Introduction
Breast cancer (BC) is the second most common
malignancy in the world, with estimated 1.67 million newly
diagnosed cases and 522,000 associated mortalities worldwide in
2012 (http://globocan.iarc.fr; accessed May 1,
2015). Despite recent efforts to improve the detection rates and
treatment of BC, the current situation, as reflected by disease
statistics, is not favorable. A better understanding of the tumor's
pathobiology will undoubtedly bring novel possibilities for
treatment and diagnosis. In an attempt to offer the best medical
approach possible for every single patient, modern medicine is
evolving towards personalized therapies, which are characterized by
a proper balance between the most radical approach possible while
avoiding undesired side effects resulting from aggressive
treatment. This attitude requires novel ideas regarding drug
development and accurate stratification of the patients; therefore,
novel prognostic factors are necessary.
Dysregulated cellular division is a key event in
cancer initiation and progression. Polo-like kinases (PLKs) are a
family of proteins that regulate the cell cycle (1). There are four PLKs with serine-threonine
kinase activity in humans (PLK1-PLK4), whereas in PLK5 [which was
first described in mice (2)] the
kinase domain is truncated and does not possess any catalytic
activity (3). However, PLK5 appears
to participate in neuronal differentiation and act as a tumor
suppressor in brain cancer (3). In
addition, PLK1-PLK4, and particularly PLK2, display other roles
beyond mitosis regulation (4,5).
PLK1, the best characterized protein of the PLK
family, is a serine-threonine kinase that plays a crucial role in
the regulation of cell division, centrosome maturation and
duplication, assembly of the bipolar spindle, sister chromatid
splitting, activation of the anaphase-promoting complex (APC),
regulation of mitotic exit and induction of cytokinesis (1,6–14). PLK1 protein comprises two main
domains: i) A conserved serine-threonine kinase domain at the
N-terminus that is crucial for its kinase activity, and ii) a
polo-box domain, a non-catalytic domain that is critical for its
spatial distribution in the cells and its molecular interactions
with specific substrates (12,13). PLK1
directly phosphorylates cell division cycle 27 protein (a component
of the APC) and cyclin B1 (15,16), and
together with other important signaling proteins such as p34
kinase, is responsible for mitotic progression (12).
PLK1 acts as a modulator of the DNA damage response
and as a novel factor in the maintenance of genome stability during
DNA replication (13). In response to
DNA damage, the checkpoint kinases ataxia telangiectasia mutated
(ATM) and ataxia telangiectasia and Rad3-related protein (ATR)
become activated and inhibit entry into mitosis via deactivation of
cyclin-dependent kinase 1 (CDK1), which is the crucial kinase that
promotes cell division (17).
Effective resumption of cell cycle progression (arrested at G2/M)
and mitotic entry upon successful repair of DNA damage are based on
the activation of PLK1 by Aurora A/Bora-mediated phosphorylation
(18,19). Additionally, activated PLK1 is
involved in the enzymatic inactivation of WEE1 (a protein kinase
that inhibits CDK1) (20) and
elimination of claspin, which functions as a key adaptor protein
for checkpoint kinase 1 activity (21). Cytophysiological downregulation of
WEE1 and claspin by enhanced activity of PLK1 promotes CDK1
activation and leads to mitotic entry (20,21).
High PLK1 expression is observed within intensively
proliferating normal tissues such as placenta and colonic
epithelium (22), and in various
types of cancer, including gastric (23), colorectal (24), hepatocellular (25), prostate (26), breast (27,28),
ovarian (29) and non-small cell lung
carcinomas (30). Notably, due to its
position as a central controller of mitosis, PLK1 has become a
potentially valuable target for antiproliferative therapies
(31). Emerging experimental results
are encouraging, and several anti-PLK1 agents are currently being
investigated in clinical trials (32). No predictive factor has been
identified thus far that could be used as a reliable qualifier to
the potential inclusion of PLK1 inhibitor therapy in BC treatment.
Similarly to the case of human epidermal growth factor receptor-2
(HER-2), immunohistochemical analysis of PLK1 expression in BC
cells and the subsequent decision of initiating therapy or
excluding the patient from therapy may be an alternative. This
hypothesis requires verification in extensive, multicentre research
studies.
Although great progress has been achieved since the
initial characterization of human PLK1 >20 years ago (22,33), there
is disagreement among researchers regarding the precise role of
this kinase in cancer pathogenesis, and the prognostic significance
of its expression in breast tumors has not been clearly established
to date.
The present study reports an association between
PLK1 expression and patient survival in 5-, 10- and 15-year
follow-ups. In addition, an analysis of the correlations between
PLK1 expression and other clinicopathological and histopathological
features is provided.
