Introduction
Epilepsy encompasses a number of different syndromes
whose cardinal feature is a predisposition to recurrent,
spontaneous seizure activity (1,2). Although
the specific mechanisms of these different syndromes may vary, at
the cellular, neurophysiological level, a common theme is a loss of
the normal balance between excitation and inhibition. As described
by Scharfman (3) using temporal lobe
epilepsy as an example, genes, developmental mechanisms and
neuronal plasticity all serve functions in creating a state of
hyperexcitability. Altered neuronal plasticity is central to the
underlying pathophysiology of epilepsy.
Among the deficits associated with epilepsy are
cognitive problems, particularly those related to memory impairment
(4,5).
In status epilepticus (SE), which is defined as ≥30 min of
continuous epileptic seizure activity, hippocampal-specific memory
is a common and serious deficit. Liu et al (6) reported an association between abnormal
place cells in the hippocampus and spatial memory in rats in which
SE had been established. Other studies using rat epilepsy models
have also demonstrated impaired spatial memory associated with
neuronal remodeling in the hippocampus (7,8).
With regard to neuronal plasticity, remodeling and
cognition, the activities of neural cell adhesion molecules (NCAMs)
and extracellular signal-regulated kinases (ERKs) appear to be
interconnected (9–11). NCAMs are cell surface glycoproteins
that mediate the recognition and adhesion of neural cells and
contribute to neurogenesis and synaptic plasticity (12,13). NCAM
expression is altered in the hippocampus of epileptic rats
(14,15).
ERKs, including ERK1 and ERK2, also known as
mitogen-activated protein kinases (MAPKs), are essential mediators
of signal transduction pathways between cell surface receptors and
the nucleus (16). ERKs are also
involved in neurogenesis and synaptic plasticity (17). As with NCAMs, studies on rodent models
of epilepsy have revealed altered ERK expression and activity in
the hippocampus (16,18,19). In
addition, during neurogenesis, NCAM expression is dependent on ERK
signaling (20). Thus, the expression
and activity of NCAM and ERK are interdependent under normal and
neuropathological conditions. However, their complex interactions
are not fully understood.
From a clinical perspective, one issue associated
with epileptogenesis and impaired cognition is how best to treat
these disorders. Although the number of pharmaceutical agents
available to treat these disorders has increased in recent decades
(21), there remain significant
problems associated with their use due to differences in their
clinical efficacy and, in particular, unwanted side effects
(22). New generation drugs may be
less toxic or better tolerated by patients with epilepsy; however,
they may also exacerbate associated conditions, particularly
cognitive impairments, for example, topiramate may cause cognitive
impairment and have effects on attention, semantic functioning and
language (23,24). Furthermore, little is known regarding
the effects of these drugs on the underlying molecular pathways
that are associated with epileptogenesis and impaired
cognition.
The current preliminary investigation sought to
determine the effects that various classes of pharmacological
agents may have on NCAM1 and ERK2 expression at the messenger RNA
(mRNA) and protein levels in the hippocampal tissues of SE rats
with spatial memory impairment. For this purpose, a
pilocarpine-induced model of SE in rats was utilized, as this
reflects a number of the neurophysiological and cognitive
alterations observed in human epilepsy (25,26).
Changes in spatial memory were evaluated using a Morris Water Maze
(MWM), and the effects of four commonly used drugs were assessed:
Two anticonvulsants (carbamazepine and oxcarbazepine); and two
drugs used to treat cognitive impairments, aniracetam (a nootropic)
and donepezil (an acetylcholinesterase inhibitor). The results
indicate that these anticonvulsants may aggravate impaired spatial
memory, whereas the cognition enhancers may improve spatial memory,
and these differential pharmacological effects on memory task
performance are associated with the differential effects on
NCAM1/ERK2 expression in the hippocampus.
Materials and methods
Animals and experimental groups
A total of 120 healthy, adult male Wistar rats (6
weeks of age; body weight, 180–200 g) were provided by Shandong
Lukang Record Pharmaceutical Co., Ltd. (Jining, China). Rats were
exposed to a 12 h light/12 h dark cycle and raised in separate
cages in a conventional environment with free access to food and
water. Rats were randomly divided into a control group (n=20) and
an experimental group (n=100) in which SE was established (model
described in subsequent paragraph). The experimental group was
randomly divided into five groups (n=20/group) as follows: i) an
epileptic (no pharmacological treatment) group; and groups treated
with ii) carbamazepine (Beijing Nuohua Pharmaceutical Co., Ltd.,
Beijing, China), iii) oxcarbazepine (Sihuan Pharmaceutical Holdings
Group Ltd., Beijing, China), iv) aniracetam (Shanghai
Pharmaceuticals Holding Co., Ltd., Shanghai, China), or v)
donepezil (Eisai China, Inc., Shanghai, China) (treatments
described below). This study was approved by the Ethics Committee
of Jining Medical College (Jining, China).
Epilepsy model and pharmacological
treatments
Established methods for generating SE in rats were
used (25,26). First, lithium chloride (Sigma-Aldrich,
St. Louis, MO, USA) was diluted in sterile saline and administered
to each rat by intraperitoneal (i.p.) injection at a dose of 127
mg/kg. After 18–24 h, methyl scopolamine (1 mg/kg in sterile
saline; MedChem Express, Princeton, NJ, USA) was administered by
i.p. injection to dampen cholinergic responses in the periphery.
After 30 min, pilocarpine (Beijing Zhongshan Jinqiao Biotechnology
Co., Ltd., Beijing, China) at 10 mg/kg in sterile saline was
administered by i.p. injection. After a further 20 min, seizure
activity was monitored with a video camera (DS126311; Canon Inc.,
Tokyo, Japan). If no seizure activity of Racine grades IV–V
appeared within 30 min (grades defined below), pilocarpine
administration was continued at 30-min intervals until SE was
established for ≥30 min. Diazepam (Beijing Shuanghe Pharmaceutical
Co., Ltd., Beijing, China) at 10 ml/kg was used to terminate the
attack following 4-h SE.
After the SE model was successfully established in
each group of mice (except for the untreated controls), the
experimental drugs or saline (for untreated SE rats; ‘epileptic
rats’) were administered. The experimental drugs were dissolved in
saline and administered by oral gavage within a 24-h period each
day. Pharmaceutical drugs or saline were given for a period of one
month after the SE model was established. Carbamazepine and
oxcarbazepine were administered at 200 mg/kg; aniracetam was
administered at 10 mg/kg; and donepezil was administered at 1
mg/kg; all drug dosages were selected based on the manufacturer's
protocol. The non-pharmacologically treated group was administered
saline at 10 ml/kg.
Behavioral observations
Seizure grading
Based on Racine's grading system (27), seizures were classified into one of
five grades: I, mouth and facial movements (wet-doggish chattering,
convulsive tics, chewing); II, rhythmic head nodding; III, forelimb
clonus (spasm in one forelimb); IV, rearing with forelimb clonus
(spasms in bilateral forelimbs, one limb standing); or V, rearing
and falling with forelimb clonus (generalized motor convulsions,
trunk imbalance, falling, myoclonus). When seizures appeared
consecutively with no normal activities (grades IV–V), this was
considered SE and indicated that the model was successfully
induced. After SE had been established and pharmacological
intervention was completed, spatial orientation and memory tests
were conducted using an MWM (28).
