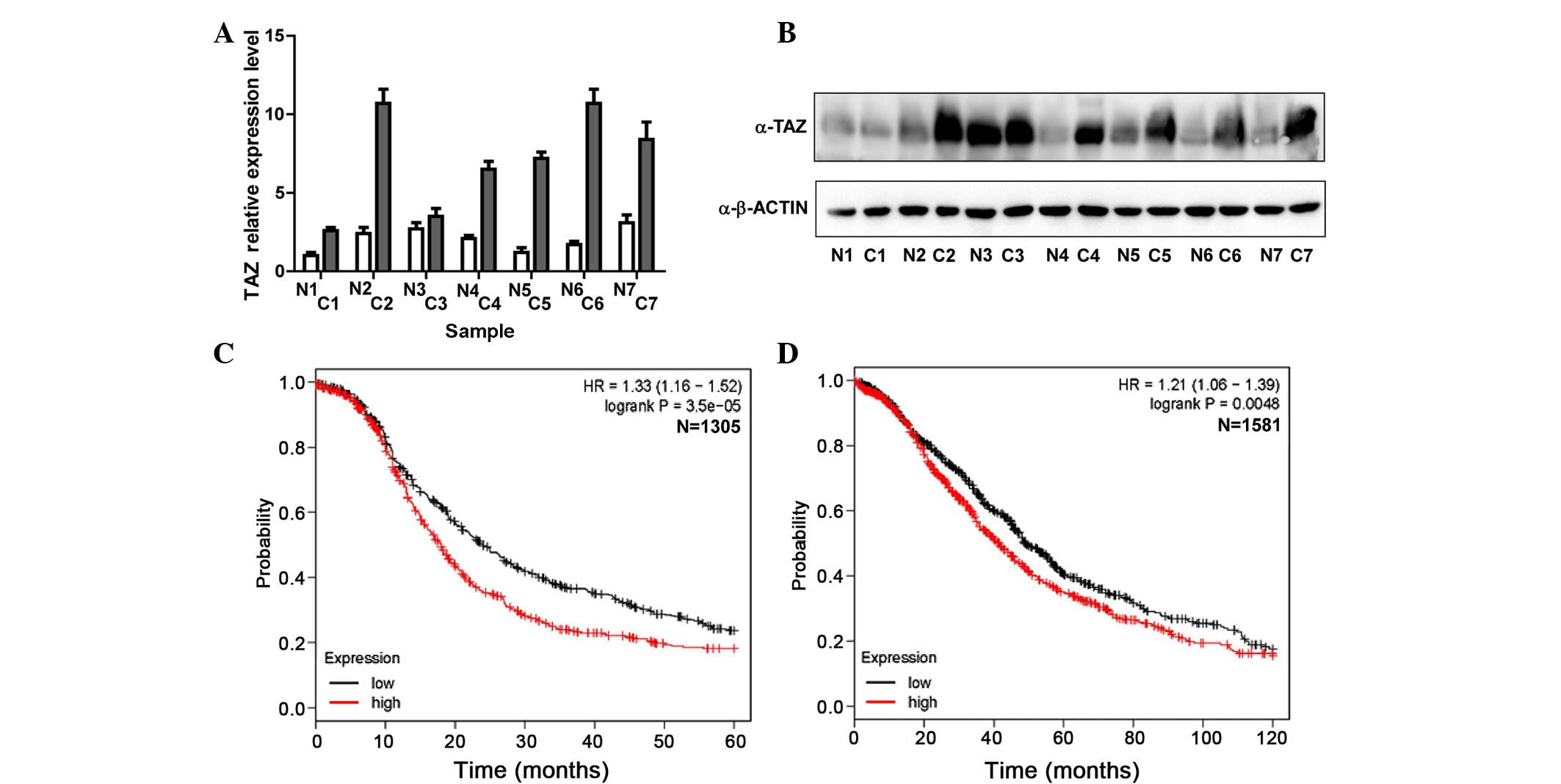
and paired normal tissue samples. Notably, as presented in Fig. 1A, TAZ mRNA was upregulated in 6/7
ovarian cancer samples compared with the paired normal tissues.