Materials and methods
Patients
Tissue samples were acquired from 83 radically
treated patients with stage II ductal BC diagnosed between 1993 and
1994 in the Lower Silesian Oncology Centre (Wroclaw, Poland). The
patients' mean age was 55.2 years. The study population was
selected based on the availability of tissues. All patients
underwent surgery (Madden mastectomy) with or without adjuvant
treatment [27% of patients were treated by chemotherapy based on
the CMF scheme (100 mg/m2 cyclophosphamide per day, days
1–14; 40 mg/m2 intravenous methotrexate, days 1 and 8;
500 mg/m2 intravenous fluorouracil, days 1 and 8; for 6
cyles of 28 days), which is no longer in use]. Following treatment,
the patients were under continuous monitoring in the Lower Silesian
Oncology Centre. Data regarding relapse and mortality were
collected using medical documentation available in the Lower
Silesian Oncology Centre. Overall survival (OS), cancer-specific
overall survival (CSOS) and disease-free survival (DFS) rates were
established for all patients. Table I
contains detailed characteristics of the cohort. The present study
was approved by the Institutional Review Board of Wroclaw Medical
University.
 | Table I.Patient and tumor characteristics,
and their association with PLK1 immunoreactivity in breast cancer
patients. |
Table I.
Patient and tumor characteristics,
and their association with PLK1 immunoreactivity in breast cancer
patients.
|
|
| Parameters of PLK1
immunoreactivity |
|
|---|
|
|
|
|
|
|---|
| Patient
characteristics | No. (%) | % | Intensity | IRS | High expression of
PLK1 (IRS ≥8) |
|---|
| All patients | 83 (100.0) | 0.888 | 0.204 | 0.480b | 0.982c |
| Age
(years)a |
|
| Mean,
55.2±10.3; median: 55 |
|
| Median,
55 |
|
|
Menopausec |
|
|
Premenopausal | 27 (32.5) | 0.316 | 0.392 | 0.935 | 0.296d |
|
Postmenopausal | 56 (67.5) |
|
| TNM stage according
to UICCc |
| 0.244 | 0.674 | 0.879 | 0.570d |
| II
A | 33 (39.8) |
|
| II
B | 50 (60.2) |
|
| Tumor size
(pT)a (mm) |
| 0.540 | 0.585 | 0.969 | 0.280c |
| Mean,
31.0±12.3 |
|
| Median,
30 |
|
| Nodal metastases
(N)c |
| 0.232 | 0.020 | 0.006 | 0.037d |
|
N_ | 47 (56.6) |
|
|
N+ | 36 (43.4) |
|
|
Gradingc |
| 0.001 | 0.746 | 0.057 | 0.014d |
| G2 | 59 (71.1) |
|
| G3 | 24 (28.9) |
|
| ER
statusa |
| 0.182 | 0.561 | 0.363 | 0.464c |
|
Negative | 22 (26.5) |
|
|
Positive | 61 (73.5) |
|
| PgR
statusa |
| 0.169 | 0.374 | 0.894 | 0.831c |
|
Negative | 22 (26.5) |
|
|
Positive | 61 (73.5) |
|
| HER-2
statusa |
| 0.735 | 0.714 | 0.875 | 0.646c |
|
Negative | 64 (77.1) |
|
|
Positive | 19 (22.9) |
|
|
Recurrencec |
| 0.394 | 0.001 | <0.001 |
<0.001d |
|
Yes | 32 (38.6) |
|
| No | 51 (61.4) |
|
Tumor samples
Tumor specimens were fixed in 10% buffered formalin
and embedded in paraffin. All hematoxylin and eosin stained
sections were evaluated by two pathologists (P.D. and A.H.,
Department of Pathomorphology and Oncological Cytology, Wroclaw
Medical University, Wroclaw). Tumor stages were assessed according
to the tumor-node-metastasis classification system (34). Tumor grades were estimated according
to the Scarff-Bloom-Richardson protocol (35), with the Elston-Ellis (36) modification (Table I).
Immunohistochemistry
Immunohistochemical analyses were performed
retrospectively on tissue samples collected for routine diagnostic
purposes. Formalin-fixed, paraffin-embedded tissue sections were
freshly prepared (4 µm thickness; Accu-Cut SRMTM; Sakura, Alphen
aan den Rijn, The Netherlands). Immunohistochemistry was performed
as previously described (37). For
the detection of PLK1, a monoclonal mouse antibody against PLK1 (BD
Transduction Laboratories™; BD Biosciences, Franklin Lakes, NJ,
USA) was diluted 1:500 in the Antibody Diluent with Background
Reducing Components (DakoCytomation; Dako, Glostrup, Denmark). For
detection of estrogen receptor (ER), an optimally pre-diluted
monoclonal mouse antibody was used (clone 1D5; DakoCytomation;
Dako), while for detection of progesterone receptor (PgR), an
optimally pre-diluted monoclonal antibody (clone PgR636;
DakoCytomation; Dako) was used. For HER-2 detection, a
semi-quantitative diagnostic immunohistochemical test was used
(HercepTest™Kit; K5207; DakoCytomation; Dako). Tissue sections were
incubated with the above antibodies for 1 h at room temperature.