Task setting
The MWM was a round pool (diameter, 1.2 m) divided
into four quadrants. The water temperature within the pool was
maintained at 20–22°C. For certain tests, a circular platform
(diameter, 12 cm) was fixed in place in one quadrant with its
surface at 1 cm above the water level. Before tests were conducted,
each rat was allowed to train by swimming freely for two 2-min
periods, one in the morning and one in the afternoon.
Spatial navigation learning
An origin was set at a fixed point in one of the
four quadrants, and the platform was the end point. Each rat was
placed into the pool facing the pool wall. The time taken for the
rat to swim from the origin to the end point was recorded as the
escape (latency) period. Each experiment allowed a maximum latency
period of 120 sec; if a rat did not locate the platform after 120
sec, it was guided to the platform to rest for 30 sec and the
latency period was recorded as 120 sec. For all groups, each rat
entered the water one time from each of the four quadrants, and the
mean latency time was recorded each day. Rats were tested for five
consecutive days at a fixed time each day.
Probe trials for spatial memory
For these trials, the platform was removed. A rat
was placed in the platform quadrant and allowed to swim. Over a 120
sec period, the amount of time spent in each quadrant, including
the ‘platform quadrant’ was recorded. These results were expressed
as the percentage (%) of time spent in each quadrant.
Data acquisition
The behaviors of the rats in the MWM were
videotaped, and data was processing using a MWM real-time
processing system that was devised in-house.
Brain specimen processing
After MWM testing was completed, rats were
anesthetized with 10% chloral hydrate (200 mg/kg; Shanghai
Pharmaceuticals Holding Co., Ltd.) by i.p. injection. The brains
were processed in two ways for each group of rats. For one
sub-group, following anesthesia, the chest was opened to expose the
heart. A syringe needle was inserted into the left ventricle, the
right atrial appendage was cut, and saline (4°C) was rapidly
infused until there was an outflow of clear liquid from the right
atrial appendage. Subsequently, 4% paraformaldehyde (Beijing
Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) was
perfused at 10 ml/min until the limbs were rigid, confirming
sacrifice. Following removal of the brain, the hippocampus was
identified and removed, and then fixed in 4% formaldehyde for 24 h.
The tissue was dehydrated with an ethanol series and xylene, and
embedded in paraffin, before serial sections were cut at 40-µm
thickness (left and right sides). These specimens were used for
immunostaining, as described subsequently. For the other sub-group,
rats were anesthetized and decapitated, and the skull was rapidly
cut open. The hippocampus was removed, rapidly frozen in liquid
nitrogen, and stored at −20°C. These specimens were used for the
reverse transcription-polymerase chain reaction (RT-PCR) analysis
as described.
RT-PCR analysis of NCAM1 and ERK2 mRNA expression
in rat hippocampus
NCAM1 and ERK2 mRNA levels were detected by RT-PCR
using the frozen stored hippocampal tissue from five rats in each
group. Total RNA from 0.5–1 g of tissue was extracted using RNeasy
Mini Kits (Qiagen, Beijing, China) according to the manufacturer's
instructions. cDNA was synthesized using a reverse transcription
kit (Invitrogen; Thermo Fisher Scientific, Beijing, China)
according to the manufacturer's instructions. The cDNA product was
used for RT-PCR. Primers were designed by Sangon Biotech Co., Ltd.
(Shanghai, China), and the sequences were as follows: ERK2 forward,
5′-CTTGAAGACACAGCACCTCAG-3′, and reverse,
5′-CTTTGGAGTCAGCGTTTGGG-3′; NCAM1 forward,
5′-CAAAAATGACGAAGCCGAAT-3′, and reverse,
5′-GTGGACGTTCTCCAGGTGAT-3′; β-actin (internal reference) forward,
5′-GCCATGTACGTAGCCATCCA-3′, and reverse,
5′-GAACCGCTCATTGCCGATAG-3′.
PCR amplification was performed using a
GeneAmp® PCR System 9700 (Applied Biosystems; Thermo
Fisher Scientific). RT-PCR reaction mixtures (25 µl total) included
0.5 µl of cDNA, 0.5 µl of each primer (forward and reverse), 11 µl
of ddH2O, and 12.5 µl of SYBR Green Supermix (Takara
Bio, Inc., Otsu, Japan). PCR amplification conditions were an
initial denaturation at 94°C for 3 min, followed by cycles of 94°C
for 50 sec, 56°C for 50 sec, and 72°C for 50 sec (33 cycles for
NCAM1; 32 cycles for ERK2). Finally, 5 µl of each amplified PCR
product was separated by electrophoresis on a 1% agarose gel.
Separated bands in the gel were photographed using a gel imaging
system (GelDoc XR+, Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Quantitative analysis was conducted using Gel-Pro Analyzer software
(Media Cybernetics, Rockville, MD, USA). Target gene mRNA
expression was normalized to that of β-actin, which was used as the
internal reference.
Immunohistochemical analysis of NCAM1 and ERK2
protein expression in rat hippocampus
Paraffin-embedded hippocampus tissue sections from
10 rats in each group (5 sections for NCAM1 staining and 5 sections
for ERK2 staining) were deparaffinized, rehydrated and treated with
citric acid. Sections were immersed in a 3%
H2O2 solution for 10 min to block endogenous
peroxidase activity, and then rinsed three times (2 min each) in
phosphate-buffered saline (PBS).
Sections were incubated with either mouse anti-human
monoclonal anti-NCAM1 (catalog no., ab9272; Abcam, Cambridge, UK)
or mouse anti-rat monoclonal anti-ERK2 (catalog no., sc-154;
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) (diluted 1:200
in PBS with 0.1% bovine serum albumin) antibodies at 37°C for 1–2
h. As a negative control, an isotype matched immunoglobulin (Wuhan
Amyjet Scientific Co., Ltd., Wuhan, China) was used in place of the
primary antibody. After incubation, the sections were rinsed 3
times (2 min each) with PBS. A secondary antibody (HRP-conjugated
goat anti-mouse IgG SABC kit; catalog no., SA1027; Boster
Biological Technology, Pleasanton, CA, USA) was added and incubated
at room temperature or 37°C for 20 min, following by 3 rinses (2
min each) with PBS. Finally, a 3,3′-diaminobenzidine solution was
added for color development. After washing, the slides were mounted
and viewed under a light microscope (BX50; Olympus Corp., Tokyo,
Japan).
The number of positive cells in five randomly
selected fields (×400 magnification) was counted and recorded as
the percentage (%) of positive cells from the total number of cells
counted. NCAM1-positive cells were stained brown, and ERK2 positive
cells were stained yellowish-brown.