Western blot analysis of these 7 paired cancer and normal tissues
also showed that TAZ protein was overexpressed in 5/7 ovarian
cancer tissues (Fig. 1B). These
results suggest that TAZ mRNA and protein expression are
upregulated in ovarian cancer. To investigate the association
between upregulated TAZ expression and the prognosis of patients
with ovarian cancer, the present study analyzed a public online
database (12) integrating 13 public
datasets with 1,305 cases with progression-free data and 1,581
cases with overall survival data, and observed that high expression
of TAZ mRNA was a significant indicator of poor prognosis in
ovarian cancer. Patients with a high expression level of TAZ mRNA
had a shorter period of progression-free (P=0.000035; Fig. 1C) and overall survival (P=0.0048;
Fig. 1D).
TAZ regulates cell proliferation,
migration and epithelial-mesenchymal transition (EMT) in ovarian
cancer cells
To investigate the function of TAZ in ovarian cancer
cells, two siRNAs targeting human TAZ were transfected into SKOV-3
cells and cell proliferation was analyzed following transfection.
Transfecting each TAZ siRNA either individually or in combination
resulted in a significant decrease in the proliferation of the
SKOV-3 cells (P=0.00004, siRNA-TAZ-1+2 vs. siRNA-CON; Fig. 2A). Next, the current study performed
Transwell assays to determine whether TAZ-knockdown affects the
migratory ability of ovarian cancer cells. As presented in Fig. 2B, knockdown of TAZ in the SKOV-3 cells