Subsequent incubations involved biotinylated antibodies (15 min,
room temperature) and a streptavidin-biotinylated peroxidase
complex (15 min, room temperature) (LSAB 2 System-HRP;
DakoCytomation; Dako). As a chromogen, 3,3′-diaminobenzidine
(DakoCytomation; Dako) was used (10 min, room temperature). All
sections were counterstained with Mayer's hematoxylin. In each
case, control reactions were included, in which the specific
antibody was substituted by a primary mouse antibody
(DakoCytomation; Dako), which served as a negative control.
Evaluation of immunohistochemical
reaction intensity
The intensity of the immunohistochemical reaction
was assessed independently by two pathologists. In doubtful cases,
a re-evaluation was performed using a double-headed microscope,
(BX45; Olympus, Tokyo, Japan) and staining was discussed until a
consensus was achieved.
PLK1 expression was evaluated using the
semi-quantitative scale of the immunoreactive score (IRS),
according to Remmele and Stegner with certain modifications
(37,38), which considers the percentage of
reactive cells (no staining=0, <25%=1, 25–50%=2, 51–75%=3 and
>75%=4) and the intensity of staining (no staining=0, weak=1,
intermediate=2 and strong=3), with the final result being the
product of both variables. Consequently, nine possible scores (0,
1, 2, 3, 4, 6, 8, 9 and 12) were obtained.
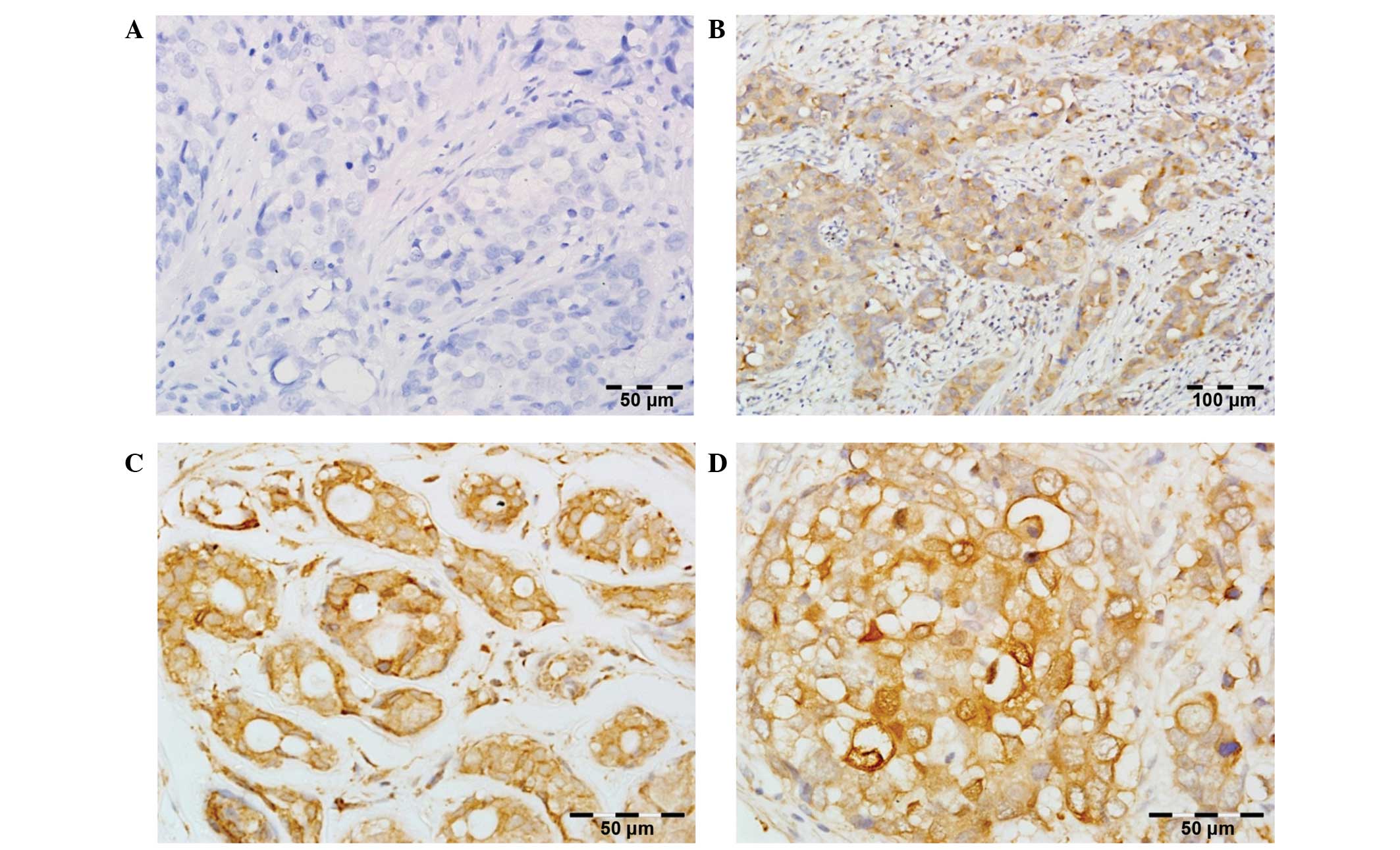
PLK1 expression was only observed in tumor tissues
of BC specimens with cytoplasmic localization. Normal breast
tissues were characterized by no or weak cytoplasmic PLK1
immunoreactivity.