Statistical analysis
RT-PCR results were analyzed using Microsoft Office
Excel® 2010 software (Microsoft, Inc., Redmond, WA,
USA). Results for spatial navigation tests were compared by
analysis of variance (ANOVA) by accounting for group and time
(experimental day) effects. Group spatial memory probe test results
were compared by ANOVA. Group results for RT-PCR and
immunohistochemical staining results were compared by t-tests.
Statistical analysis used SPSS version 13.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Spatial navigation learning
A total of 20 rats comprised a control group for
normal behavioral observations, and SE was successfully established
in 100 rats. Following pharmaceutical treatments, an MWM was used
to test each rat for spatial navigation learning on 5 successive
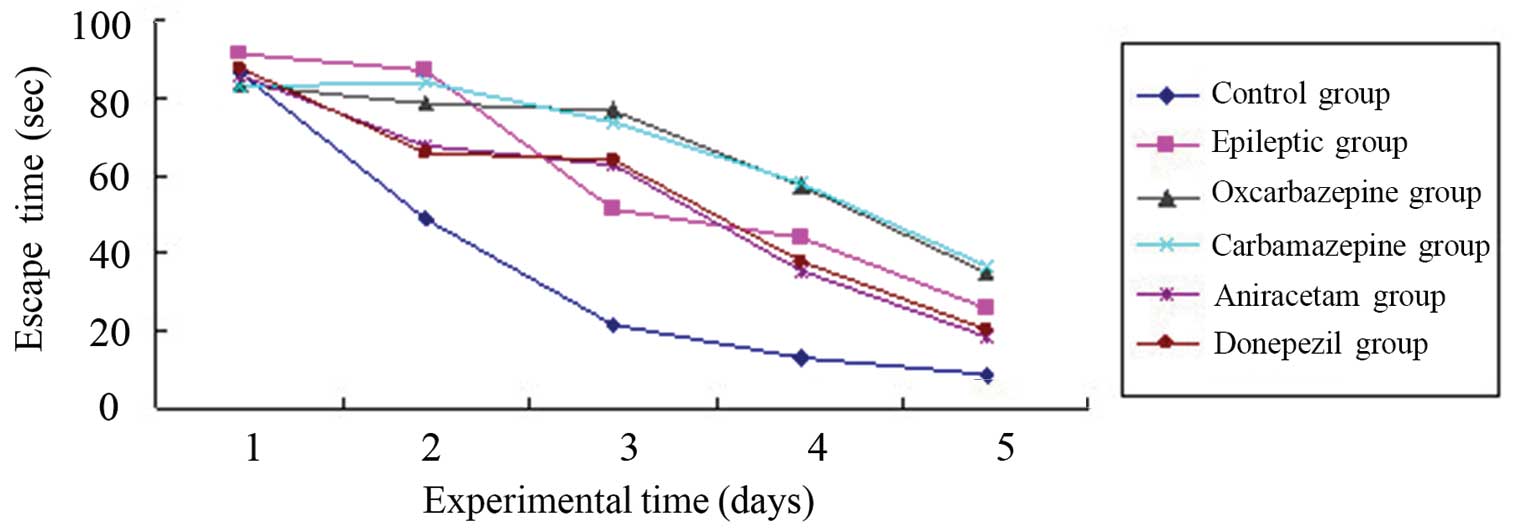
days. Figure 1 shows the mean escape
(latency) times for each group of rats per day. As expected, the
normal (control) group adapted well to this task, with the group's
mean latency time decreasing each day for 5 days. By contrast, all
groups of rats in which SE was established had a significantly
longer mean latency time compared with the control group, even
after 5 days (P=0.002 compared to control group). Thus, SE
significantly altered memory associated with the spatial navigation
learning task in the rats.
Among the experimental (SE) rat groups, the
time-course learning curves for this task were nearly identical for
the saline-treated ‘epileptic’ group and the groups treated with
aniracetam and donepezil. Thus, these agents used for cognitive
impairment did not improve the SE-associated memory impairment. In
addition, the time-course learning curves indicated significantly
poorer memory in the groups treated with the anticonvulsants
carbamazepine and oxcarbazepine (P=0.001 vs. saline-treated SE
group).
Probe trials for spatial memory
The MWM was also used for probe trials for spatial
memory following the removal of the escape platform from the pool.
Table I shows the mean percentages of
time that rats in each group spent swimming in each pool quadrant
over a 120 sec period (one trial per rat). Quadrant IV was the
‘platform quadrant’. Normal control rats had the best retained
spatial memory as demonstrated by spending the longest time
swimming in quadrant IV (mean, 50.62% of swimming time). By
contrast, all groups of rats in which SE was established spent
significantly less time in quadrant IV than control rats (<50%
of swimming time; P<0.01).
 | Table I.Results of Morris Water Maze tasks in
rats of each group (n=20/group). |
Table I.
Results of Morris Water Maze tasks in
rats of each group (n=20/group).
|
|
| Escape
(latency) | Swimming in
‘platform quadrant’ |
|---|
|
|
|
|
|
|---|
| Group | Group number | Time (sec; mean ±
SD) |
P-valuea |
P-valueb | Time (%; mean ±
SD) |
P-valuea |
P-valueb |
|---|
| Control | 1 | 35.78±4.84 | – | 0.002 | 50.62±11.47 | – | 0.004 |
| Epileptic | 2 | 67.14±7.37 |
0.002 | – | 36.65±13.28 | 0.004 | – |
| Carbamazepine | 3 | 81.23±9.46 | <0.001 | 0.001 | 26.84±08.68 | 0.001 | 0.005 |
| Oxcarbazepine | 4 | 71.59±8.81 | <0.001 | 0.241 | 31.55±14.13 | 0.002 | 0.153 |
| Aniracetam | 5 | 68.96±6.73 |
0.001 | 0.630 | 41.55±11.17 | 0.026 | 0.009 |
| Donepezil | 6 | 53.75±6.74 |
0.031 | 0.001 | 46.65±10.38 | 0.529 | 0.007 |
With regard to pharmacological intervention in the
SE rats, compared to the ‘epileptic’ group (saline treatment; mean,
36.65% of swimming time in quadrant IV), rats treated with
donepezil had significantly better spatial memory (mean,
46.65±13.28% of swimming time in quadrant IV; P=0.007), whereas
rats treated with carbamazepine had significantly worse spatial
memory (mean, 26.84±8.68% of swimming time in quadrant IV;
P=0.005). Other drugs did not demonstrate a significant difference
in their effect on spatial memory compared with that of saline
treatment.
Thus, based on the two MWM tasks, the current rat SE
model exhibited significant hippocampus-associated spatial learning
and memory impairments, and the different classes of
pharmacological agents investigated had varying effects on these
impairments (Table I).
Hippocampal expression of NCAM1
mRNA
RT-PCR analysis of NCAM1 and β-actin mRNA expression
was conducted in frozen hippocampal tissue from each group of rats.