largely decreased their migratory ability compared with control
cells (P=0.000089). Overexpression of TAZ in breast cancer promotes
EMT (13,14), therefore, the protein expression
levels of several EMT markers were also examined in the
TAZ-knockdown cancer cells. Although no changes were observed in
canonical EMT markers, including E-cadherin and N-cadherin (data
not shown), a moderate increase in the expression level of
epithelial marker ZO-1 and a decrease in the level of mesenchymal
marker vimentin were identified in TAZ-knockdown versus control
cells (Fig. 2C). These results
indicate that mesenchymal-epithelial transition was induced by
TAZ-knockdown in the SKOV-3 cells. Taken together, the data
suggests that TAZ serves a vital role in promoting proliferation,
migration and EMT in ovarian cancer cells.
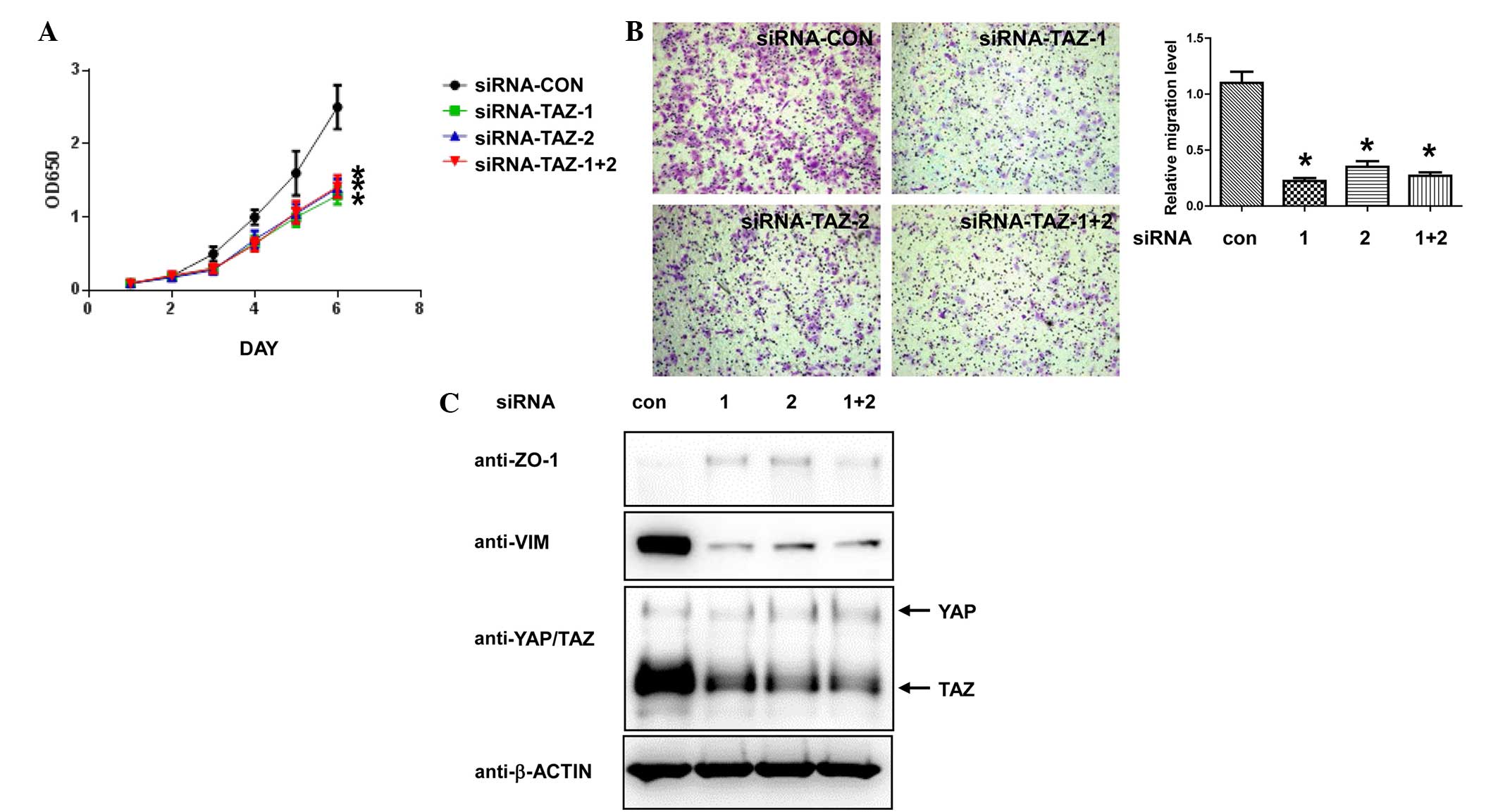 | Figure 2.TAZ-knockdown inhibits cell
proliferation and migration, and induces epithelial-mesenchymal
transition in ovarian cancer cells. SKOV-3 cells were transfected
with siRNA targeting human TAZ and control siRNA. (A) At 48 h
post-transfection, the cells were plated into 96-well plates and
the cell numbers were detected by Cell Counting kit-8 assay. (B)
Migratory ability of the SKOV-3 cells, as detected by Transwell
assay. Representative images (×100 magnification) of each group are
shown. Crystal violet staining. *P<0.05 vs. con, Student's