For subsequent statistical analyses, a two-grade
scale system was applied, allocating 0 points for expression of
PLK1 <8 (low PLK1 immunoreactivity) and 1 for expression of PLK1
≥8 (high PLK1 immunoreactivity). Definition of these two groups and
determination of the cut-off point is a specific consensus of
histopathological observations and statistical analyses.
Evaluation of ER, PgR and HER-2 expression was
performed using standard methods described in a previous study
(36).
Statistical analysis
Statistical analysis was performed using the
Statistica 10.0 software package (StatSoft Inc., Tulsa, OK, USA).
OS was defined as the time between primary surgical treatment and
mortality, and it was censored at the last follow-up for those
patients who were alive. DFS was defined as the time between
primary surgical treatment and date of relapse or mortality,
whichever occurred first. DFS was censored at the last follow-up
for patients who survived without disease recurrence. CSOS was
defined as the time between primary surgical treatment and
cancer-associated mortality, and was censored at the last follow-up
for surviving patients.
To analyze the associations between PLK1 protein
expression and clinicopathological parameters, the Pearson linear
correlation coefficient in case of quantitative variables, the
Kendall rank correlation in case of ordinal variables, the Pearson
χ2 test of independence in case of categorical variables
and the exact Fisher test in case of 2×2 tables, were used.
Differences between two groups were tested with the Mann-Whitney U
test, while the log-rank test was used for comparison of survival
in two groups. The OS rate was estimated by the Kaplan-Meier
method, and the influence of explanatory variables on mortality
risk was analyzed by Cox proportional hazard regression and
logistic regression in case of binary survival. P<0.05 was
considered to indicate a statistically significant difference.
Results
PLK1 immunostaining in BC
specimens
PLK1 expression defined as IRS >0 was detected in
all 83 BC patients. The average IRS was 6.55±3.10, and the median
was 6.00. For statistical analysis, augmented immunoreactivity of
PLK1 was defined as IRS ≥8 (38 patients, 45.8%), while low
immunoreactivity was assigned to IRS=0–6 (45 patients, 54.2%)
(Fig. 1).
Association between PLK1 expression
and clinicopathological parameters
Overexpression of PLK1 and high intensity of
immunohistochemical reaction were significantly correlated with the
presence of regional lymph node metastases (P=0.03700 and 0.02000,
respectively) (Table I). Disease
recurrence was observed more frequently in patients with increased
PLK1 expression and with high intensity of PLK1 immunoreactivity
(P<0.00100 and P=0.00100, respectively). Paradoxically,
increased PLK1 expression and high percentage of PLK1+
cells were associated with lower histological grade (P=0.01400 and
0.00100, respectively). No significant correlations were observed
between PLK1 expression and hormone receptor/HER-2 status, primary
tumor size, menopausal status or age at the time of diagnosis
(Table I).
PLK1 immunoreactivity and patient
survival at 5-, 10- and 15-year follow-ups
Univariate logistic regression analysis of PLK1
expression in the context of 5-, 10- and 15-year survival revealed
highly negative prognostic significance of PLK1 overexpression in
patients with early stage BC in all the follow-up periods analyzed
(Table II).
 | Table II.Univariate analysis of correlations
between immunohistochemical parameters of PLK1 expression and 5-,
10- and 15-year CSOS, and multivariate Cox regression analysis of
PLK1 expression and 15-year CSOS in groups with and without lymph
node metastases and in the whole cohort of patients. |
Table II.
Univariate analysis of correlations
between immunohistochemical parameters of PLK1 expression and 5-,
10- and 15-year CSOS, and multivariate Cox regression analysis of
PLK1 expression and 15-year CSOS in groups with and without lymph
node metastases and in the whole cohort of patients.