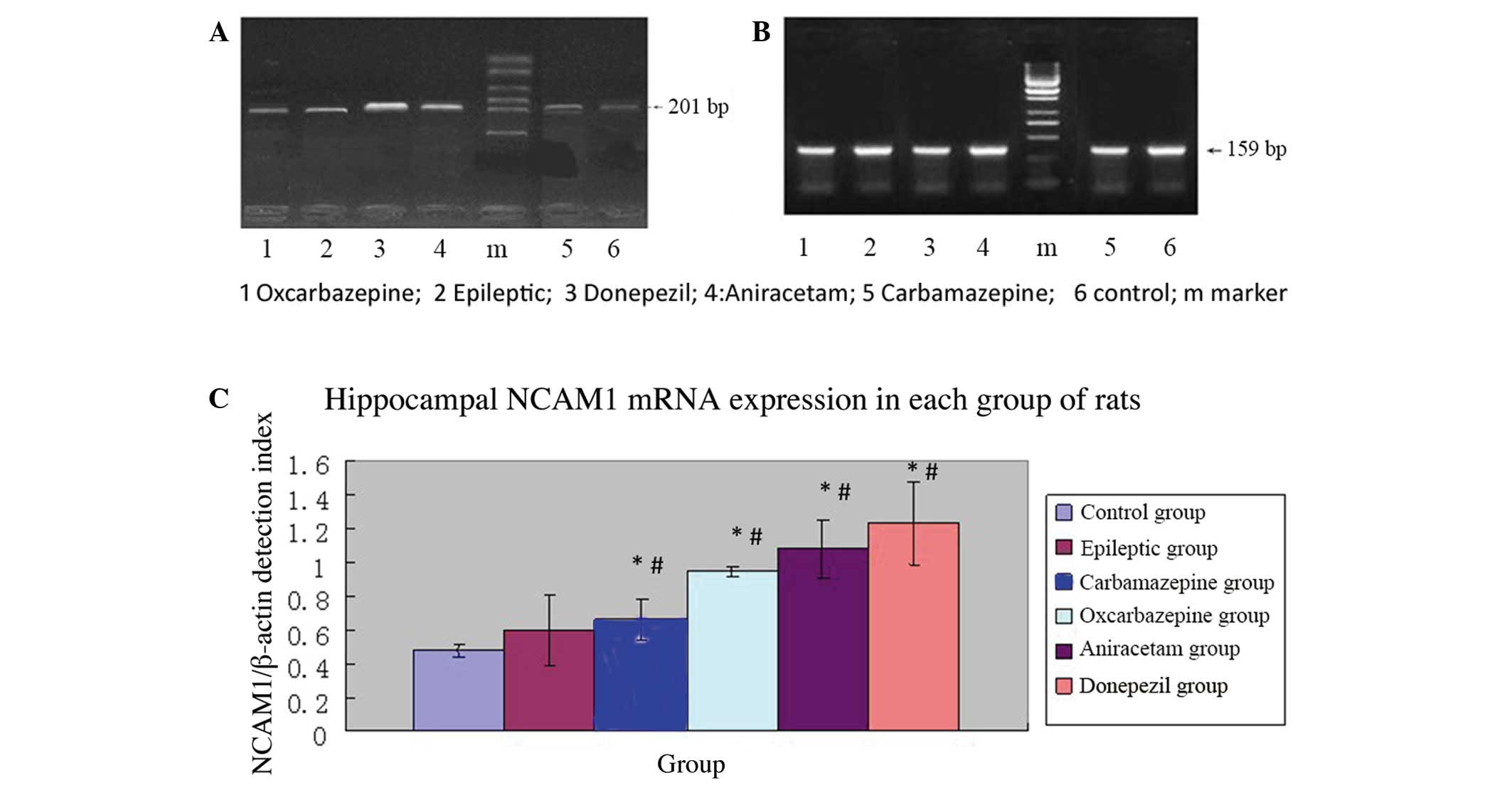
Figure 2 shows the mean relative
NCAM1 expression (NCAM1/β-actin mRNA ratios) for each group (5
rats/group). The mean NCAM1 mRNA expression was significantly
higher in the epileptic group (saline treatment) than in the normal
control group (P<0.01). Thus, the SE model resulted in increased
NCAM1 mRNA expression in the hippocampus.
Among SE rats subjected to pharmacological
intervention, those treated with carbamazepine had significantly
lower relative NCAM1 mRNA expression compared to the epileptic
group (P=0.005), whereas rats treated with donepezil had
significantly higher relative NCAM1 mRNA expression compared to the
epileptic group (P=0.001). Thus, among SE rats, a drug used to
treat cognitive impairment resulted in relatively higher NCAM1 mRNA
expression in the hippocampus, whereas an anticonvulsant agent
resulted in relatively lower NCAM1 mRNA expression.
In SE rats, relative NCAM1 mRNA expression in the
hippocampus was observed to be highest in those treated with
donepezil, followed by those treated with aniracetam, saline,
oxcarbazepine and carbamazepine (Table
II).
 | Table II.Comparison of hippocampal NCAM1 mRNA
and ERK2 mRNA expression in each group rats (n=5/group). |
Table II.
Comparison of hippocampal NCAM1 mRNA
and ERK2 mRNA expression in each group rats (n=5/group).