t-test. (C) Western blot analysis of SKOV-3 cells transfected with
indicated siRNA 72 h post-transfection. OD, optical density; siRNA,
small interfering RNA; CON, control; TAZ, tafazzin; ZO, zonula
occludens; VIM, vimentin; YAP, yes-associated protein 1. |
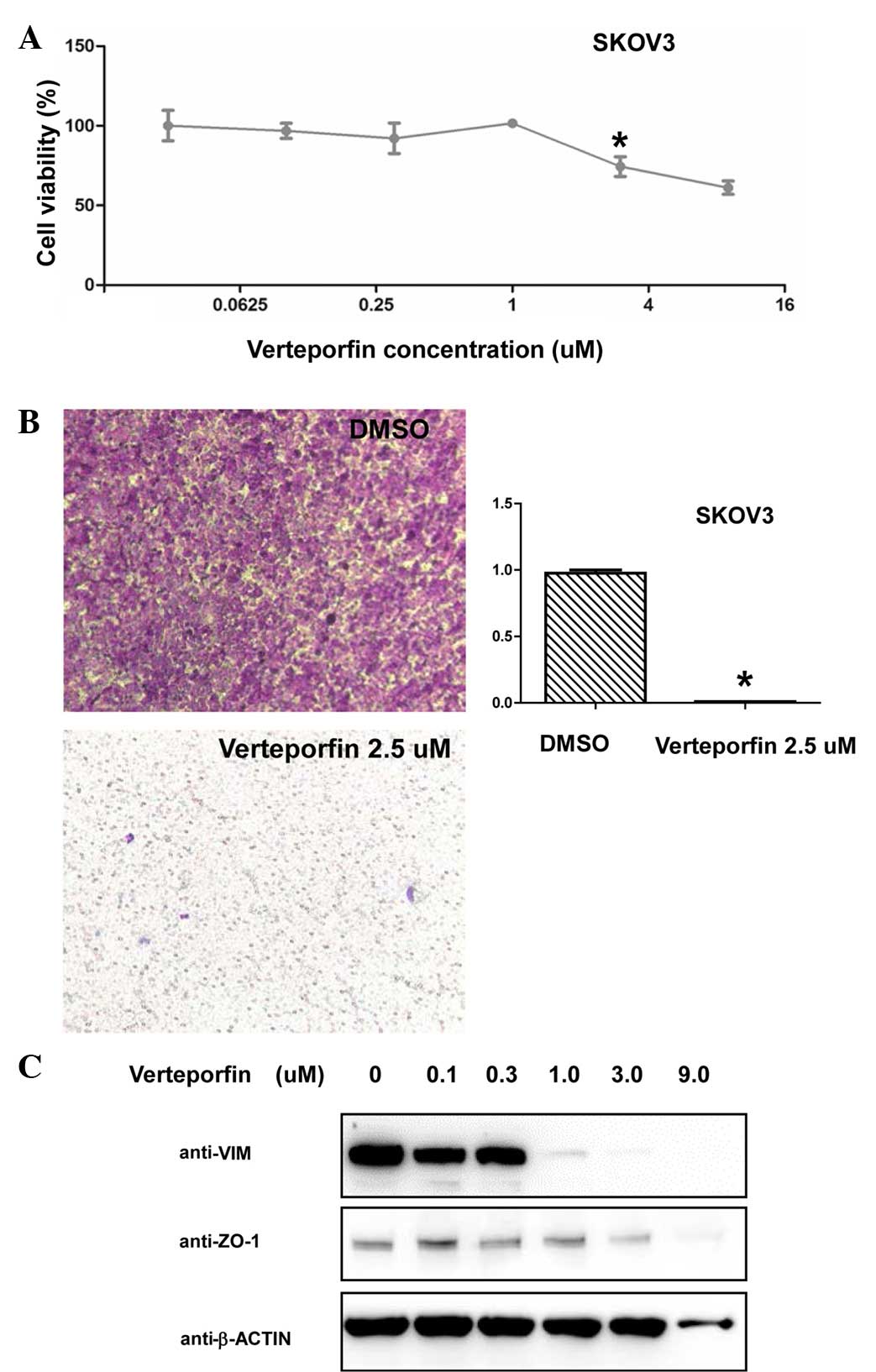
Pharmacological disruption of the
interaction between YAP/TAZ and TEAD decreases cell viability and
migration, and induces EMT in ovarian cancer cells
YAP/TAZ have been demonstrated to promote the
tumorigenesis and progression of multiple types of cancer primarily
through TEAD family members (14,15),
therefore, disrupting the interaction between YAP/TAZ and TEAD may
serve as a promising target for new drugs. Verteporfin, a drug used
previously for the treatment of macular degeneration, was
identified to disrupt the YAP/TEAD complex (16,17). The
present study observed that verteporfin treatment results in
decreased SKOV-3 cell viability (P=0.0014, 3 vs. 0 µM) Fig. 3A). Furthermore, verteporfin treatment
significantly decreased and nearly abolished the migratory ability
of the SKOV-3 cells (P=0.0000043; Fig.
3B). Similar to the effect of TAZ-knockdown on the SKOV-3
cells, the current study observed markedly decreased vimentin
expression levels in the SKOV-3 cells following verteporfin
treatment (Fig. 3C). These results
indicate that disruption of the YAP/TAZ/TEAD complex mimics the
effect of TAZ-knockdown in SKOV-3 ovarian cancer cells.