| A, Univariate
logistic regression |
|---|
|
|---|
|
| 5-year
survival | 10-year
survival | 15-year
survival |
|---|
|
|
|
|
|
|---|
| Parameters of PLK1
expression | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) |
|---|
| Positive cells
(%) | 0.20700 | 1.85
(0.71–4.87) | 0.18800 | 1.62
(0.79–3.33) | 0.11400 | 1.79
(0.87–3.68) |
| Intensity | 0.01200 | 3.14
(1.29–7.65) | 0.00200 | 3.33
(1.59–7.01) | 0.00040 | 4.51
(2.01–10.10) |
| IRS | 0.00700 | 1.35
(1.09–1.67) | 0.00080 | 1.40
(1.16–1.70) | 0.00020 | 1.54
(1.24–1.92) |
| High expression
(IRS ≥8) | 0.00500 | 9.92
(2.01–49.05) | 0.00060 | 7.80
(2.48–24.51) | 0.00010 | 12.18
(3.75–39.62) |
|
| B, Multivariate Cox
regression analysis of 15-year survival |
|
|
| All patients | Without lymph node
metastases | With lymph node
metastases |
|
|
|
|
|
| Clinicopathological
parameters | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) |
|
| High expression of
PLK1 | 0.00030 | 6.13
(2.30–16.33) | 0.01200 | 19.21
(1.91–193.28) | 0.01400 | 4.02
(1.32–12.20) |
| Tumor size
(pT) | 0.12100 | 1.03
(0.99–1.07) | 0.05100 | 1.07
(1.00–1.14) | 0.52600 | 1.01
(0.97–1.06) |
| Lymph node
metastases | 0.00300 | 3.57
(1.55–8.24) | – | – | – | – |
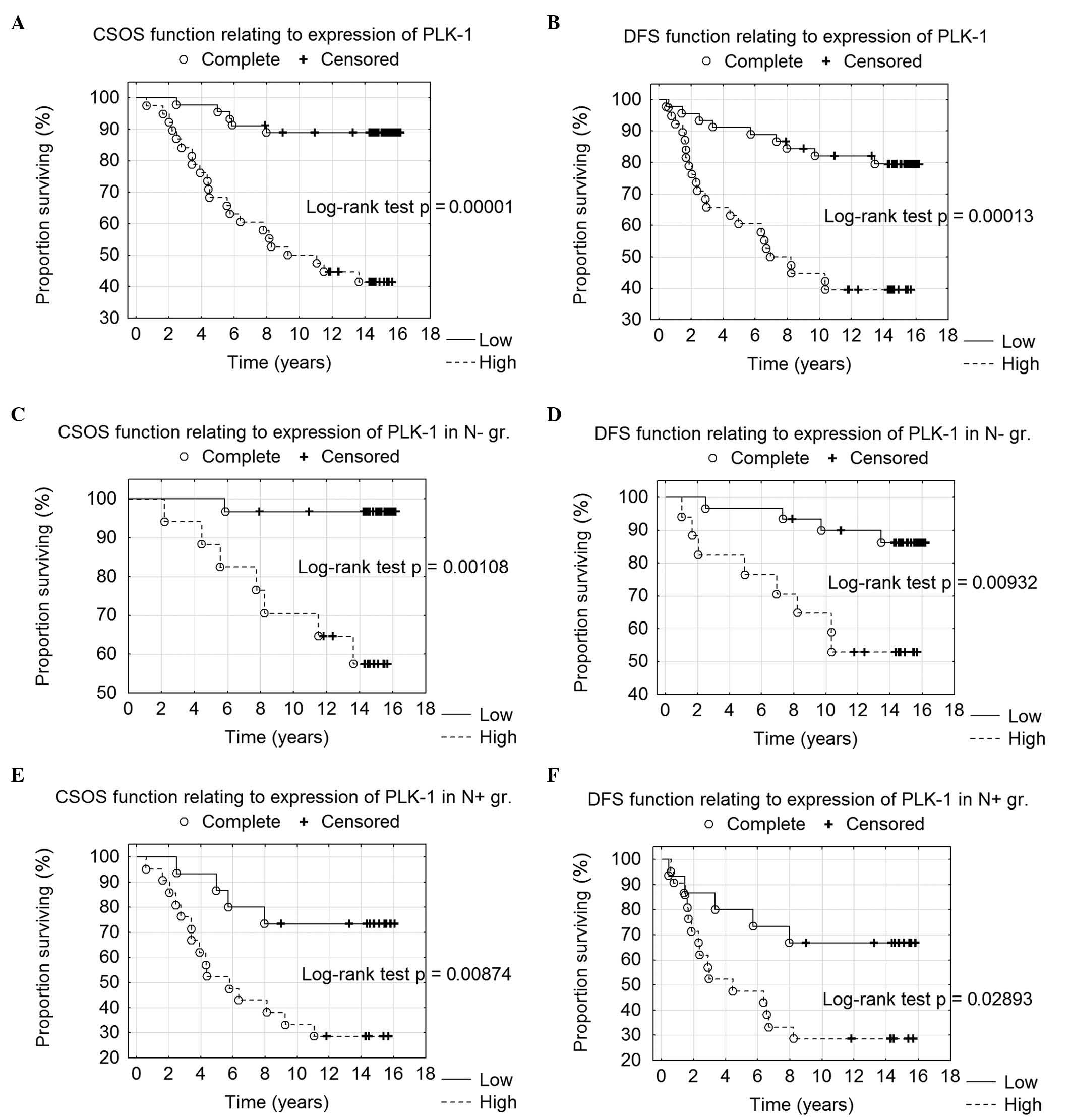
Kaplan-Meier analysis confirmed the correlation of
PLK1 overexpression with long-term survival, as high PLK1
immunoreactivity (IRS ≥8) was associated with shorter CSOS and DFS
(P=0.00001 and 0.00013, respectively) (Fig. 2A and B). Additionally, high PLK1
immunoreactivity was correlated with shorter CSOS and DFS in
patients without local lymph node metastases (P=0.00110 and
0.00900, respectively) (Fig. 2C and
D) and in patients with diagnosed nodal metastatic foci
(P=0.00900 and 0.03000, respectively) (Fig. 2E and F).