|
|
| NCAM mRNA
expression | ERK2 mRNA
expression |
|---|
|
|
|
|
|
|---|
| Group | Group number | Relative to β-actin
(mean ± SD) |
P-valuea |
P-valueb | Relative to β-actin
(mean ± SD) |
P-valuea |
P-valueb |
|---|
| Control | 1 | 0.48±0.04 | – | <0.001 | 1.33±0.16 | – | 0.005 |
| Epileptic | 2 | 0.95±0.21 | <0.001 | – | 0.96±0.21 | 0.005 | – |
| Carbamazepine | 3 | 0.60±0.12 | 0.002 | 0.005 | 0.59±0.24 | 0.001 | 0.005 |
| Oxcarbazepine | 4 | 0.66±0.03 | <0.001 | 0.005 | 0.75±0.23 | 0.001 | 0.077 |
| Aniracetam | 5 | 1.18±0.17 | <0.001 | 0.013 | 1.06±0.21 | 0.011 | 0.357 |
| Donepezil | 6 | 1.23±0.24 | <0.001 | 0.001 | 1.27±0.18 | 0.358 | 0.005 |
Hippocampal expression of ERK2
mRNA
Frozen hippocampal tissue from each group of rats
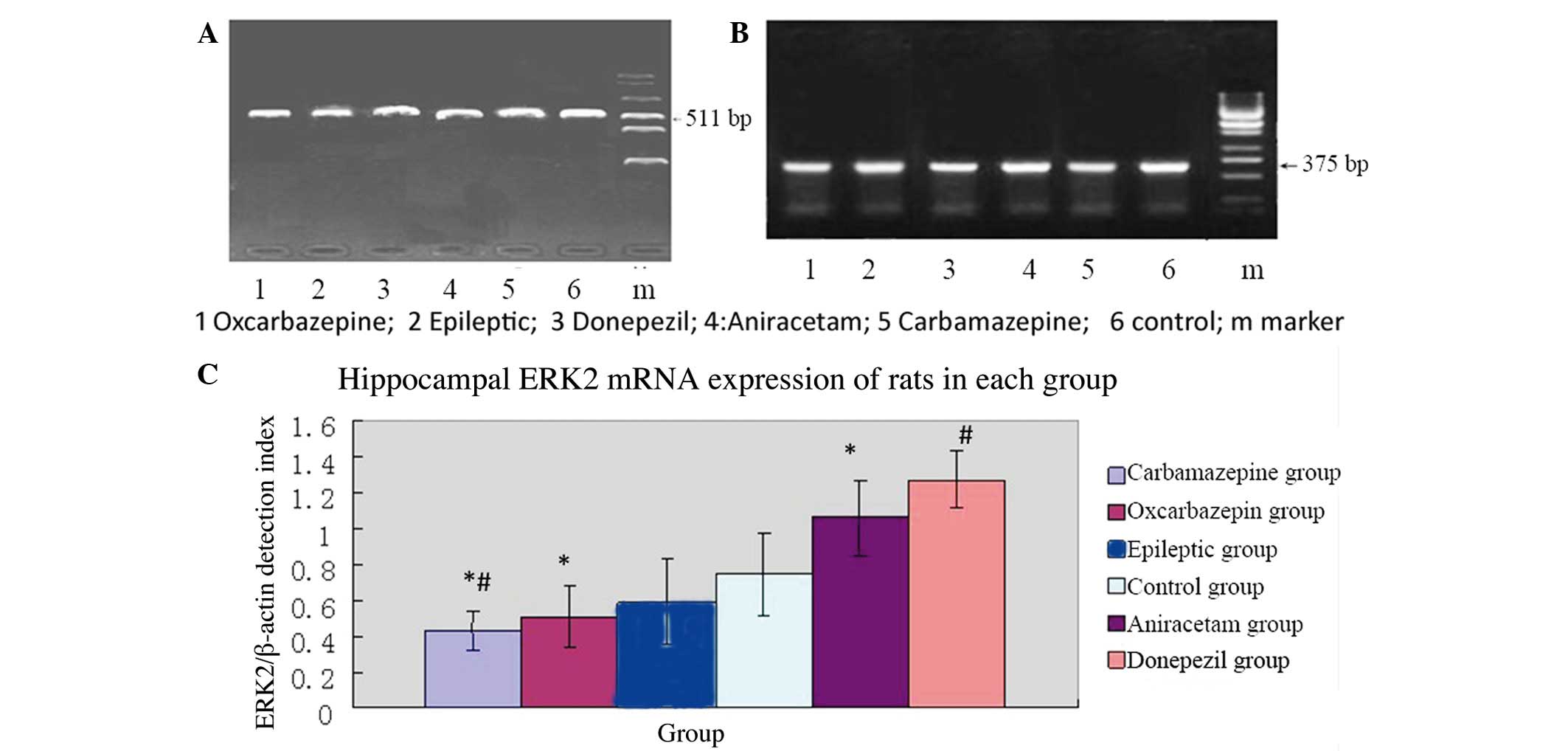
was also used for RT-PCR analysis of ERK2 mRNA expression. Figure 3 shows the mean relative ERK2
expression (ERK2/β-actin mRNA ratios) results per group (5
rats/group). In contrast to NCAM1 mRNA expression, the mean
relative ERK2 mRNA expression was significantly lower in the
epileptic group (saline treatment) than in the normal control group
(P=0.005). Thus, the SE model resulted in decreased ERK2 mRNA
expression in the hippocampus.
In SE rats subjected to pharmacological
intervention, those treated with carbamazepine had significantly
lower relative ERK2 mRNA expression compared with the epileptic
group (P=0.005). However, rats treated with donepezil had
significantly higher relative ERK2 mRNA expression compared to the
epileptic group (P=0.005). Thus, among SE rats, drugs used for the
treatment of cognitive impairment resulted in relatively higher
ERK2 mRNA expression in the hippocampus, whereas treatment with
anticonvulsant agents resulted in relatively lower ERK2 mRNA
expression in the hippocampus.
As with NCAM1 mRNA expression, the relative ERK2
mRNA expression was highest in the hippocampus of SE rats treated
with donepezil, followed by aniracetam, saline, oxcarbazepine and
carbamazepine.
Regarding the trends in NCAM1 and ERK2 mRNA
expression, compared to normal rats, saline-treated SE rats had
relatively higher NCAM1 mRNA and relatively lower ERK2 mRNA
expression in the hippocampus. Among SE rats subjected to
pharmacological intervention, compared with saline-treated SE rats,
those treated with anticonvulsants (carbamazepine and
oxcarbazepine) had relatively lower NCAM1 and ERK2 mRNA expression
in the hippocampus, whereas rats treated with drugs to treat
cognitive impairment (donepezil and aniracetam) had relatively
higher NCAM1 and ERK2 mRNA expression in the hippocampus (Table II).
Hippocampal expression of ERK2 and
NCMA1 protein
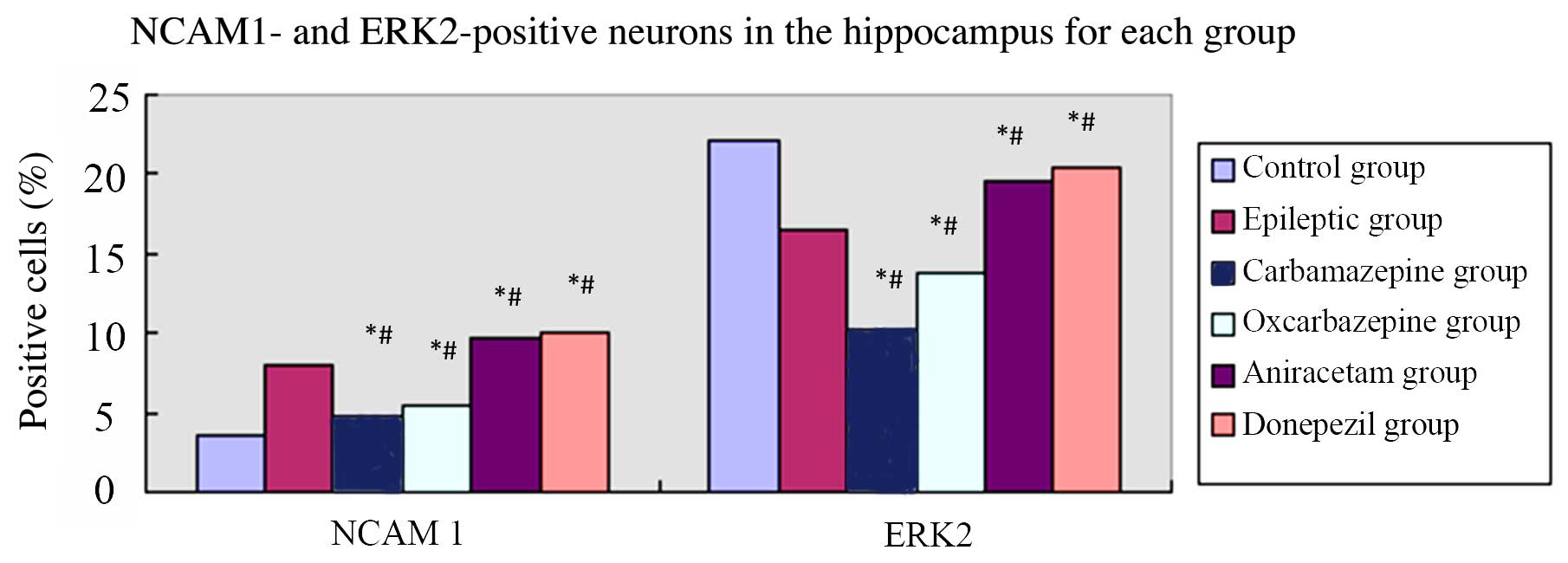
Figure 4 shows the
results of the immunohistochemical analysis of NCMA1 and ERK2
protein expression in hippocampal tissue. Similar to the mRNA
results, saline-treated SE rats had a greater percentage of
NCAM1-positive cells in the hippocampus compared to control rats
(P=0.001). Among the groups of SE rats, the percentages of
NCAM1-positive cells in the hippocampus were ordered high to low as
follows: Donepezil, aniracetam, saline, oxcarbazepine and
carbamazepine. Furthermore, as with the mRNA results,
saline-treated SE rats had relatively fewer ERK2-positive cells in
the hippocampus compared to control rats (P=0.003). Among the
groups of SE rats, the percentages of ERK2-positive cells in the
hippocampus were ordered high to low as follows: Donepezil,
aniracetam, saline, oxcarbazepine and carbamazepine. Thus, the
effects of pharmacological intervention on NCAM1 and ERK2
expression in the hippocampus of epileptic rats with cognitive
dysfunction was confirmed at the mRNA and protein levels (Table III).