Discussion
Dysregulation of the Hippo tumor suppressor pathway
has been observed in multiple types of human cancer. Inactivation
of upstream tumor suppressors (including hypermethylation of Mst1
or decreased expression of LATS1/2) or activation of downstream
oncogenes YAP/TAZ result in enhanced cell proliferation, inhibition
of cell apoptosis and promotion of metastasis (3,4). In
ovarian cancer, nuclear expression of YAP, indicative of YAP
activation, was identified to correlate with poor prognosis
(7). Furthermore, the overexpression
of Drosophila YkiS168A or human
YAPS127A, a constitutively active Yki/YAP mutant, was
reported to induce tumorigenesis in the Drosophila ovary
(5). Such data indicates the vital
role of YAP/TAZ activation in ovarian cancer.
TAZ, first identified as a 14-3-3 binding protein,
shares 50% of its amino acid sequence with YAP in mammalian cells
(18). Although the biochemical
regulation of YAP/TAZ by the Hippo pathway is similar, the
functions of YAP/TAZ are different in certain aspects. For example,
in mice, Taz knockout leads to the development of polycystic kidney
disease and emphysema (19), while
Yap knockout results in embryonic lethality (20). The transcriptional regulation of YAP
and TAZ also differ from one another. Gender determining region Y
box 2 (21) and GA binding protein
(22) have been reported to regulate
YAP transcription; however, no transcription factor has yet been
identified to regulate the transcription of TAZ. Although the TAZ
gene locus amplification has been identified in 10% of ovarian
cancer samples in The Cancer Genome Atlas datasets (23), further efforts are warranted to
determine whether additional transcription factors are implicated
in the overexpression of TAZ in ovarian cancer. In addition,
despite similarities between the biochemical regulatory mechanisms
and primary downstream target genes of YAP and TAZ, it was reported
that YAP and TAZ also regulate different downstream target genes
(14), which may result in the
proteins exerting distinct functions in ovarian cancer development
and progression. The identification of more TAZ downstream target
genes in ovarian cancer may elucidate novel functions of the
protein in the development of this disease.
Overexpression of TAZ and activation of YAP have
been observed in ovarian cancer, therefore, TAZ/YAP may function as
a potential drug target for the treatment of ovarian cancer.
Furthermore, disruption of the YAP/TAZ/TEAD complex has been
reported to inhibit YAP/TAZ-induced tumorigenesis in liver cancer
models (16). The present study
demonstrated that verteporfin treatment induced a similar phenotype
to that observed following TAZ-knockdown in the SKOV-3 cells,
further indicating that disruption of the YAP/TAZ/TEAD complex may
function as a therapeutic target in patients with ovarian
cancer.
In conclusion, the results of the current study
indicate that overexpression of TAZ at the mRNA and protein level
promotes the tumorigenesis and progression of ovarian cancer, and
may, therefore, serve as a potential therapeutic drug target for
this disease.
Acknowledgements
The present study was supported by the Key
Specialized Research Funds of Songgang People's Hospital (Shenzhen,
China).
References
|
1
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cho KR and Shih IeM: Ovarian cancer. Annu
Rev Pathol. 4:287–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Plouffe SW, Hong AW and Guan KL: Disease
implications of the Hippo/YAP pathway. Trends Mol Med. 21:212–222.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hall CA, Wang R, Miao J, Oliva E, Shen X,
Wheeler T, Hilsenbeck SG, Orsulic S and Goode S: Hippo pathway
effector Yap is an ovarian cancer oncogene. Cancer Res.
70:8517–8525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang X, George J, Deb S, Degoutin JL,
Takano EA, Fox SB, Bowtell DD and Harvey KF: AOCS Study group: The
Hippo pathway transcriptional co-activator, YAP, is an ovarian
cancer oncogene. Oncogene. 30:2810–2822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia Y, Chang T, Wang Y, Liu Y, Li W, Li M
and Fan HY: YAP promotes ovarian cancer cell tumorigenesis and is
indicative of a poor prognosis for ovarian cancer patients. PLoS
One. 9:e917702014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia Y, Zhang YL, Yu C, Chang T and Fan HY:
YAP/TEAD co-activator regulated pluripotency and chemoresistance in
ovarian cancer initiated cells. PLoS One. 9:e1095752014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He C, Lv X, Hua G, Lele SM, Remmenga S,
Dong J, Davis JS and Wang C: YAP forms autocrine loops with the
ERBB pathway to regulate ovarian cancer initiation and progression.