Multivariate Cox regression
analysis
In the multivariate Cox regression analysis, two
clinicopathological parameters were noticed to have independent
prognostic value in patients with early stage BC, namely high
expression of PLK1 (P=0.00030) and presence of local lymph node
metastases (P=0.00300). Other clinicopathological features had no
significance in the multivariate Cox model.
Since lymph node metastases had a significant
prognostic impact, multivariate analysis was performed individually
in N− and N+ patients (Table II). It was demonstrated that, in both
lymph node-negative and positive groups, high expression of PLK1
was an independent unfavorable prognostic factor (P=0.01200 and
0.01400, respectively), which confirms the findings of univariate
analysis.
Discussion
In the present study, a homogeneous group of
patients with stage II invasive ductal BC was investigated with
regard to expression levels of PLK1 and patient survival in a
15-year follow-up period. The associations between PLK1 reactivity
in BC specimens and the status of HER-2 and steroid receptors were
also evaluated.
Overexpression of PLK1 (defined as IRS ≥8) was
detected in 45.8% of patients (38 patients), while low
immunoreactivity of PLK1 was observed in 54.2% of patients (45
patients). PLK1 expression was only observed in the tumoral
compartment of BC specimens, with cytoplasmic localization. With
regard to the cytoplasmic expression pattern of PLK1, the present
results were similar to those reported by other studies (27,28). By
contrast, the findings regarding the cut-off value for high PLK1
immunoreactivity and the incidence of PLK1 overexpression were less
concordant (27,28). King et al (28) demonstrated PLK1 overexpression in only
11% of analyzed patients, whereas Weichert et al (27) reported overexpression in 42.2% cases
of BC, a value close to the present observations (45.8%). Likely
reasons for these dissimilarities are methodological differences in
PLK1 expression assessment between the studies and a highly
homogenous study population (stage II, according to the Union for
International Cancer Control classification) in the present study
(34).
In the current study, overexpression of PLK1 was
significantly correlated with the presence of regional lymph node
metastases (P=0.03700) and disease recurrence (P<0.00100).
Kaplan-Meier analysis confirmed the correlation of PLK1
overexpression with long-term survival, as high PLK1
immunoreactivity was strongly associated with shorter CSOS and DFS
(P=0.00001 and 0.00013, respectively). In a multivariate Cox
regression analysis, two clinicopathological parameters were
observed to have independent prognostic value in patients with
early stage BC: High expression of PLK1 (P=0.00030) and presence of
regional lymph node metastases (P=0.00300).
Highly negative impact of increased PLK1 expression
on patient prognosis was also observed by King et al
(28), who demonstrated significantly
shorter OS of patients with PLK1 overexpression in their analysis
of 215 subjects. In addition, a positive correlation between PLK1
expression and the presence of a mutant version of the tumor
protein p53 gene was also revealed in that study (28). Weichert et al (27) did not confirm the prognostic
significance of enhanced PLK1 immunoreactivity in BC cells, and
only PLK3 overexpression was observed by the authors to be a
negative predictor of OS and recurrence-free survival.
An important point in the interpretation of the
present results is the significant correlation between PLK1
overexpression and the presence of regional lymph node metastases,
which is commonly accepted as an independent predictor of negative
prognosis (38). King et al
(28) and Weichert et al
(27) did not observe any significant
associations between increased PLK1 immunoreactivity and regional
nodal metastases. The absence of associations between PLK1
overexpression and PgR/HER-2 status in the current results are in
agreement with those of other authors (27,28).
Notably, King et al (28) and
Weichert et al (27)
demonstrated that negative ER status and high histological grade
correlated with PLK1 overexpression, which was not confirmed in the
present study. This is probably due to the highly homogenous
population (comprising only early BC patients) in the current
study, whereas the study groups in the aforementioned reports
contained patients in all stages of the disease.
Another aspect worth considering is the role of PLK1
expression as a potential marker of cell proliferation, since
strong expression of PLK1 (which has been associated with enhanced
mitotic activity) is detectable in actively proliferating cells
(those in phase G2/M) (39). In the
present study and in the study conducted by Weichert et al
(27), there were cases of BC in
which 100% of cells exhibited strong PLK1 immunoreactivity. This
observation is difficult to interpret and requires further
investigation. PLK1 overexpression is closely associated with the
G2/M phase of the cell cycle in in vitro models (40). However, such a remarkably high
proportion of PLK1+ cells does not necessarily imply
that all the positive cells are in the G2/M phase (which is the
active phase of proliferation) at the same time. The above
observation may indicate a pleiotropic significance of PLK1 in
cytophysiology; thus, its expression may not only be a symptom of
ongoing cellular divisions, but may also reflect a cellular
response to DNA damage in cancer cells and the subsequent attempts
to repair it by numerous enzymes, including ATM, ATR and
poly(ADP-ribose) polymerase 1 (PARP-1). This postulate is in line
with the results of the present study, which identified a positive
correlation between enhanced PLK1 and PARP-1 expression in BC cells
(data not shown).