 | Table III.Comparison of NCAM1- and
ERK2-positive neurons in the hippocampus for each group of rats
(n=5/group). |
Table III.
Comparison of NCAM1- and
ERK2-positive neurons in the hippocampus for each group of rats
(n=5/group).
|
|
| NCAM1 | ERK2 |
|---|
|
|
|
|
|
|---|
| Group | Group number | Number of positive
neurons (mean ± SD) |
P-valuea |
P-valueb | Number of positive
neurons (mean ± SD) |
P-valuea |
P-valueb |
|---|
| Control | 1 | 3.54±0.52 | – | 0.001 | 22.14±1.34 | – | 0.003 |
| Epileptic | 2 | 7.92±0.46 | 0.001 | – | 16.47±0.54 | 0.003 | – |
| Carbamazepine | 3 | 4.74±0.58 | 0.013 | 0.008 | 10.28±1.67 | 0.001 | 0.001 |
| Oxcarbazepine | 4 | 5.42±0.82 | 0.009 | 0.015 | 13.65±1.64 | 0.001 | 0.010 |
| Aniracetam | 5 | 9.78±0.83 | 0.003 | 0.004 | 19.57±1.72 | 0.008 | 0.017 |
| Donepezil | 6 | 10.13±0.79 | 0.001 | 0.002 | 20.36±1.28 | 0.016 | 0.002 |
Discussion
The purpose of the present preliminary investigation
was to assess the effects of various classes of pharmacological
agents on cognitive dysfunction (spatial memory impairment) in a
pilocarpine-induced rat model of status epilepticus (SE), as this
model reflects some of the neurophysiological and cognitive
deficits observed in human epilepsy (25,26). Using
Morris Water Maze (MWM) trials, the SE rat groups demonstrated
significant impairments in spatial learning and memory tasks. As
expected, poor performance was associated with altered NCAM1 and
ERK2 expression in hippocampal tissues of SE rats. It has been
reported that NCAM1 and ERK2 expressions, which are associated with
neuronal remodeling and synaptic plasticity (12,13,17), may
be altered in the hippocampal tissues of rodent models of epilepsy
(14–16,18,19). Thus,
this model was appropriate for investigating the effects of
pharmacological interventions.
Epilepsy is a clinical syndrome caused by abnormal,
highly synchronous discharges of brain neurons (1,2); this
condition is often accompanied by a decline in learning and memory
ability (4,5). With regard to treatment, the primary aim
is to improve seizure control (22).
However, while good clinical efficacy may be obtained with few
toxic side effects, certain patients may still be resistant to a
particular pharmacological agent. In addition, although seizure
control may be achieved, certain agents may worsen cognitive
impairments (23,24). One issue underlying these differential
responses to pharmacological interventions is the current limited
understanding of the effects of these agents on specific cellular
and molecular mechanisms.
The results of the current study revealed that,
compared with control rats with good spatial learning and memory in
MWM trials, saline-treated SE rats with impaired MWM trial results
had significantly reduced ERK2 expression and significantly
increased NCAM1 expression in hippocampal tissue at the mRNA and
protein levels. That these expressions were altered in opposite
directions was somewhat surprising, as NCAM expression is dependent
on ERK signaling during neurogenesis, at least in vitro
(20). However, these changes in
expression were evaluated at 1 month after SE generation; thus, it
is not known what shorter term effects may have been involved. In
addition, this preliminary investigation did not determine which
cell types expressed these molecules. Thus, the results may have
been due to different expressions levels of NCAM1 and ERK2 on
different cell types. This requires further investigation.
Nonetheless, the current results regarding
intervention with different pharmacological agents revealed the
same trends for NCAM1 and ERK2 expression in the hippocampal
tissues of SE rats. The trends for NCAM1 and ERK2 expressions in
the hippocampus (at both the mRNA and protein levels) paralleled
the trends for spatial memory impairments, by either worsening
(carbamazepine and oxcarbamazine) or improving (aniracetam and
donepezil) MWM spatial learning/memory performance.
In general, extracellular signal-regulated protein
kinases (ERKs), including ERK1 and ERK2, also called
mitogen-activated protein kinases (MAPKs), are critically involved
in signal transduction from cell surface receptors to the cell
nucleus, and regulate numerous cell functions, including
proliferation, differentiation, apoptosis and others. Thus, among
other functions in the nervous system, they are essential in
neurogenesis and synaptic plasticity (17). Total ERK expression and, particularly,
phosphorylated forms (p-ERK) are significantly higher in the brains
of patients with refractory epilepsy compared to healthy controls
(18). In rodent models, ERK
expression and signaling are required for generating epileptiform
discharges (16,19). Altered ERK (MAPK) expression/activity
is also associated with poor MWM spatial learning task performance
in rats (29). Although the
expression and activity of ERKs may vary between different
observation periods, it is involved with epilepsy and
hippocampus-associated spatial learning/memory.