Oncogene. 34:6040–6054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calvo F, Ege N, Grande-Garcia A, Hooper S,
Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary
E, Charras G and Sahai E: Mechanotransduction and YAP-dependent
matrix remodelling is required for the generation and maintenance
of cancer-associated fibroblasts. Nat Cell Biol. 15:637–646. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gyorffy B, Lánczky A and Szállási Z:
Implementing an online tool for genome-wide validation of
survival-associated biomarkers in ovarian-cancer using microarray
data from 1287 patients. Endocr Relat Cancer. 19:197–208. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lei QY, Zhang H, Zhao B, Zha ZY, Bai F,
Pei XH, Zhao S, Xiong Y and Guan KL: TAZ promotes cell
proliferation and epithelial-mesenchymal transition and is
inhibited by the hippo pathway. Mol Cell Biol. 28:2426–2436. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Liu CY, Zha ZY, Zhao B, Yao J,
Zhao S, Xiong Y, Lei QY and Guan KL: TEAD transcription factors
mediate the function of TAZ in cell growth and
epithelial-mesenchymal transition. J Biol Chem. 284:13355–13362.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu
J, Lin JD, Wang CY, Chinnaiyan AM, et al: TEAD mediates
YAP-dependent gene induction and growth control. Genes Dev.
22:1962–1971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brodowska K, Al-Moujahed A, Marmalidou A,
Meyer Zu Horste M, Cichy J, Miller JW, Gragoudas E and Vavvas DG:
The clinically used photosensitizer Verteporfin (VP) inhibits
YAP-TEAD and human retinoblastoma cell growth in vitro without
light activation. Exp Eye Res. 124:67–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu-Chittenden Y, Huang B, Shim JS, Chen
Q, Lee SJ, Anders RA, Liu JO and Pan D: Genetic and pharmacological
disruption of the TEAD-YAP complex suppresses the oncogenic
activity of YAP. Genes Dev. 26:1300–1305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanai F, Marignani PA, Sarbassova D, Yagi
R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC
and Yaffe MB: TAZ: A novel transcriptional co-activator regulated
by interactions with 14-3-3 and PDZ domain proteins. EMBO J.
19:6778–6791. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morin-Kensicki EM, Boone BN, Howell M,
Stonebraker JR, Teed J, Alb JG, Magnuson TR, O'Neal W and Milgram
SL: Defects in yolk sac vasculogenesis, chorioallantoic fusion and
embryonic axis elongation in mice with targeted disruption of
Yap65. Mol Cell Biol. 26:77–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hossain Z, Ali SM, Ko HL, Xu J, Ng CP, Guo
K, Qi Z, Ponniah S, Hong W and Hunziker W: Glomerulocystic kidney
disease in mice with a targeted inactivation of Wwtr1. Proc Natl
Acad Sci USA. 104:1631–1636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seo E, Basu-Roy U, Gunaratne PH, Coarfa C,
Lim DS, Basilico C and Mansukhani A: SOX2 regulates YAP1 to
maintain stemness and determine cell fate in the osteo-adipo
lineage. Cell Reports. 3:2075–2087. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu H, Xiao Y, Zhang S, Ji S, Wei L, Fan F,
Geng J, Tian J, Sun X, Qin F, et al: The Ets transcription factor
GABP is a component of the hippo pathway essential for growth and
antioxidant defense. Cell Reports. 3:1663–1677. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bell D, Berchuck A, Birrer M, Chien J,
Cramer DW, Dao F, Dhir R, DiSaia P, Gabra H, Glenn P, et al: Cancer
Genome Atlas Research Network: Integrated genomic analyses of
ovarian carcinoma. Nature. 474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|