Additionally, PLK1 overexpression in cancer cells
may result from chromosomal overrepresentation of the PLK1
gene locus, which leads to increased protein production. This is
consistent with the observations of Tirkkonen et al
(41), who detected chromosomal
amplification of the 16p12 region (which contains the PLK1
locus) in 38% of BC patients, a rate that is close to the 45.8% of
tumors overexpressing PLK1 detected in the present study.
In conclusion, there is a significant and
independent association between PLK1 overexpression and unfavorable
prognosis in the 15-year follow-up of early BC patients. The
results of the present study suggest a potential role for PLK1 in
the progression of BC. The present findings may aid to generate
molecular targeted therapies based on PLK1 inhibitors.
Acknowledgements
The present study was supported by funding from the
Wroclaw Medical University (Wroclaw, Poland; research grant nos.
ST-593 and Pbmn157).
References
|
1
|
Glover DM, Hagan IM and Tavares AA:
Polo-like kinases: A team that plays throughout mitosis. Genes Dev.
12:3777–3787. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andrysik Z, Bernstein WZ, Deng L, Myer DL,
Li YQ, Tischfield JA, Stambrook PJ and el Bahassi M: The novel
mouse polo-like kinase 5 responds to DNA damage and localizes in
the nucleolus. Nucleic Acids Res. 38:2931–2943. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Cárcer G, Escobar B, Higuero AM, García
L, Ansón A, Pérez G, Mollejo M, Manning G, Meléndez B,
Abad-Rodríguez J and Malumbres M: Plk5, a polo box domain-only
protein with specific roles in neuron differentiation and
glioblastoma suppression. Mol Cell Biol. 31:1225–1239. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Cárcer G, Manning G and Malumbres M:
From Plk1 to Plk5: Functional evolution of polo-like kinases. Cell
Cycle. 10:2255–2262. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seeburg DP, Pak D and Sheng M: Polo-like
kinases in the nervous system. Oncogene. 24:292–298. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hansen DV, Loktev AV, Ban KH and Jackson
PK: Plk1 regulates activation of the anaphase promoting complex by
phosphorylating and triggering SCFbetaTrCP-dependent destruction of
the APC Inhibitor Emi1. Mol Biol Cell. 15:5623–5634. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Golsteyn RM, Mundt KE, Fry AM and Nigg EA:
Cell cycle regulation of the activity and subcellular localization
of Plk1, a human protein kinase implicated in mitotic spindle
function. J Cell Biol. 129:1617–1628. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lane HA and Nigg EA: Antibody
microinjection reveals an essential role for human polo-like kinase
1 (Plk1) in the functional maturation of mitotic centrosomes. J
Cell Biol. 135:1701–1713. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petronczki M, Glotzer M, Kraut N and
Peters JM: Polo-like kinase 1 triggers the initiation of
cytokinesis in human cells by promoting recruitment of the RhoGEF
Ect2 to the central spindle. Dev Cell. 12:713–725. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lowery DM, Lim D and Yaffe MB: Structure
and function of Polo-like kinases. Oncogene. 24:248–259. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Vugt MA and Medema RH: Getting in and
out of mitosis with Polo-like kinase-1. Oncogene. 24:2844–2859.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takai N, Hamanaka R, Yoshimatsu J and
Miyakawa I: Polo-like kinases (Plks) and cancer. Oncogene.
24:287–291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takaki T, Trenz K, Costanzo V and
Petronczki M: Polo-like kinase 1 reaches beyond
mitosis-cytokinesis, DNA damage response, and development. Curr
Opin Cell Biol. 20:650–660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
el Bahassi M: Polo-like kinases and DNA
damage checkpoint: Beyond the traditional mitotic functions. Exp
Biol Med (Maywood). 236:648–657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kotani S, Tugendreich S, Fujii M,
Jorgensen PM, Watanabe N, Hoog C, Hieter P and Todokoro K: PKA and
MPF-activated polo-like kinase regulate anaphase-promoting complex
activity and mitosis progression. Mol Cell. 1:371–380. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nigg EA: Mitotic kinases as regulators of
cell division and its checkpoints. Nat Rev Mol Cell Biol. 2:21–32.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartek J and Lukas J: DNA damage
checkpoints: From initiation to recovery or adaptation. Curr Opin
Cell Biol. 19:238–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Macůrek L, Lindqvist A, Lim D, Lampson MA,
Klompmaker R, Freire R, Clouin C, Taylor SS, Yaffe MB and Medema
RH: Polo-like kinase-1 is activated by aurora A to promote
checkpoint recovery. Nature. 455:119–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seki A, Coppinger JA, Jang CY, Yates JR
and Fang G: Bora and the kinase Aurora A cooperatively activate the
kinase Plk1 and control mitotic entry. Science. 320:1655–1658.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Vugt MA, Brás A and Medema RH:
Polo-like kinase-1 controls recovery from a G2 DNA damage-induced
arrest in mammalian cells. Mol Cell. 15:799–811. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mamely I, van Vugt MA, Smits VA, Semple
JI, Lemmens B, Perrakis A, Medema RH and Freire R: Polo-like
kinase-1 controls proteasome-dependent degradation of Claspin
during checkpoint recovery. Curr Biol. 16:1950–1955. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holtrich U, Wolf G, Bräuninger A, Karn T,
Böhme B, Rübsamen-Waigmann H and Strebhardt K: Induction and
down-regulation of PLK, a human serine/threonine kinase expressed
in proliferating cells and tumors. Proc Natl Acad Sci USA.