NCAMs are cell surface glycoproteins that mediate
the recognition and adhesion of neural cells and are involved in
axonal growth, neuronal synaptic reconstruction, loop formation and
neuronal migration processes. These are important in axon
regeneration and synaptic reconstruction following brain injury
(30). During seizures, NCAM
overexpression may lead to abnormal neuronal loop formation, which
results in abnormal neuronal discharges (13). NCAMs are involved in regulating
hippocampal synaptic plasticity, which is essential in regulating
cognitive functions, including spatial learning and memory.
Increased NCAM expression has been detected in the hippocampus in
human temporal lobe epilepsy (31)
and in hippocampus mossy fiber axonal sprouts in
pilocarpine-induced epilepsy in rodents (32). In addition, conditional silencing of
NCAM expression in the mouse hippocampus is associated with
impaired spatial learning (33).
The present results from saline-treated SE rats were
consistent with the general trends observed in previous reports on
NCAM1 and ERK2 expression in the hippocampus of epileptic humans
and rodents and their associations with impaired spatial learning.
Thus, the present SE rat model was useful for, at least, evaluating
the general trends for intervention with different pharmacological
agents. However, these are general trends only, as the hippocampus
comprises different types of cholinergic and other systems and
cells, whose complex interrelationships must be taken into account
(34).
Carbamazepine and oxcarbazepine are stabilizers that
inactivate voltage-gated sodium channels, rendering them
unavailable for subsequent opening and reducing neuron
excitability. While both carbamazepine (35) and oxcarbazepine (36) are reasonably well tolerated,
oxcarbazepine is less toxic over long-term usage. However, the
effects of these anti-epileptic drugs on cognitive function have
been a major concern, particularly carbamazepine (37). The current results indicated that
these two drugs were associated with poorer spatial learning and
memory compared with saline-treated SE rats (Fig. 1; Table
I). In addition, relative to saline-treated SE rats, these
trends were associated with reducing NCAM1 and ERK2 expression in
the hippocampus; however, only the results of carbamazepine
treatment were statistically significant (Figs. 2–4). The
effects of these agents on worsening epilepsy-associated cognitive
impairment appears to be related to their inhibiting the activities
associated with NCAM1 and ERK2 expression.
Aniracetam, a so-called nootropic, is an anxiolytic
agent. Although its mechanism of action is uncertain,
investigations in mouse models suggest that it can act on
cholinergic, dopaminergic and serotonergic systems (38). It is thought that aniracetam may be an
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)
receptor agonist (glutamate analogue) that could potentially
mediate against fast synaptic transmission by competing with
glutamate for receptor transmission. Increased glutamate release is
associated with epilepsy-related changes in the hippocampus
(39). Although not statistically
significant, the present results suggest that aniracetam treatment
for SE rats could improve performance on spatial learning/memory
tasks by increasing hippocampal tissue expression of NCAM1 and
ERK2.
Donepezil is a reversible acetylcholinesterase
inhibitor. By increasing the availability of acetylcholine, it is
able to increase the activities of muscarinic acetylcholine
receptors, which are abundant in the hippocampus (34). Thus, it may have a neuroprotective
role. While donepezil has been demonstrated to be efficacious for
treating cognitive impairments associated with certain
neurodegenerative disorders, such as Alzheimer's disease, its
effects on cognitive impairments in epilepsy are not yet firmly
established (40). In the present rat
SE model, however, there were significant improvements in spatial
learning/memory tasks following donepezil treatment (Fig. 1; Table
I), and these improvements were associated with significantly
increased NCAM1 and ERK2 expression in the hippocampus at the mRNA
(Figs. 2 and 3) and protein levels (Fig. 4). However, it must be considered that
these effects of pharmacological intervention were for a 1 month
period after SE was established.
The current preliminary investigation had several
limitations. As previously noted, the specific cell types in the
hippocampus expressing NCAM1 and ERK2 were not determined. In
addition, only total NCAM1 and ERK2 expression, and not that of
their ‘activated’ forms, was evaluated; in the hippocampus,
epileptic activity is associated with highly polysialated NCAMs
(31) and p-ERKs (18). Also not investigated were the possible
dose-dependent effects of these pharmacological agents, or if
shorter or longer term use may have altered the results. Finally,
NCAM expression is dependent on ERK signaling during neurogenesis,
at least in vitro (20);
studies on NCAM1 and ERK2 co-expression in the same or different
cell types also warrants investigation.
In summary, in this preliminary investigation, a rat
model of SE was successfully established, and demonstrated
significantly impaired hippocampus-associated spatial learning and
memory. Treatment with two commonly used, first-line anticonvulsant
agents (carbamazepine and oxcarbazepine) resulted in poorer
performance on spatial memory tasks, whereas the nootropic
aniracetam and the acetylcholinesterase inhibitor donepezil
improved spatial memory task performance in SE rats. Compared to
non-pharmacologically (saline) treated SE rats, these trends were
paralleled by decreased (carbamazepine and oxcarbazepine) or
increased (aniracetam and donepezil) expression of NCAM1 and ERK2
in the hippocampus at the mRNA and protein levels.
Acknowledgements
Financial support was provided by the Shandong
Provincial Natural Science Foundation (grant no. 2009ZRB02602).
References
|
1
|
Browne TR and Holmes GL: Epilepsy. New
Engl J Med. 344:1145–1151. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang BS and Lowenstein DH: Epilepsy. New
Engl J Med. 349:1257–1266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scharfman HE: The neurobiology of
epilepsy. Curr Neurol Neurosci Rep. 7:348–354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hermann B and Seidenberg M: Epilepsy and
cognition. Epilepsy Curr. 7:1–6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou JL, Shatskikh TN, Liu X and Holmes
GL: Impaired single cell firing and long-term potentiation
parallels memory impairment following recurrent seizures. Eur J
Neurosci. 25:3667–3677. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu X, Muller RU, Huang LT, Kubie JL,
Rotenberg A, Rivard B, Cilio MR and Holmes GL: Seizure-induced
changes in place cell physiology: Relationship to spatial memory. J
Neurosci. 23:11505–11515. 2003.PubMed/NCBI
|
|
7
|
Parent JM, Tada E, Fike JR and Lowenstein
DH: Inhibition of dentate granule cell neurogenesis with brain
irradiation does not prevent seizure-induced mossy fiber synaptic
reorganization in the rat. J Neurosci. 19:4508–4519.
1999.PubMed/NCBI
|
|
8
|
Rutten A, van Albada M, Silveira DC, Cha
BH, Liu X, Hu YN, Cilio MR and Holmes GL: Memory impairment
following status epilepticus in immature rats: Time-course and
environmental effects. Eur J Neurosci. 16:501–513. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doherty P, Fazeli MS and Walsh FS: The
neural cell adhesion molecule and synaptic plasticity. J Neurobiol.
26:437–446. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Dai G, Cheng YB, Qi X and Geng MY:
Polysialylation promotes neural cell adhesion molecule-mediated
cell migration in an fibroblast growth factor receptor-dependent
manner, but independent of adhesion capability. Glycobiology.