91:1736–1740. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jang YJ, Kim YS and Kim WH: Oncogenic
effect of Polo-like kinase 1 expression in human gastric
carcinomas. Int J Oncol. 29:589–594. 2006.PubMed/NCBI
|
|
24
|
Takahashi T, Sano B, Nagata T, Kato H,
Sugiyama Y, Kunieda K, Kimura M, Okano Y and Saji S: Polo-like
kinase 1 (PLK1) is overexpressed in primary colorectal cancers.
Cancer Sci. 94:148–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He ZL, Zheng H, Lin H, Miao XY and Zhong
DW: Overexpression of polo-like kinase1 predicts a poor prognosis
in hepatocellular carcinoma patients. World J Gastroenterol.
15:4177–4182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weichert W, Schmidt M, Gekeler V, Denkert
C, Stephan C, Jung K, Loening S, Dietel M and Kristiansen G:
Polo-like kinase 1 is overexpressed in prostate cancer and linked
to higher tumor grades. Prostate. 60:240–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weichert W, Kristiansen G, Winzer KJ,
Schmidt M, Gekeler V, Noske A, Müller BM, Niesporek S, Dietel M and
Denkert C: Polo-like kinase isoforms in breast cancer: Expression
patterns and prognostic implications. Virchows Arch. 446:442–450.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
King SI, Purdie CA, Bray SE, Quinlan PR,
Jordan LB, Thompson AM and Meek DW: Immunohistochemical detection
of Polo-like kinase-1 (PLK1) in primary breast cancer is associated
with TP53 mutation and poor clinical outcome. Breast Cancer Res.
14:R402012. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weichert W, Denkert C, Schmidt M, Gekeler
V, Wolf G, Köbel M, Dietel M and Hauptmann S: Polo-like kinase
isoform expression is a prognostic factor in ovarian carcinoma. Br
J Cancer. 90:815–821. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang ZX, Xue D, Liu ZL, Lu BB, Bian HB,
Pan X and Yin YM: Overexpression of polo-like kinase 1 and its
clinical significance in human non-small cell lung cancer. Int J
Biochem Cell Biol. 44:200–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weiß L and Efferth T: Polo-like kinase 1
as target for cancer therapy. Exp Hematol Oncol. 1:382012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yim H: Current clinical trials with
polo-like kinase 1 inhibitors in solid tumors. Anticancer Drugs.
24:999–1006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hamanaka R, Maloid S, Smith MR, O'Connell
CD, Longo DL and Ferris DK: Cloning and characterization of human
and murine homologues of the Drosophila polo
serine-threonine kinase. Cell Growth Differ. 5:249–257.
1994.PubMed/NCBI
|
|
34
|
Bloom HJ and Richardson WW: Histological
grading and prognosis in breast cancer; a study of 1409 cases of
which 359 have been followed for 15 years. Br J Cancer. 11:359–377.
1957. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours (7th). Wiley-Blackwell.
Hoboken, NJ: 2009.
|
|
36
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Halon A, Donizy P, Surowiak P and
Matkowski R: ERM/Rho protein expression in ductal breast cancer: A
15 year follow-up. Cell Oncol (Dordr). 36:181–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fitzgibbons PL1, Page DL, Weaver D, Thor
AD, Allred DC, Clark GM, Ruby SG, O'Malley F, Simpson JF, Connolly
JL, et al: Prognostic factors in breast cancer. College of American
Pathologists Consensus Statement 1999. Arch Pathol Lab Med.
124:966–978. 2000.PubMed/NCBI
|
|
39
|
Degenhardt Y and Lampkin T: Targeting
Polo-like kinase in cancer therapy. Clin Cancer Res. 16:384–389.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
41
|
Tirkkonen M, Tanner M, Karhu R,
Kallioniemi A, Isola J and Kallioniemi OP: Molecular cytogenetics
of primary breast cancer by CGH. Genes Chromosomes Cancer.
21:177–184. 1998. View Article : Google Scholar : PubMed/NCBI
|