21:1010–1018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu GY, Deisseroth K and Tsien RW: Spaced
stimuli stabilize MAPK pathway activation and its effects on
dendritic morphology. Nat Neurosci. 4:151–158. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kiss JZ and Muller D: Contribution of the
neural cell adhesion molecule to neuronal and synaptic plasticity.
Rev Neurosci. 12:297–310. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seidenfaden R, Krautzer A and Hildebrandt
H: The neural cell adhesion molecule NCAM regulates neurogenesis by
multiple mechanisms of interaction. Neurochem Int. 49:1–11. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niquet J, Jorquera I, Ben-An Y and Represa
A: NCAM immunoreactivity on mossy fibers and reactive astrocytes in
the hippocampus of epileptic rats. Brain Res. 626:106–116. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parent JM, Yu TW, Leibowitz RT, Geschwind
DH, Sloviter RS and Lowenstein DH: Dentate granule cell
neurogenesis is increased by seizures and contributes to aberrant
network reorganization in the adult rat hippocampus. J Neurosci.
17:3727–3738. 1997.PubMed/NCBI
|
|
16
|
Zhao W, Bianchi R, Wang M and Wong RK:
Extracellular signal-regulated kinase 1/2 is required for the
induction of group I metabotropic glutamate receptor-mediated
epileptiform discharges. J Neurosci. 24:76–84. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukunaga K and Miyamoto E: Role of MAP
kinase in neurons. Mol Neurobiol. 16:79–95. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xi ZQ, Wang XF, He RQ, Li MW, Liu XZ, Wang
LY, Zhu X, Xiao F, Sun JJ, Li JM, et al: Extracellular
signal-regulated protein kinase in human intractable epilepsy. Eur
J Neurol. 14:865–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Houser CR, Huang CS and Peng Z: Dynamic
seizure-related changes in extracellular signal-related kinase
activation in a mouse model of temporal lobe epilepsy.
Neuroscience. 156:222–237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mariggiò MA, Morabito C, Guarnieri S,
Gentile A, Kolkova K and Fanò G: IgIII (270-280)-fragment-like
H2N-DDSDEEN-COOH peptide modulates N-CAM expression via
Ca2+-dependent ERK signaling during ‘in vitro
neurogenesis’. Peptides. 29:1486–1497. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rowles J and Olsen M: Perspectives on the
development of antioxidant antiepileptogenic agents. Mini Rev Med
Chem. 12:1015–1027. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shorvon S: The treatment of chronic
epilepsy: A review of recent studies of clinical efficacy and side
effects. Curr Opin Neurol. 20:159–163. 2007.PubMed/NCBI
|
|
23
|
Beyenburg S, Bauer J and Reuber M: New
drugs for the treatment of epilepsy: A practical approach. Postgrad
Med J. 80:581–587. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wahab A: Difficulties in treatment and
management of epilepsy and challenges in new drug development.
Pharmaceuticals. 3:2090–2110. 2010. View Article : Google Scholar
|
|
25
|
Curia G, Longo D, Biagini G, Jones RS and
Avoli M: The pilocarpine model of temporal lobe epilepsy. J
Neurosci Methods. 172:143–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Freitas RM, Oliveira AA, Sousa FCF,
Vasconcelos SMM, Viana GSB and Fonteles FMM: Pathophysiology of
status epilepticus induced by pilocarpine. Cent Nerv Syst Agents
Med Chem. 7:11–15. 2007. View Article : Google Scholar
|
|
27
|
Racine RJ, Steingart M and McIntyre DC:
Development of kindling-prone and kindling-resistant rats:
Selective breeding and electrophysiological studies. Epilepsy Res.
35:183–195. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hemb M, Cammarota M and Nunes ML: Effects
of early malnutrition, isolation and seizures on memory and spatial
learning in the developing rat. Int J Dev Neurosci. 28:303–307.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shang X, Xue Y and Cai K: Expression of
N-methyl-1 D-asparate receptor (NMDAR) and mitogen activated
protein kinase (MAPK) in an Alzheimer disease (AD) rat model. Acta
Anatomica Sinica. 36:241–245. 2005.
|
|
30
|
Sato K, Iwai M, Nagano I, Shoji M and Abe
K: Temporal and spatial changes of highly polysialated neural cell
adhesion molecule immunoreactivity in amygdala kindling
development. Neurol Res. 25:79–82. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mikkonen M, Soininen H, Kälviänen R,
Tapiola T, Ylinen A, Vapalahti M, Paljärvi L and Pitkänen A:
Remodeling of neuronal circuitries in human temporal lobe epilepsy:
Increased expression of highly polysialated neural cell adhesion
molecule in the hippocampus and the entorhinal cortex. Ann Neurol.
44:923–934. 1988. View Article : Google Scholar
|
|
32
|
Shan W, Yoshida M, Wu XR, Huntley GW and
Colman DR: Neural (N-) cadherin, a synaptic adhesion molecule, is
induced in hippocampal mossy fiber axonal sprouts by seizure. J
Neurosci Res. 69:292–304. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bukalo O, Fentrop N, Lee AY, Salmen B, Law
JW, Wotjak CT, Schweizer M, Dityatev A and Schachner M: Conditional
ablation of the neural cell adhesion molecule reduces precision of
spatial learning, long-term potentiation and depression in the CA1
subfield of mouse hippocampus. J Neurosci. 24:1565–1577. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Woolf NJ: Cholinergic systems in mammalian
brain and spinal cord. Prog Neurobiol. 37:475–524. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marson AG, Al-Kharusi AM, Alwaidh M,
Appleton R, Baker GA, Chadwick DW, Cramp C, Cockerell OC, Cooper
PN, Doughty J, et al: The SANAD study of effectiveness of
carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or
topiramate for treatment of partial epilepsy: An unblinded
randomised controlled trial. Lancet. 369:1000–10015. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grant SM and Faulds D: Oxcarbazepine: A
review of its pharmacology and therapeutic potential in epilepsy,
trigeminal neuralgia and affective disorders. Drugs. 43:873–888.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Salinsky MC, Binder LM, Oken BS, Storzbach
D, Aron CR and Dodrill CB: Effects of gabapentin and carbamazepine
on the EEG and cognition in healthy volunteers. Epilepsia.
43:482–490. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakamura K and Kurusawa M: Anxiolytic
effects of aniracetam in three different mouse models of anxiety
and the underlying mechanism. Eur J Pharmacol. 420:33–43. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee TS, Mane S, Eid T, Zhao H, Lin A, Guan
Z, Kim JH, Schweitzer J, King-Stevens D, Weber P, et al: Gene
expression in temporal lobe epilepsy is consistent with increased
release of glutamate by astrocytes. Molec Med. 13:1–13. 2007.
View Article : Google Scholar
|
|
40
|
Hamberger MJ, Palmese CA, Scarmeas N,
Weintraub D, Choi H and Hirsch LJ: A randomized, double-blind,
placebo-controlled trial of donepezil to improve memory in
epilepsy. Epilepsia. 48:1283–1291. 2007. View Article : Google Scholar : PubMed/NCBI
|